Open Access Article
Open Access ArticleThe peculiar effect of water on ionic liquids and deep eutectic solvents
Chunyan
Ma
 ab,
Aatto
Laaksonen
ab,
Aatto
Laaksonen
 bcde,
Chang
Liu
bcde,
Chang
Liu
 b,
Xiaohua
Lu
b,
Xiaohua
Lu
 *b and
Xiaoyan
Ji
*b and
Xiaoyan
Ji
 *ab
*ab
aEnergy Engineering, Division of Energy Science, Luleå University of Technology, Luleå, 971 87, Sweden. E-mail: xiaoyan.ji@ltu.se; Tel: +46-725393298
bState Key Laboratory of Material-Oriented Chemical Engineering, Nanjing Tech University, Nanjing, 210009, P. R. China. E-mail: xhlu@njtech.edu.cn
cDepartment of Materials and Environmental Chemistry, Arrhenius Laboratory, Stockholm University, Stockholm, SE-106 91, Sweden
dDepartment of Chemistry-Ångström Laboratory, Uppsala University, Box 538, SE-75121 Uppsala, Sweden
eCentre of Advanced Research in Bionanoconjugates and Biopolymers, Petru Poni Institute of Macromolecular Chemistry Aleea Grigore Ghica-Voda, 41A, 700487 Iasi, Romania
First published on 9th October 2018
Abstract
Ionic liquids (ILs) and deep eutectic solvents (DESs) have been suggested as eco-friendly alternatives to organic solvents. A trace amount of water is often unavoidable as impurity, and water is also added on purpose to reduce their problematically high viscosity and lower their high price. Understanding the distinct effects of water on the properties of ILs/DESs is highly important. In this review, we collect published experimental and theoretical results for IL/DES–H2O systems at varied water concentrations and analyze them. Results from mechanistic studies, thermodynamic modelling and advanced experiments are collected and critically discussed. Six commonly studied IL/DES–H2O systems were selected to map experimental observations onto microscopic results obtained in mechanistic studies. A great variety of distinct contours of the excess properties can be observed over the entire compositional range, indicating that the properties of IL/DES–H2O systems are highly unpredictable. Mechanistic studies clearly demonstrate that the added H2O rapidly changes the heterogeneous 3D structures of pure ILs/DESs, leading to very different properties and behaviour. There are similarities between aqueous electrolytes and IL/DES solutions but the bulky and asymmetric organic cations in ILs/DESs do not conform to the standard salt dissolution and hydration concepts. Thermodynamic modelling previously assumes ILs/DESs to be either a neutral ion-pair or completely dissociated ions, neglecting specific ion hydration effects. A new conceptual framework is suggested for thermodynamic modelling of IL/DES–H2O binary systems to enable new technologies for their practical applications.
1. Introduction
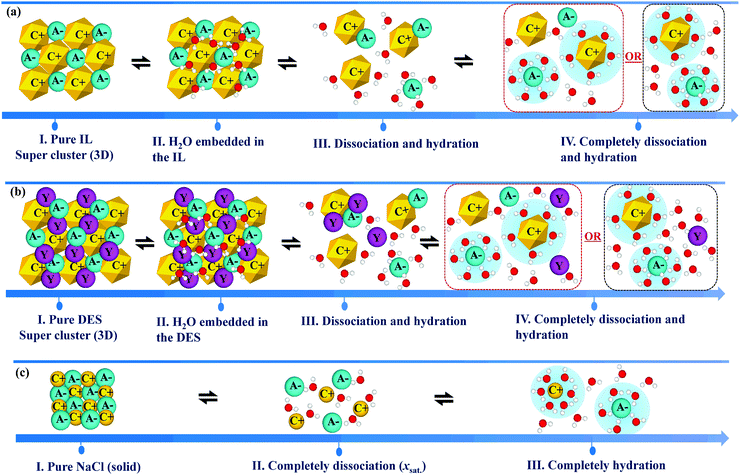
Even though ionic liquids (ILs) were discovered more than a century ago, it is only over the last several decades that they have become promising alternative replacements to the volatile and hazardous organic solvents. This is due to the unique properties of ILs, which include negligible vapor pressure, high thermal stability, non-flammability, the ability to dissolve both organic and inorganic compounds, and so on.1,2 Deep eutectic solvents (DESs) are eutectic mixtures of two or more components, and have been identified as either a new class of ILs or IL analogues.3 DESs share many characteristics and properties with ILs, although these two are different types of solvents based on their molecular structures.3Fig. 1 demonstrates that IL is composed primarily of an anion and a cation. In contrast, DES is typically formed with a hydrogen-bond acceptor (HBA), for example a quaternary ammonium halide salt, and a hydrogen-bond donor (HBD), which can be a variety of species. It has been suggested that ILs and/or DESs may revolutionize various industrial processes in the future, and much research has been carried out with