Enhanced visible-light-driven photocatalysis from WS2 quantum dots coupled to BiOCl nanosheets: synergistic effect and mechanism insight†
Peiyuan
Xiao
a,
Jufeng
Lou
a,
Huixian
Zhang
a,
Weili
Song
a,
Xi-Lin
Wu
*a,
Hongjun
Lin
 a,
Jianrong
Chen
a,
Jianrong
Chen
 *a,
Shoujie
Liu
c and
Xiangke
Wang
*a,
Shoujie
Liu
c and
Xiangke
Wang
 ab
ab
aCollege of Geography and Environmental Science, Zhejiang Normal University, Jinhua, 321004, China. E-mail: dbwxl@zjnu.cn; cjr@zjnu.cn; Fax: +86 579 82282273; Tel: +86 579 82291275
bSchool of Environment and Chemical Engineering, North China Electric Power University, Beijing 102206, China
cCollege of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241000, China
First published on 2nd November 2017
Abstract
Quantum dots (QDs) derived from two-dimensional (2D) nanosheets (NSs), especially ultrathin transition metal dichalcogenide (TMD, e.g. WS2, WSe2, MoS2, MoSe2) NSs, have attracted great attention due to their broad band absorption and high charge mobility. Herein, WS2 QDs, one of the emerging extraordinary zero-dimensional (0D) TMD materials, were applied to the preparation of novel 0D/2D heterojunctions of WS2 QDs/BiOCl nanosheets. The obtained WS2/BiOCl composites exhibited significantly enhanced visible-light-driven photocatalytic activity as compared with pure BiOCl. The results indicated that the holes (h+) and O2˙− are the main active species generated by the catalysts under visible light irradiation. The enhanced photocatalytic performance could be due to the broad band absorption and up-conversion properties of the WS2 QDs as well as the band alignment and the strong coupling between the WS2 QDs and BiOCl NSs, leading to a broadened light absorption range and enhanced efficiency for electron–hole pair production and separation. These findings offer exciting opportunities using the extraordinary 2D TMD material-derived quantum dots for the fabrication of novel 0D/2D composites and may provide new insights into the application of the novel 0D/2D composites in catalysis.
Introduction
Graphene, a classic two-dimensional (2D) material, has stimulated a wide range of interests of materials scientists and physicists in the past decade. The extensive study of graphene has opened a new stream of research for other 2D materials, including transition metal dichalcogenides (e.g. WS2, WSe2, MoS2, MoSe2), several carbide- and nitride-based materials (e.g. h-BN, g-C3N4) and mono-elemental 2D sheets (e.g. black phosphorus).1–3 As emerging zero-dimensional (0D) materials, quantum dots derived from these 2D materials have drawn increasing attention due to their unique properties and unprecedented merits.1 Tungsten disulfide (WS2) is one of the transition metal dichalcogenides which is composed of 2D single layer WS2 building blocks stacked together by van der Waals forces.3,4 By reducing the lateral size of WS2 nanosheets, abundant defects produced at the edge of the small WS2 nanoparticles combined with the changed edge structure and stronger quantum confinement effects endow the WS2 QDs with unique electronic properties and tunable bandgaps, leading to extraordinary electrical and optical properties.4,5 Although, WS2 QDs hold many distinct and enormous advantages, the study of WS2 QDs is still at its early stage. In recent years, several studies on the fabrications, properties and applications of WS2 QDs have been published;4–8 however, their properties are still far from being fully understood and their applications are largely unexploited.Semiconductor-based photocatalysts have drawn great attention and have been widely used as catalysts for environmental remediation, energy harvesting/production and water splitting.9–11 TiO2, the first example of a semiconductor based photocatalyst, has been studied for applications in pollution clean-up since the 1970s.12 However, over 96% of sunlight cannot be utilized by pure TiO2 due to the fact that pure TiO2 can only be excited by ultraviolet (UV) light (Eg = 3.2 eV for anatase).13 After this, other semiconductor based photocatalysts such as ZnO,14,15 SnO2,15 ZnS,15 CdS,15 g-C3N4,16 Ag3PO4,17 and Bi2WO6 (ref. 18) have been developed and applied for environmental management. Although many attempts have been made in the past two decades at developing novel photocatalysts, achieving high efficiency remains a great challenge due to the easy electron–hole recombination and limited visible-light absorption. In recent years, bismuth oxyhalides have been demonstrated to be promising photocatalysts for photocatalytic energy conversion and environment remediation because of their layered-structure mediated unique physicochemical properties, high chemical/optical stability, low-cost and nontoxicity.19 BiOCl, one of the bismuth oxyhalides, has a wide band gap (UV region) which is theoretically not suitable for visible-light-driven photocatalysts.20,21 In addition, the photocatalytic performance of BiOCl is still far from sufficient for potential industrial applications. Till now, methods such as internal electric field tuning, dehalogenation, surface functionalization, doping and heterojunction construction have been applied for improving the photocatalytic efficiencies of bismuth oxyhalides.19 Among these methods, construction of a semiconductor heterojunction is considered as the most promising approach toward highly efficient photocatalysis due to the improved spectrum absorption and increased effectiveness for electron–hole pair separation.13 Intuitively, 0D WS2 quantum dots could be suitable candidates for coupling with 2D BiOCl, forming 0D/2D semiconductor–semiconductor heterojunctions.
