Coke distribution determines the lifespan of a hollow Mo/HZSM-5 capsule catalyst in CH4 dehydroaromatization†
Abstract
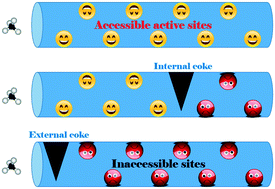
The effect of coke distribution (external versus internal coke) on the deactivation behavior was investigated in methane dehydroaromatization (MDA) using benchmark metal-modified HZSM-5 catalysts. A hollow HZSM-5 capsule zeolite (HC) was facilely prepared by a one-step hydrothermal strategy with the assistance of Na2EDTA and n-butylamine, and a commercial HZSM-5 (CZ) with a similar Si/Al molar ratio was used as a reference support. Four catalysts (Mo/HC, W/HC, Mo/CZ, and W/CZ) were synthesized via an incipient wetness impregnation method, and tested in the MDA reaction. The properties of coke species were characterized by means of the TG, TPO, HRTEM, and UV-Raman techniques, and different locations of coking over the spent catalysts were obtained. The results showed that external coke can give rise to a more severe deactivation than internal coke owing to the blockage of the pore mouths with external coke that decreased the diffusion length of feed gas in the zeolite channels. This phenomenon could be explained by the excellent catalytic stability over the hollow Mo/HZSM-5 capsule catalyst during the MDA reaction, which is associated with the lower external coke formation rate. Finally, the evolution of Mo species and coke species over the coked Mo/HC catalyst was determined by in situ UV-Raman spectroscopy during the TPO process, and the regeneration by O2 was conducted for the hollow Mo/HZSM-5 capsule catalyst.


 Please wait while we load your content...
Please wait while we load your content...