regard to certain applications. Examples include CO2 capture and separation,4–6 lubrication and tribology,7–9 biomass dissolution10–16 and absorption heat transformers.17,18 Herein, we briefly introduce some of these applications.Blanchard et al.4 first reported the use of ILs as promising absorbents for CO2 separation in 1999. Since then, developing ILs for CO2 capture and separation has become an intense research area because of the significant solubility of CO2 in these solvents and their high selectivity, as well as the low energy demand for solvent regeneration compared with current technologies, such as those based on aqueous amine solutions. Recently, task-specific ILs (TSILs) have been proposed to further increase the CO2 absorption capacity. The first TSIL was reported by Bates et al.19 A review by Cui et al.20 stresses that the presence of strong CO2-philic reactive sites is essential for the development of ILs capable of capturing CO2 from post-combustion flue gases via chemisorption. DESs have also been widely developed for CO2 separation because of their many appealing properties compared with conventional ILs, such as low cost, biodegradability and simple preparation processes.21 Because both ILs and DESs are highly tunable, it is possible to incorporate CO2-philic functional groups into these substances to promote their CO2 absorption capacity, and functionalized ILs have been found to be as efficient as alkanolamine solvents in terms of CO2 absorption capacity, while being much more energy efficient.22 In addition, some ILs and DESs exhibit high physisorption capacities.23 However, the CO2 absorption rate of ILs/DESs is generally low because of their high viscosities, and the costs associated with the large-scale manufacturing of these substances are still too high.
Using ILs as lubricants was first proposed by Ye et al.7 in 2001. Since then, ILs have attracted considerable attention in the field of tribology because of their remarkable lubrication and anti-wear properties compared with those of fossil-based and synthetic lubricants.9 In addition, many new materials (that is, other than metal surfaces) require new types of lubricants, and ILs have been found to be better alternatives for such novel surfaces. Because of their high price, however, ILs are primarily used as additives to conventional lubrication oils at present.9 The number of publications, including both journal papers and patents, regarding this topic has increased dramatically, even though ILs remain too expensive for industrial applications. In the case of DESs, many reports have noted that their lubrication properties are superior to those of mineral oils.24,25
Biomasses are typically dissolved by conventional techniques such as cooking in strong alkaline or acidic solutions, which is both expensive and polluting. In 2002, Rogers and co-workers first reported that cellulose, which is insoluble in water and in most organic solvents, can be dissolved in ILs to form a solution of cellulose at concentrations greater than 10 wt%, providing a new platform for the efficient utilization of this resource (Swatloski et al.10). In fact, both ILs and DESs have been shown to be excellent solvents for dissolving and further processing of biomass and cellulose in particular.11–16 However, again, ILs are expensive and difficult to synthesize on a large scale, making their practical use on an industrial scale difficult at the moment. In contrast, DESs are usually less expensive than ILs and have also been tested as an alternative solvent for the extraction of cellulose from biomass and for further processing to generate fuels or plastic substitutes.12–15 The use of either ILs or DESs is expected to provide environmentally sustainable chemical processes giving biodegradable products; however, their high cost remains an obstacle to practical applications.