Herein, we fabricate 0D/2D heterojunctions of WS2 QDs/BiOCl nanosheets (NSs) for the first time via a facile one-pot solvothermal method. The WS2 QDs with an average size of about 7 nm were uniformly incorporated in the phase of BiOCl NSs. The optical properties, morphologies, structures and photocatalytic performance of WS2/BiOCl were investigated in detail. The photocatalytic activity of the WS2/BiOCl materials was evaluated by using water-soluble organic dyes (Rhodamine B (RhB) and Congo Red (CR)) as model pollutant compounds. It was found that the WS2/BiOCl heterojunctions showed much higher photocatalytic activity for the degradation of organic dyes compared with the bare BiOCl NSs under visible light irradiation. The catalytic properties of WS2/BiOCl can be tuned by varying the amount of WS2 QDs added to the precursor. The possible structure–activity relationships are discussed in detail and related photocatalytic mechanisms are proposed.
Experimental section
Materials
All the materials used for the experiments were of analytical grade and received from the supplier without any further purification. Tungsten sulfide (WS2, 99.9%), Rhodamine B (RhB) and Congo Red (CR) were purchased from Aladdin Reagent Co. (China). N,N-Dimethylformamide (DMF), sodium chloride (NaCl), bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) and mannitol were supplied by Sinopharm Chemical Reagent Co., Ltd.Synthesis of 0D/2D heterojunctions of WS2/BiOCl
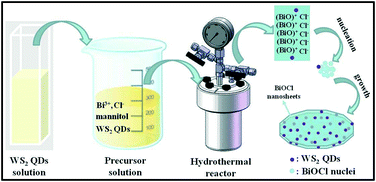
The WS2 quantum dots were prepared via a sonication–solvothermal method. Typically, 1 g of WS2 powder was added to 100 mL DMF and subjected to sonication for 3 h with an output power of 250 W. Then the top 2/3 of the mixture was transferred into a flask and heated for 6 h at 140 °C under vigorous stirring. After that, the suspension was cooled to room temperature and centrifuged at 2000 rpm for 10 min. The yellow supernatant is the WS2 QDs that we need.The 0D/2D heterojunction of WS2/BiOCl was prepared by a one-pot solvothermal approach (Scheme 1). Typically, 0.24 g of Bi(NO3)3·5H2O was dissolved in 15 mL mannitol solution (0.1 M) by sonication at room temperature. Afterwards, the desired amount (5, 10 and 15 mL, respectively) of WS2 QDs was slowly added to the above solution under magnetic stirring. Then, 5 mL of NaCl solution (0.1 M) was added dropwise to the above suspension and kept stirring for another 1 h. The obtained solution was transferred into a 50 mL Teflon-lined autoclave and heated at 140 °C for 3 h. Finally, the autoclave was then cooled down to room temperature. The product was collected by centrifugation, washed with deionized water and ethanol three times and dried at 60 °C under vacuum for 24 h. The final products were denoted as WS2/BiOCl-1, WS2/BiOCl-2, and WS2/BiOCl-3 according to the different amounts (5, 10 and 15 mL, respectively) of WS2 QDs added to the precursor. The contents of WS2 in the above WS2/BiOCl-1, WS2/BiOCl-2, and WS2/BiOCl-3 samples are ∼2 wt%, 4 wt% and 6 wt%, respectively (Table S1†).
The proposed formation process of the 0D/2D heterojunction of WS2/BiOCl is shown in Scheme 1. During the hydrothermal process, Bi3+ was firstly hydrolyzed to produce BiO+.21 Then, the BiO+ ions were bonded to each other to form larger layered [Bi2O2]2+ nanocrystals and Cl− was inserted to the layered structure.20 At the same time, the negatively charged WS2 QDs were attracted by positively charged BiO+ to form the WS2/BiOCl heterojunctions. The layered BiOCl nanocrystals continued to grow by combining with the remaining BiOCl nuclei, finally forming the 2D BiOCl nanosheets (NSs) through the Ostwald ripening process.22 During the formation of BiOCl NSs, the mannitol may act as a cross linker and soft template to promote the assembly and growth of the BiOCl nanocrystals.21
Characterization
The X-ray diffraction (XRD) patterns were recorded on a Shimadzu XRD-6000 X-ray diffractometer equipped with Cu Kα radiation (λ = 1.54178 Å). The Raman scattering measurement was conducted on a LabRAM-HR confocal Raman microprobe equipped with a 514.5 nm argon ion laser. The scanning electron microscopy (SEM) images were collected using a scanning electron microscope (Hitachi S-4800) operated at an accelerating voltage of 10 kV. The transmission electron microscopy (TEM) images were obtained from a JEM-2100 transmission electron microscope with an accelerating voltage of 200 kV. The X-ray photoelectron spectroscopy (XPS) measurements were performed on an ESCALAB 250 system with an Al Kα source. The UV/vis absorption of WS2 was measured using a lambda 25 UV/vis spectrometer (Perkin-Elmer, USA). The photoluminescence (PL) spectra and lifetimes of WS2/BiOCl were obtained using an LS-55 system (Perkin Elmer, USA) and an FLS 980 fluorescence spectrophotometer (Edinburgh instruments, UK). The UV-vis-NIR diffuse reflectance spectra were displayed on a UV-2450 spectrophotometer (Shimadzu Corporation, Japan) with BaSO4 as the reference. The content of WS2 in the WS2/BiOCl heterojunction was determined by inductively coupled plasma (ICP) atomic emission spectrometric analysis (POEMS, TJA). The electron spin resonance (ESR) spectra were recorded using an ESR spectrometer at a frequency of about 9.8 GHz (JES-FA200, Japan). The photocurrent was measured in phosphate buffer solution (PBS, 0.1 M, pH 7.0) with an electrochemical workstation (CHI660 B, Shanghai Chenhua, Inc.).Catalytic degradation experiments
