Use of stability diagrams to predict catalyst speciation during Fischer Tropsch reduction stage: a mini-review
Abstract
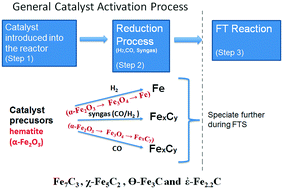
An understanding of the speciation of iron catalyst precursor during reduction is an important piece of knowledge in Fischer–Tropsch Synthesis (FTS) reaction. The thermodynamics of the precursor speciation is governed by the reducing gases used, and this tends to influence the activity, selectivity and the catalyst life span. During catalyst reduction or FT synthesis, the interaction of reducing agents with Fe-based catalyst results in the formation of several gaseous components such as CO, H2, CO2 and H2O. The partial pressure of each gaseous component and operating conditions determine the predominant state of the catalyst. Catalyst speciation happens due to different partial pressures of the gaseous components, and as a result, the stable iron phases formed during catalyst reduction are those that are in equilibrium with the gas composition. Although the ratio of PH2/PH2O and PCO/PCO2 or the partial pressure of water and carbon dioxide does not have any appreciable effect on the deactivation by oxidation on the FT reaction rate in Cobalt catalysts, in the iron based catalyst deactivation by oxidation is predominant. Based on thermodynamic calculations of the FTS system, the maximum allowable oxygen partial pressure during catalyst activation is 1 × 10−45 bars. The oxygen partial pressure is therefore dependent on the PH2/PH2O and PCO2/PCO ratios, and the equilibrium constants.


 Please wait while we load your content...
Please wait while we load your content...