ILs can also be used as absorbents when combined with H2O to formulate new working pairs that may replace currently-used working fluids, such as lithium bromide–H2O or ammonia–H2O, in absorption cycles meant to recover waste heat and utilize low-grade energy.17,18,26,27 Since Kim et al.26 proposed working pairs composed of imidazolium-based ILs in 2004, much research has been carried out to identify new IL–H2O working pairs. As such, developing novel and suitable ILs has lately become one of the most active research topics in the field of cooling technology.17,18,27 DESs have also been assessed for use in absorption refrigeration systems in combination with the widely used hydrofluorocarbon refrigerant R134a to develop more energy-efficient refrigeration (or heat pump) systems.28
Although emerging ILs/DESs-based technologies are promising in many applications, the drawbacks of high cost and/or high viscosity are still impediments to further development and implementation.29,30 Adding suitable co-solvents has been proposed as one of the most promising options to developing IL/DES-based processes. Among the potential co-solvents, H2O is both environmentally friendly and has low viscosity. H2O is also a common impurity in various CO2-enriched gas streams, and has been used as a physical absorbent for the removal of CO2 from biogas during biogas upgrading.31 H2O is also an important component of biomass. Additionally, in tribology, H2O can serve as a lubricant with very low friction, while, in absorption cycles, H2O is frequently used as one component in working pairs.17 The addition of H2O could, therefore, be a viable approach to developing IL/DES-based technologies for practical applications.32 For this reason, it is crucial to study the properties of IL/DES–H2O systems.
The addition of H2O can drastically alter the properties of ILs/DESs, as can be seen from the large amount of experimental data reported for such systems. As an example, the viscosities of ILs/DESs can be decreased significantly by the addition of only a trace amount of water.33 The mixing of water with ILs/DESs can be either endothermic, exothermic or both, depending on the water content.34 In addition, the excess volume can be either positive, negative or both, thus generating an S-shaped curve.35 It is, therefore, evident that mixture of H2O and ILs/DESs can be very different from ordinary binary solvent mixtures. ILs are molten salts and thus similar to electrolytes, but are still liquids over a wide range of temperatures below 373 K. DESs also contain cations and anions,25 and so again are similar to electrolytes, although they are more complex because they contain at least one additional neutral compound. The electrical conductivity of IL/DES–H2O systems generally shows a maximum value at a relatively low IL/DES concentration.36 All these observations imply that IL/DES–H2O systems are unique compared with either organic solvent mixtures or aqueous electrolyte solutions. However, no attempts have been made to systematically assess the extent to which these systems differ from binary solvent mixtures or aqueous electrolyte solutions by comprehensively summarizing the available literature.
It has been pointed out that it would be desirable to develop theoretical models to describe the properties of IL/DES–H2O systems. It should be possible to determine empirical correlations based on experimental work and the corresponding results from high-quality computational studies. Such work could lead to the development of empirical models describing, for example, densities and viscosities at various temperatures and concentrations. However, interestingly, we have found no attempts to summarize and rationalize the available experimental results as a means of obtaining new insights into mechanistic studies and, ultimately, devising predictive models.
Mechanistic studies of IL/DES–H2O systems on the microscopic level are crucial to gaining an understanding of the effects of adding H2O to ILs/DESs. Such studies are also important in terms of further developing an appropriate model that describes and predicts the properties of such systems over the entire compositional range from a pure IL/DES to an infinitely dilute IL/DES solution. To study the mechanisms of IL/DES–H2O systems, both molecular simulations and advanced experiments have been conducted for more than a decade, the majority of which have examined imidazolium-based ILs.37,38
Several review articles have been published focusing on the importance of ILs/DESs to future process development in the field of chemical engineering as well as their industrial and environmental significance. Although DESs and ILs are two different types of solvents considering their molecular structures, their thermophysical properties are similar. Nearly all of these concentrate on the properties and applications of pure ILs,1,2,39–52 and DESs are usually not included. There have been some reviews only regarding pure DESs.3,53–61 For IL/DES–H2O systems, only a handful of reviews have been published with the focus on a single property and/or only a limited number of ILs.35,48,62 Tariq et al.62 discussed the surface tension of ILs and IL solutions, with regard to the effects of temperature, the addition of water or other impurities, and the IL structure on the surface tension. Bahadur et al.35 assessed the excess molar volumes of binary IL–H2O mixtures, using approximately 50 ILs. Podgorsek et al.48 focused on the mixing enthalpies of binary mixtures containing ILs and proposed that thermodynamic quantities of mixing could provide valuable insights into molecular interactions and microscopic structures in such mixtures. However, summarizing the research work on both experimental observations and mechanistic studies of various types of IL/DES–H2O systems is virtually nonexistent.