The photocatalytic activity of the pure BiOCl and WS2/BiOCl was investigated by using Rhodamine B (RhB) and Congo Red (CR) as the model organic pollutants and a 300 W Xe lamp with a piece of UV cutoff filter (λ > 420 nm) was used as the simulated visible light source. For the photocatalytic experiments, 20 mg of the catalyst was dispersed into 100 mL of dye solution and then the suspension was placed in the dark with continuous stirring for 1 h to achieve the adsorption–desorption equilibrium. After that, the suspension was subjected to irradiation and 2 mL of the solution was sampled at certain time intervals and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to separate the catalysts. The concentration of organic dyes in the separated supernatant was determined with a UV-vis spectrometer. The degradation efficiency (DE) was calculated using the following equation:
000 rpm for 10 min to separate the catalysts. The concentration of organic dyes in the separated supernatant was determined with a UV-vis spectrometer. The degradation efficiency (DE) was calculated using the following equation:| DE(%) = (C0 − Ct)/Ct × 100% | (1) |
Results and discussion
Structure and morphology
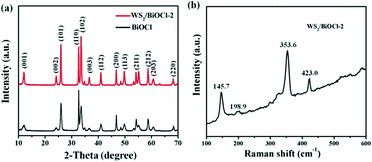
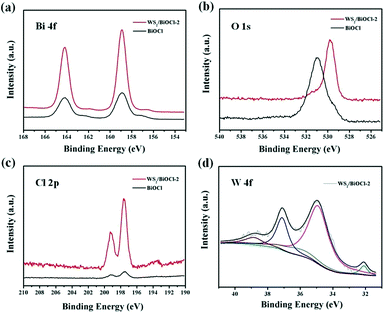
The composition and structure of the pure BiOCl and 0D/2D heterojunctions of WS2/BiOCl were investigated by X-ray diffraction (XRD) and Raman scattering techniques. The XRD patterns of the pure BiOCl and WS2/BiOCl are shown in Fig. 1a. All the peaks which appeared in the patterns can be assigned to the tetragonal phase of BiOCl (JCPDS 73-2060),21 and the peaks of WS2/BiOCl match well with those of the pure BiOCl. The results indicate that the formation of well-crystalline BiOCl was not influenced by WS2 QDs in the precursor. The absence of characteristic peaks of WS2 in the XRD pattern of WS2/BiOCl could be due to the fact that the amount of WS2 QDs added to the precursor is small, leading to the low loading of WS2 QDs onto the BiOCl nanosheets (∼4 wt% WS2 loading for WS2/BiOCl-2). Similar results were also found in CQDs/Bi2WO6,18 CQDs/BiOI (ref. 23) and Co3O4/BiOCl composites.24 To further identify the phase compositions, Raman scattering measurement was conducted. As shown in Fig. 1b, the strong bands at 145.7 and 198.9 cm−1 can be assigned to the A1g and Eg internal stretching vibrational modes of Bi–Cl,25 while the two characteristic bands at 353.6 and 423.0 cm−1 are correspond to E12g and A1g modes in WS2,8,26 respectively. The presence of the bands from both BiOCl and WS2 phases suggests the successful preparation of WS2/BiOCl-2. The Raman spectra for the pure BiOCl and WS2 QDs are shown in Fig. S1.† As compared with the Raman spectra of the pure BiOCl and WS2 QDs, the bands produced by the vibration motions of bismuth atoms shift to lower wavenumbers, and the bands derived from the vibration motions of sulfur atoms shift to higher wavenumbers, suggesting the strong coupling between the bismuth and sulfur atoms.The composition and chemical state of the surface elements in WS2/BiOCl-2 were further investigated by XPS analysis. Fig. S2† shows the XPS surveys of BiOCl and WS2/BiOCl-2. The presence of Bi, O and Cl elements in both of the samples is clearly observed. The high-resolution XPS spectra of Bi 4f (Fig. 2a) show two well resolved peaks at binding energies of 164.9 and 159.6 eV, corresponding to the characteristic peaks of Bi 4f5/2 and Bi 4f7/2, respectively.22Fig. 2c shows the O 1s core level spectra of BiOCl and WS2/BiOCl-2. It is noteworthy that the binding energy of O 1s in WS2/BiOCl (529.8 eV) shifts to lower binding energy as compared to the pure BiOCl (531.0 eV), indicating the strong interaction between WS2 QDs and BiOCl. The high resolution Cl 2p spectra are shown in Fig. 2c. Two peaks with binding energies at about 199.2 and 197.6 eV are clearly observed, which can be assigned to Cl 2p1/2 and Cl 2p3/2, respectively. The existence of the W element in WS2/BiOCl-2 is confirmed by the high resolution W 4f XPS spectra (Fig. 2d). The peaks located at 32.1 and 35.0 eV correspond to the W 4f7/2 and W 4f5/2 lines of the W4+ atoms, while the peaks at higher energy values (37.1 and 38.9 eV) are ascribed to the high valence state W atoms (W6+),5,8 indicating that WS2 is partially oxidized. The shifted O 1s XPS peaks and the presence of W6+ in WS2/BiOCl-2 suggest that the implanted WS2 QDs may be coupled to BiOCl via the strong Bi–O–W bonds, demonstrating the successful preparation of the WS2/BiOCl heterojunctions.
