C–H bond cleavage occurring on a Rh(v) intermediate: a theoretical study of Rh-catalyzed arene azidation†
Abstract
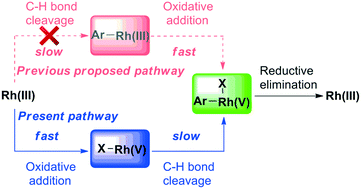
The sequence of C–H bond cleavage, oxidative addition, and reductive elimination is often proposed to account for Rh(III)-catalyzed arene functionalization. Invariably, C–H bond cleavage is the rate-limiting step. In our present work, the sequence of steps in Rh-catalyzed C–H azidation of arenes is theoretically studied by the density functional theory method M11-L. Theoretical calculations indicated that the oxidation of Rh(III) to Rh(V) by PhI(OAc)OTs is a facile process. Subsequent electrophilic deprotonation, which is the rate-determining step, was shown to occur from a Rh(V) intermediate rather than a Rh(III) intermediate. Finally, the C–N3 reductive elimination from a cyclometalated Rh(V) complex could give the azidation product and regenerate the Rh(III) catalyst. Moreover, the natural population analysis (NPA) of the oxidation process clarified that Rh(III) is oxidized to Rh(V) by trivalent iodine.


 Please wait while we load your content...
Please wait while we load your content...