The purpose of the present review is to summarize the state-of-the-art research of IL/DES–H2O systems. This review covers both experimental and mechanistic studies concerning various types of ILs/DESs to present a broad picture of the present body of knowledge. The experimental data for IL/DES–H2O systems, primarily consisting of density, viscosity, electrical conductivity and excess/mixing enthalpy values, is surveyed and analyzed. In addition, mechanistic studies of IL/DES–H2O systems using molecular simulations, quantum-chemical calculations or thermodynamic analysis are addressed, and relationships between the experimental data on the macroscopic properties and mechanistic phenomena on the microscopic molecular structure are discussed. The properties and mechanisms of various IL/DES–H2O systems are compared with one another as well as with those of common aqueous electrolyte solutions. Furthermore, current developments in theoretical modelling are discussed. Finally, a framework for developing new theoretical models is proposed to study the macroscopic properties and phase equilibria for IL/DES–H2O systems, as a means of developing practical applications in clean process technologies and designing new eco-friendly solvents.
2. A survey of experimental data for IL/DES–H2O systems
Considering the increasing technological and scientific importance of IL/DES–H2O systems, much research and development work has been carried out during recent years, as reflected in the steadily increasing number of publications. The density, viscosity, electrical conductivity, excess/mixing enthalpy, surface tension and interfacial tension of IL/DES–H2O systems have all been extensively studied experimentally. In this section, we report and discuss the available data regarding the physicochemical properties for a wide variety of such systems.2.1 Density ρ
Density is one of the fundamental properties and often plays a key role in understanding the behaviour of ILs/DESs in the presence of water. It is easy to measure so that extensive amounts of experimental data can be found in the literature on the IL/DES–H2O systems. Mostly, the densities ρ and the excess molar volumes VE are reported simultaneously to analyze the mixing of ILs/DESs and H2O. The VE is calculated from the density according to the following equation: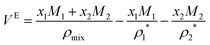 | (1) |
In total, 700+ articles have been published until August 2018 reporting the density of IL/DES–H2O binary systems based on the databases including Scopus, Web of science and the database on ionic liquid properties (NIST Standard Reference Database no. 147, or so-called IL Thermo v2.0). Some of the reported results cover the densities in a wide compositional range or even the whole compositional range of IL/DES–H2O systems, and some results focus on an infinitely dilute IL/DES solution63,64 or an IL/DES with a trace amount of water.65–67 The studied ILs cover both conventional ILs and TSILs. To the best of our knowledge, only one review article has been published summarizing the excess molar volumes VE of about 50 ILs.35 In this work, the experimental results for both the ILs and DESs (excluding those have been summarized in the review by Bahadur et al.35) are collected in Table 1. Also, it should be mentioned that the information on the data over the infinitely dilute IL/DES solutions or the IL/DES with a trace amount of water is excluded in Table 1.