The morphology and structure of BiOCl and WS2/BiOCl-2 were observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM images of the pure BiOCl are shown in Fig. S3;† the BiOCl nanosheets possess a smooth surface and irregular shape. The TEM image of the WS2 QDs is shown in Fig. S4,† where the size of the WS2 QDs is in the range of ∼4–8 nm. The WS2 incorporation may alter the morphology of BiOCl. As can be seen from Fig. 3a and b, WS2/BiOCl-2 also exhibits a plate-like structure with smaller size as compared with the pure BiOCl. In Fig. 3c, the TEM image shows that the WS2 QDs with a size of about 5–7 nm are incorporated into the phase of BiOCl nanosheets, implying the successful preparation of the 0D/2D heterojunctions of WS2/BiOCl. The surface of WS2/BiOCl-2 is rougher than that of the pure BiOCl, which could lead to increased defects and active sites. Thus, we suspect that the WS2/BiOCl heterojunctions may possess improved catalytic properties as compared with the pure BiOCl. In Fig. 3d, the HR-TEM image shows that the WS2 nanodots are coupled with the well crystalline BiOCl. The lattice fringes with spacings of 0.21 and 0.275 nm are observed, which can be assigned to the (104) planes of WS2 (ref. 8) and (110) planes of BiOCl,25 respectively. The continuity of the lattice fringes between WS2 and BiOCl suggests the strong coupling between the two phases, confirming the successful preparation of the WS2/BiOCl heterojunctions.
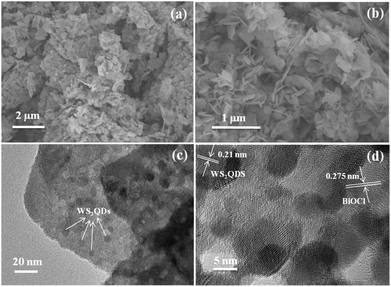 | ||
| Fig. 3 (a) Low magnification and (b) high magnification SEM images, (c) TEM image and (d) HR-TEM image of WS2/BiOCl-2. | ||
Optical properties
The UV-vis absorption spectrum and photoluminescence (PL) emission spectrum of the WS2 QDs are shown in the ESI† Fig. S5. In Fig. S5a,† the absorption bands at ∼631 and ∼523 nm are ascribed to the direct band to band excitonic transition and spin-split valence band to conduction band transition, respectively.27,28 In the PL spectrum (Fig. S5b†), the emission at ∼437 nm is due to the strong quantum confinement effect and the peak at ∼468 nm is attributed to the defect state emission by the exfoliated QDs.28 Smaller peaks at ∼656 (1.9 eV) and ∼668 nm (1.86 eV) are observed which can be attributed to the direct excitonic transitions in the monolayer WS2 occuring between the lowest conduction band and the highest spin-split valence band, located at the K-point of the Brillouin zone.7,29 In Fig. 4, the up-conversion properties of the WS2 QDs were observed with emission peaks centered at ∼650 nm under long-wavelength light excitation (800 to 900 nm). Previous studies also demonstrated that the WS2 nanosheets can absorb UV to near infrared (NIR) light.4 As is well known, NIR light accounts for over 50% of the solar energy, the combination of UV to NIR light absorption with the up-conversion properties may further expand the light absorption of the WS2/BiOCl heterojunctions.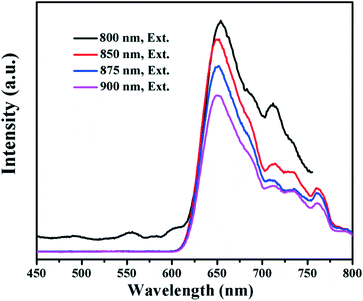 | ||
| Fig. 4 PL emission spectra of the WS2 quantum dots under various excitation wavelengths (Ex = 800, 850, 875 and 900 nm). | ||
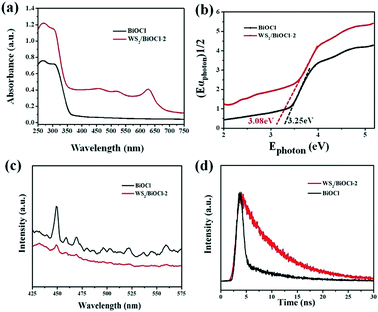
The optical properties of the pure BiOCl and WS2/BiOCl-2 were studied by UV-vis-NIR diffuse reflectance spectroscopy and PL spectroscopy. As can be seen in Fig. 5a, the UV-vis-NIR diffuse reflectance spectra (DRS) of the pure BiOCl show that the strong absorption in the UV region (<365 nm) agrees well with the theoretical band gap of BiOCl (Eg = 3.4 eV, corresponding to λ = 365 nm).19,24 The UV-vis-NIR DRS of WS2/BiOCl-2 exhibits three peaks located at ∼460, ∼521 and ∼629 nm, which is in accordance with the results of the UV-vis absorption spectrum of the WS2 QDs, demonstrating the successful coupling of WS2 QDs with BiOCl. As compared with the pure BiOCl, WS2/BiOCl-2 displayed much broader and stronger light absorption with the absorption edge extending to 700 nm. In addition, WS2/BiOCl-2 also exhibits weak but significant absorption in the broad IR to NIR region due to the contribution from the incorporated WS2.4 The enhanced light-harvesting by WS2/BiOCl-2 can facilitate the production of electron–hole pairs and finally improve the photocatalytic activity. The band gaps of the pure BiOCl and WS2/BiOCl-2 were evaluated using the plots of (αEphoton)1/2vs. Ephoton (Fig. 5b). It was found that the band gap of WS2/BiOCl-2 (3.08 eV) is slightly smaller than that of BiOCl (eV), which could be due to the existence of oxygen vacancies in WS2/BiOCl-2.14 The narrower band gap of WS2/BiOCl-2 is beneficial to the photoabsorption and excited electron production, leading to enhanced photocatalytic activity.
PL spectroscopy was applied to study the charge transfer and recombination of the photoinduced electron–hole pairs of pure BiOCl and WS2/BiOCl-2. The PL spectra of BiOCl and WS2/BiOCl-2 are collected with an excitation wavelength of 360 nm (Fig. 5c). The pure BiOCl PL spectrum exhibits an intense emission peak at ∼450 nm accompanied with several smaller peaks at higher wavelengths, indicating the multiple emission properties of BiOCl. In contrast, only very weak emission peaks at ∼450 nm and ∼465 nm could be observed in the PL spectrum of WS2/BiOCl-2 (Fig. 5c, red curve). It is known that the recombination of excited electrons and holes can result in enhanced PL signals. Accordingly, the quenched emission peaks of WS2/BiOCl-2 indicate that the sample features restrained recombination of the photoinduced electron–hole pairs due to the improved charge separation between strongly coupled BiOCl-2 and WS2. In addition, time-resolved transient PL decay was also used to further study electron–hole pair production and transfer. The decay curves of BiOCl and WS2/BiOCl-2 were fitted by using a double-exponential model:30
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2) | (2) |
| Sample | A 1 | τ 1(ns) | A 2 | τ 2(ns) | τ average(ns) |
|---|---|---|---|---|---|
| BiOCl | 161.51 | 8.61 | 980.08 | 0.75 | 5.89 |
| WS2/BiOCl-2 | 859.97 | 6.65 | 95.02 | 13.19 | 7.83 |
Photocatalytic activity
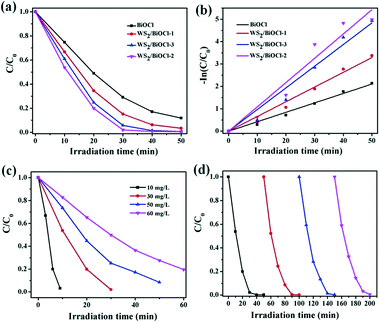
Rhodamine B (RhB), a cationic dye, was applied to evaluate the photocatalytic activity of the catalysts under visible light irradiation. Before the photocatalytic degradation of the organic dye, a blank experiment was carried out and the results demonstrated that RhB can hardly be degraded without adding photocatalysts under visible light irradiation, suggesting that the photobleaching of RhB can be ignored. As can be seen in Fig. 6a, ∼51.0% of RhB was degraded by pure BiOCl after 20 min irradiation, while 65.5%, 75.3% and 80.1% of RhB was decomposed in the presence of WS2/BiOCl-1, WS2/BiOCl-3 and WS2/BiOCl-2, respectively, with the same initial RhB concentrations and irradiation time, suggesting the enhanced photocatalytic activities of WS2-decorated BiOCl as compared with the pure BiOCl. It was found that WS2/BiOCl-2 (∼4 wt% WS2 load) exhibits the highest catalytic activities toward RhB among all the WS2/BiOCl samples and a higher WS2 loading will lead to the decreased photocatalytic activity. This is because the loaded WS2 QDs are beneficial for the charge transfer and inhibit the recombination of the photoinduced electron–hole pairs, but too much WS2 QD loading would decrease the light absorption by BiOCl and finally reduce the exciton production. The decomposition percentage of RhB reaches ∼80% and ∼99.3% after 30 and 50 min irradiation, respectively, demonstrating the high catalytic activity of WS2/BiOCl-2. The plots of −ln(C/C0) vs. t are shown in Fig. 6b. The good linearity between −ln(C/C0) and t suggests that the data are well fitted by the pseudo-first-order kinetic model:31| −ln(C/C0) = kt | (3) |