| At atmospheric pressure | |||||
|---|---|---|---|---|---|
| ILs & DESs | T/K | Shapea (VE–xIL/DES) | V E/cm3 mol−1 at xIL/DES = extreme position | (∂|VE|/∂T)P,x/range (xIL/DES) | Ref. |
| a ‘u’: negative single hump, ‘n’: positive single hump, ‘m’: double hump, ‘w’: negative double hump, and ‘s’: sinusoidal or S-shape. | |||||
| DESs | |||||
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–333.15 | u | [−0.20, −0.12] at xDES ∼ 0.3 | <0/(0–1) | 71 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
293.15–363.15 | u | [−0.16, −0.11] at xDES ∼ 0.3 | <0/(0–1) | 78 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
303.15 | — | — | (0–1) | 79 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
293.15–323.15 | u | [−0.18, −0.15] at xDES ∼ 0.3 | <0/(0–1) | 80 |
ChCl/TG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
303.15–353.15 | u | [−0.90, −0.70] at xDES ∼ 0.3 | <0/(0–1) | 73 |
ChCl/Gluc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
303.15–353.15 | w | [−0.75, −0.70] at xDES ∼ 0.7; [−0.60, −0.58] at xDES ∼ 0.3 | <0/(0–1) | 73 |
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
283.15–363.15 | u | [−0.33, −0.25] at xDES ∼ 0.4 | <0/(0–1) | 81 |
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–333.15 | u | [−0.33, −0.25] at xDES ∼ 0.4 | <0/(0–1) | 82 |
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
303.15–323.15 | — | — | — | 83 |
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
303.15–313.15 | — | — | (0.17–1) | 84 |
ChCl/MA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
303.15–313.15 | — | — | (0.13–1) | 84 |
ChCl/MA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
283.15–363.15 | u | — | <0/(0–1) | 81 |
ChCl/MA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
303.15–323.15 | — | — | — | 83 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
283.15–363.15 | u | [−0.35, −0.30] at xDES ∼ 0.35 | <0/(0–1) | 85 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–333.15 | u | [−0.35, −0.30] at xDES ∼ 0.4 | <0/(0–1) | 82 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
303.15–313.15 | — | — | (0.19–1) | 84 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
283.15–343.15 | u | [−0.4, −0.30] at xDES ∼ 0.4 | <0/(0–1) | 86 |
ChCl/LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–363.15 | — | — | (0.68–1) | 87 |
CrCl3/ChCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) 2.5) |
298.15–353.15 | — | — | (0.053–0.143) | 88 |
CrCl3/ChCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–353.15 | — | — | (0.053–0.143) | 89 |
DEAC/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–343.15 | u | [−0.45, −0.40] at xDES ∼ 0.4 | <0/(0–1) | 90 |
DEAC/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–343.15 | u | [−0.54, −0.45] at xDES ∼ 0.4 | <0/(0–1) | 90 |