To evaluate the practical applicability of the WS2/BiOCl-2 catalyst, we further studied the effect of concentrations on the RhB degradation and the reusability of WS2/BiOCl-2. As shown in Fig. 6c, the decomposition percentage decreased as the RhB concentrations increased from 10 to 60 mg L−1 under the same conditions of visible light irradiation. It was found that 10 and 30 mg L−1 RhB were almost completely decomposed within 10 and 30 min, respectively, and over 90% and 80% of the 50 and 60 mg L−1 RhB were decomposed after 50 and 60 min irradiation, respectively. The results suggest that WS2/BiOCl-2 is suitable for the dyeing wastewater treatment over a wide dye concentration range. Reusability is one of the most important factors for evaluating the practical applicability of a photocatalyst. As shown in Fig. 6d, WS2/BiOCl-2 exhibits high stability with the degradation percentage maintained over 99.6% after four cycle degradation processes. There was no obvious loss of the degradation efficiency after each of the cyclic degradation experiments, suggesting the good reusability of the catalyst. After the cycling experiments, WS2/BiOCl-2 was further investigated by XRD and TEM. As can be seen from Fig. S8,† the XRD pattern matches with the original WS2/BiOCl-2. The TEM image (Fig. S9†) of recycled WS2/BiOCl-2 showed that the WS2 QDs are firmly anchored on BiOCl. The above results demonstrated the good stability of the WS2/BiOCl-2 catalysts. The wide application range, good stability and excellent reusability of the WS2/BiOCl-2 heterojunctions make them promising candidates for application in visible light-driven photocatalysis.
Proposed mechanisms for the enhanced photocatalytic activity
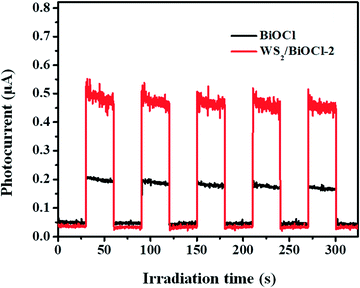
The above PL studies suggest the restrained recombination of the photoinduced electron–hole pairs in WS2-loaded BiOCl. To further understand the improved electron production and transfer, the transient photocurrent response of the pure BiOCl and WS2/BiOCl-2 was recorded for several cycles under visible-light irradiation. As can be seen in Fig. 7, the photocurrent of WS2/BiOCl-2 is ∼2.5 times that of the pure BiOCl, which is in accordance with the result of the degradation experiments that the rate constant (k) of WS2/BiOCl-2 is ∼2.7 times that of the pure BiOCl. The enhanced photocurrent response indicates the efficient charge separation at the 0D/2D interface in the WS2/BiOCl heterojunctions, leading to the enhanced photocatalytic activity.In order to identify the active species produced by the catalysts, ESR spectroscopy was employed to probe the active oxygen species during the visible-light driven photocatalytic processes. The DMPO spin-trapping ESR spectra of the pure BiOCl and WS2/BiOCl are shown in Fig. 8. As can be seen from Fig. 8a, the ESR signals of DMPO-˙OH for all the samples are just a little stronger than the background noise under both dark and light conditions, suggesting that the production of ˙OH by the catalysts is negligible. In Fig. 8b, four characteristic peaks of DMPO-O2˙− are observed under visible light irradiation, indicating that the O2˙− and holes (h+) are the main active species produced during the photocatalytic processes. It can also be mentioned that the ESR signals of DMPO-O2˙− obtained from WS2/BiOCl are much stronger than those from the pure BiOCl, which further demonstrated the enhanced catalytic properties of WS2/BiOCl.
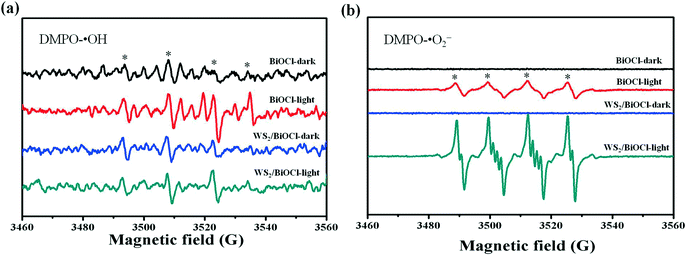 | ||
| Fig. 8 DMPO spin-trapping ESR spectra of the pure BiOCl and WS2/BiOCl (a) in the aqueous dispersion (for DMPO-˙OH) and (b) in the methanol dispersion (for DMPO-O2˙−). | ||
To better understand the mechanisms for the enhanced catalytic activities of WS2/BiOCl, the XPS valence band (VB) spectra of the samples were measured. As can be seen from Fig. S10,† the VB maxima of the pure BiOCl and WS2/BiOCl are at 2.4 and 2.6 eV, accordingly. As is well known, the VB of BiOCl primarily resulted from the hybridization of O 2p and Cl 3p orbitals, while the conduction band (CB) is mainly dominated by the Bi 6p orbitals.32 The little shift of the VB maximum for WS2/BiOCl suggests that the electronic structure of the BiOCl matrix was not affected by the incorporated WS2 QDs. The band gap (Eg) of WS2/BiOCl obtained from the DRS analysis is 3.02 eV. The conduction band edge potential (ECB) can be calculated using the formula: ECB = EVB − Eg.33 Thus, the ECB minimum will occur at −0.42 eV, which is more negative than the E0(O2/O2˙−) value (−0.046 eV (vs. NHE)), indicating that O2 can be reduced to O2˙−.33 While the value of E0(˙OH/OH−) is 2.38 eV (vs. NHE) which means that WS2/BiOCl is not able to produce active ˙OH.33 The results presented here are in good consistency with the above ESR spectroscopy analysis.