ATPPB/DEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
293.15–343.15 | w | [−0.72, −0.44] at xDES ∼ 0.25 | <0/(0–1) | 91 |
ATPPB/DEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
293.15–343.15 | u | [−0.66, −0.44] at xDES ∼ 0.3 | <0/(0–1) | 91 |
ATPPB/DEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16) 16) |
293.15–343.15 | u | [−0.74, −0.53] at xDES ∼ 0.3 | <0/(0–1) | 91 |
| Imidazolium-based ILs | |||||
| [C4C2im][Br] | 293.15–323.15 | u | [−0.6, −0.5] at xIL ∼ 0.4–0.5 | <0/(0–1) | 92 |
| [Mmim][DMP] | 298.15 | — | — | (0.252–1) | 93 |
| [Mmim][MeSO4] | 298.15–328.15 | s | [−0.11, −0.047] at xIL ∼ 0.4–0.5; [0.07, 0.122] at xIL ∼ 0.05 | <0/(0.32–1); >0/(0–0.32) | 94 |
| [Emim][BuSO4] | 288–318 | u | [−0.42, −0.23] at xIL ∼ 0.3–0.4 | <0/(0–1) | 95 |
| [Emim][HexSO4] | 288–318 | u/s | [−0.16, −0.087] at xIL ∼ 0.58; [0.028, 0.042] at xIL ∼ 0.93 | <0/(0–0.85); >0/(0.85–0.1) | 95 |
| [Emim][OcSO4] | 298–318 | u/s | [−0.11, −0.10] at xIL ∼ 0.39–0.74; 0.045 at xIL ∼ 0.1 | <0/(0–1); — | 95 |
| [Emim][TFA] | 278.15–348.15 | u | [−0.76, −0.3] at xIL ∼ 0.25–0.35 | <0/(0–1) | 96 |
| [Emim][TCM] | 298.15 | s | x IL ∼ 0.45; xIL ∼ 0.013 | >0/(0–0.05); <0/(0.05–1) | 97 |
| [Emim][Ac] | 298.15–343.15 | u | [−1.57, −1.49] at xIL ∼ 0.39 | <0/(0–1) | 98 |
| [Emim][Ac] | 293.15–353.15 | — | — | (0.347–0.705) | 99 |
| [Emim][Ac] | 298.15–353.15 | u | — | (0.665–0.955) | 33 |
| [Emim][DCA] | 298.15–343.15 | m | [0.21, 0.41] at xIL ∼ 0.46; [0.18, 0.43] at xIL ∼ 0.26 | >0/(0–1) | 98 |
| [Emim][L-lactate] | 298.15 | n | 1.3 at xIL ∼ 0.5 | — | 100 |
| [Emim][Glyci] | 300 | u | −2.6 at xIL ∼ 0.2 | (0–1) | 101 |
| [Emim][DMP] | 293.15–333.15 | u | [−1.6, −1.5] at xIL ∼ 0.3–0.4 | (0–1) | 102 |
| [Emim][Et2PO4] | 293.15–373.15 | u | [−1.75, −1.3] at xIL ∼ 0.24 | (0–1) | 103 |
| [Bmim][DMP] | 293.15–333.15 | u | [−1.4, −1.17] at xIL ∼ 0.3 | <0/(0–1) | 102 |
| [Bmim][L-lactate] | 298.15–318.15 | n | [0.72, 1.1] at xIL ∼ 0.40 | <0/(0–1) | 104 |
| [HDBU][IM] | 293.15–313.15 | u | [−1.61, −1.50] at xIL ∼ 0.3 | <0/(0–1) | 105 |
| [BDBU][IM] | 293.15–313.15 | u | [−1.36, −1.21] at xIL ∼ 0.3 | <0/(0–1) | 105 |
| [TMG][IM] | 293.15–313.15 | u | [−1.5, −1.4] at xIL ∼ 0.3 | <0/(0–1) | 106 |
| [Amim][Cl] | 298.15 | — | — | (0–1) | 107 |
| [Amim][Cl] | 298.15–353.15 | u | — | (0.665–0.955) | 33 |
| Pyrrolidinium-based ILs | |||||
| [Pyrr][HCOO] | 298.15 | u | −0.5 at xIL ∼ 0.19 | — | 68 |
| [Pyrr][NO3] | 298.15–333.15 | n | [2.33, 2.48] at xIL ∼ 0.12 | >0/(0.4–1) | 68 |
| [Pyrr][HSO4] | 298.15 | n | 3.90 at xIL ∼ 0.096 | — | 68 |
| [Empyr][EtSO4] | 298.15–343.15 | s | [−0.42, −0.08] at xIL ∼ 0.4; [0.033, 0.40] at xIL ∼ 0.89 | <0/(0–0.4); >0/(0.4–1) | 108 |