The proposed mechanisms for the enhanced photocatalytic activities of the WS2/BiOCl heterojunctions are shown in Fig. 9. As is well-known, BiOCl is not an efficient visible-driven photocatalyst due to its large band gap. And the production of active oxygen species is limited by the low efficiency of the electron–hole pair production and separation by BiOCl. After the incorporation of WS2 QDs in the BiOCl matrix, the band gap of WS2/BiOCl is narrowed and the absorption properties are improved. The EVB and ECB minima of the WS2 nanoparticles are about 1.61 eV and −1.03 eV, respectively.34 The ECB value of the WS2 QDs is more negative than that of BiOCl, indicating that the electrons generated by WS2 QDs can transfer to the CB of BiOCl. Also the EVB value of the WS2 QDs is also smaller than that of BiOCl, suggesting that the holes in the BiOCl lean toward WS2. Thus, the electrons and holes move in reverse directions in the strongly coupled WS2/BiOCl system, resulting in the facilitated electron–hole transportation and separation. Moreover, the ECB value of WS2 QDs is also more negative than the E0 (O2/O2˙−) value, suggesting that the electrons in the CB of the WS2 QDs can also react with the absorbed O2 to generate O2˙− radicals. The active O2˙− radicals can continuously react with the pollutants and decompose them into smaller molecules such as H2O and CO2. The improved photocatalytic performance of WS2 QDs/BiOCl materials was ascribed to the crucial role of WS2 QDs, which enhanced molecular oxygen activation ability and worked as the light-harvesting center and charge separation center as well as active center for degrading the pollutants.
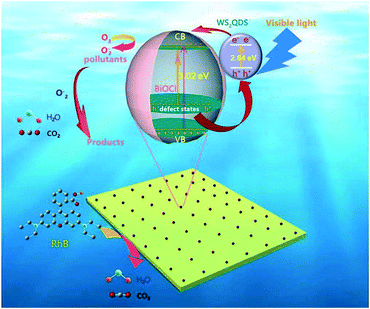 | ||
| Fig. 9 Schematic of the separation and transfer of photogenerated charges in the WS2/BiOCl heterojunctions and the proposed photodegradation mechanisms. | ||
Conclusions
In summary, novel 0D/2D heterojunctions of WS2 QDs/BiOCl were fabricated via facile one-pot hydrothermal processes. The strongly coupled WS2 QDs/BiOCl heterojunctions exhibit superior visible-light response and enhanced photocatalytic activity toward both cationic and anionic dyes. The narrow band gap of the WS2 QDs makes them great photosensitizers for capturing visible light. Moreover, the up conversion properties of the WS2 QDs further boost the light-harvesting efficiency, which finally leads to enhanced electron–hole pair production. The band alignment between the WS2 QDs and BiOCl is beneficial to the charge separation and facilitates the production of active O2˙− radicals. This study provides new insights into the synergistic combination of the novel TMD QDs and 2D nanosheets for application in photocatalysis and may further promote the application of the novel TMD QD-based composites in catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21775138, 21607135) and Zhejiang Environmental Protection Bureau (No. 2013A025).Notes and references
- X. Wang, G. Sun, N. Li and P. Chen, Chem. Soc. Rev., 2016, 45, 2239–2262 RSC.
- Z. Lin, A. McCreary, N. Briggs, S. Subramanian, K. Zhang, Y. Sun, X. Li, N. J. Borys, H. Yuan, S. K. Fullerton-Shirey, A. Chernikov, H. Zhao, S. McDonnell, A. M. Lindenberg, K. Xiao, B. J. LeRoy, M. Drndić, J. C. M. Hwang, J. Park, M. Chhowalla, R. E. Schaak, A. Javey, M. C. Hersam, J. Robinson and M. Terrones, 2D Mater., 2016, 3, 042001 CrossRef.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886–1893 CrossRef CAS PubMed.
- Y. Sang, Z. Zhao, M. Zhao, P. Hao, Y. Leng and H. Liu, Adv. Mater., 2015, 27, 363–369 CrossRef CAS PubMed.
- Y. Yan, C. Zhang, W. Gu, C. Ding, X. Li and Y. Xian, J. Phys. Chem. C, 2016, 120, 12170–12177 CAS.
- Y. Yong, X. Cheng, T. Bao, M. Zu, L. Yan, W. Yin, C. Ge, D. Wang, Z. Gu and Y. Zhao, ACS Nano, 2015, 9, 12451–12463 CrossRef CAS PubMed.
- H. Long, L. Tao, C. P. Chiu, C. Y. Tang, K. H. Fung, Y. Chai and Y. H. Tsang, Nanotechnology, 2016, 27, 414005 CrossRef PubMed.
- X. Zhao, X. Ma, J. Sun, D. Li and X. Yang, ACS Nano, 2016, 10, 2159–2166 CrossRef CAS PubMed.
- W.-J. Ong, L.-L. Tan, S.-P. Chai, S.-T. Yong and A. R. Mohamed, ChemSusChem, 2014, 7, 690–719 CrossRef CAS PubMed.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- R. Marschall, Adv. Funct. Mater., 2014, 24, 2421–2440 CrossRef CAS.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., 2005, 44, 8269–8285 CrossRef CAS.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- H.-L. Guo, Q. Zhu, X.-L. Wu, Y.-F. Jiang, X. Xie and A.-W. Xu, Nanoscale, 2015, 7, 7216–7223 RSC.
- S. Kansal, M. Singh and D. Sud, J. Hazard. Mater., 2007, 141, 581–590 CrossRef CAS PubMed.
- H. Wang, X. Zhang, J. Xie, J. Zhang, P. Ma, B. Pan and Y. Xie, Nanoscale, 2015, 7, 5152–5156 RSC.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- J. Di, J. Xia, Y. Ge, H. Li, H. Ji, H. Xu, Q. Zhang, H. Li and M. Li, Appl. Catal., B, 2015, 168–169, 51–61 CrossRef CAS.
- J. Li, Y. Yu and L. Zhang, Nanoscale, 2014, 6, 8473 RSC.
- Y. Peng, D. Wang, H.-Y. Zhou and A.-W. Xu, CrystEngComm, 2015, 17, 3845–3851 RSC.
- J. Xiong, G. Cheng, F. Qin, R. Wang, H. Sun and R. Chen, Chem. Eng. J., 2013, 220, 228–236 CrossRef CAS.
- X. Zhang, X.-B. Wang, L.-W. Wang, W.-K. Wang, L. L. Long, W.-W. Li and H.-Q. Yu, ACS Appl. Mater. Interfaces, 2014, 6, 7766–7772 CAS.
- J. Di, J. Xia, M. Ji, L. Xu, S. Yin, Q. Zhang, Z. Chen and H. Li, Carbon, 2016, 98, 613–623 CrossRef CAS.
- C. Tan, G. Zhu, M. Hojamberdiev, K. Okada, J. Liang, X. Luo, P. Liu and Y. Liu, Appl. Catal., B, 2014, 152–153, 425–436 CrossRef CAS.
- J. Hu, W. Fan, W. Ye, C. Huang and X. Qiu, Appl. Catal., B, 2014, 158–159, 182–189 CrossRef CAS.
- A. L. Elías, N. Perea-López, A. Castro-Beltrán, A. Berkdemir, R. Lv, S. Feng, A. D. Long, T. Hayashi, Y. A. Kim, M. Endo, H. R. Gutiérrez, N. R. Pradhan, L. Balicas, T. E. Mallouk, F. López-Urías, H. Terrones and M. Terrones, ACS Nano, 2013, 7, 5235–5242 CrossRef PubMed.
- C. L. Choi, J. Feng, Y. Li, J. Wu, A. Zak, R. Tenne and H. Dai, Nano Res., 2013, 6, 921–928 CrossRef CAS.
- A. Ghorai, S. Bayan, N. Gogurla, A. Midya and S. K. Ray, ACS Appl. Mater. Interfaces, 2017, 9, 558–565 CAS.
- N. Peimyoo, W. Yang, J. Shang, X. Shen, Y. Wang and T. Yu, ACS Nano, 2014, 8, 11320–11329 CrossRef CAS PubMed.
- F.-T. Li, Y.-L. Li, M.-J. Chai, B. Li, Y.-J. Hao, X.-J. Wang and R.-H. Liu, Catal. Sci. Technol., 2016, 6, 7985–7995 CAS.
- X. Meng and Z. Zhang, Appl. Catal., B, 2017, 209, 383–393 CrossRef CAS.
- H. Cheng, B. Huang and Y. Dai, Nanoscale, 2014, 6, 2009 RSC.
- J. Xia, J. Di, H. Li, H. Xu, H. Li and S. Guo, Appl. Catal., B, 2016, 181, 260–269 CrossRef CAS.
- J.-P. Zou, J. Ma, J.-M. Luo, J. Yu, J. He, Y. Meng, Z. Luo, S.-K. Bao, H.-L. Liu, S.-L. Luo, X.-B. Luo, T.-C. Chen and S. L. Suib, Appl. Catal., B, 2015, 179, 220–228 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cy01784g |
| This journal is © The Royal Society of Chemistry 2018 |