| [Epmpyr][Cl] | 293.15–313.15 | u | [−1.1, −0.70] at xIL ∼ 0.4 | <0/(0–1) | 109 |
| [Epmpyr][Ac] | 288.15–313.15 | u | [−1.15, −1.0] at xIL ∼ 0.3 | <0/(0–1) | 110 |
| [Epmpyr][SAL] | 293.15–313.15 | u | [−1.3, −0.70] at xIL ∼ 0.3 | <0/(0–1) | 111 |
| [Empyr][Glyc] | 298.15–348.15 | u | [−1.19, −1.0] at xIL ∼ 0.29–0.35 | <0/(0–1) | 112 |
| [Empyr][Ac] | 298.15–348.15 | u | [−1.15, −0.97] at xIL ∼ 0.32–0.41 | <0/(0–1) | 112 |
| Piperidinium-based ILs | |||||
| [Empip][EtSO4] | 298.15–343.15 | u | [−0.69, −0.45] at xIL ∼ 0.26–0.36 | <0/(0–1) | 108 |
| Piperazinium-based ILs | |||||
| [(OH)2C2pi][EtCOO] | 293.15–323.15 | — | — | (0–0.12) | 36 |
| [MP][Glyci] | 300 | u | −3 at xIL ∼ 0.25 | (0–1) | 101 |
| Guanidinium-based ILs | |||||
| [(C2)22(C1)2(C1)23gu][EtSO4] | 293.15–323.15 | u | [−0.6, −0.2] at xIL ∼ 0.2 | <0/(0–1) | 113 |
| [TMG][BEN] | 298.15 | — | — | (0–0.224) | 114 |
| [TMG][SAL] | 298.15 | — | — | (0–0.224) | 114 |
| [TMG][Lac] | 303.15–328.15 | u | [−1.2, −1.0] at xIL ∼ 0.3 | <0/(0–1) | 115 |
| Morpholinium-based ILs | |||||
| [Emmor][EtSO4] | 298.15–343.15 | u | [−0.26, −0.12] at xIL ∼ 0.32–0.44 | <0/(0–1) | 108 |
| [Emmor][Ac] | 298.15–348.15 | u | [−1.1, −1.0] at xIL ∼ 0.3 | <0/(0–1) | 116 |
| [Emmor][Pro] | 298.15–348.15 | u | [−1.2, −1.0] at xIL ∼ 0.3 | <0/(0–1) | 116 |
| [Emmor][Glyc] | 298.15–348.15 | u | [−0.86, −0.66] at xIL ∼ 0.29 | <0/(0–1) | 112 |
| Collidinium-based ILs | |||||
| [Col][HCOO] | 298.15 | u | −2.8 at xIL ∼ 0.3 | — | 68 |
| Cholinium-based ILs | |||||
| [Cho][Lac] | 293.15 | u | −1.2 at xIL ∼ 0.25 | — | 117 |
| [Cho][Prop] | 293.15 | u | −1.28 at xIL ∼ 0.25 | — | 117 |
| [Cho][Mal] | 293.15 | u | −0.8 at xIL ∼ 0.2 | — | 117 |
| [Cho][Glyci] | 300 | u | −2.3 at xIL ∼ 0.25 | (0–1) | 101 |
| Ammonium-based ILs | |||||
| TMEAC | 293.15–323.15 | w | [−0.75, −0.35] at xIL ∼ 0.16; [−0.50, −0.10] at xIL ∼ 0.02–0.46 | >0/(0–1) | 118 |
| [DEMA][MeSO3] | 293.15–353.15 | — | — | (0.347–0.705) | 99 |
| [DEMA][OTf] | 293.15–353.15 | — | — | (0–0.964) | 119 |
| PAF | 293.15–333.15 | u | [−1.34, −1.26] at xIL ∼ 0.4 | <0/(0–1) | 120 |
| 3HPAF | 293.15–333.15 | s | [−0.48, −0.42] at xIL ∼ 0.19; [1.20, 1.38] at xIL ∼ 0.91 | <0/(0–0.4); >0/(0.4–1) | 120 |
| 3HPATFAc | 293.15–333.15 | u | [−7.0, −6.86] at xIL ∼ 0.8 | >0/(0–1) | 120 |
| PAAc | 293.15–333.15 | u | [−1.69, −1.67] at xIL ∼ 0.5 | <0/(0–1) | 120 |
| 3HPAAc | 293.15–333.15 | u | [−1.18, −1.13] at xIL ∼ 0.3 | <0/(0–1) | 120 |
| [PN][NO3] | 298.15 | u | −0.6 at xIL ∼ 0.3 | — | 69 |
| 2-HEAB | 298.15/323.15 | u | −1.2 at xIL ∼ 0.22 | — | 121 |
| 2-HEAP | 298.15/323.15 | u | −1.1 at xIL ∼ 0.30 | — | 121 |
| 2-HEAH | 298.15/323.15 | u | x IL ∼ 0.29 | — | 121 |
| 2-HDEAH | 298.15/323.15 | u | x IL ∼ 0.18 | — | 121 |
| [DIPEA][HCOO] | 298.15 | u | −0.80 at xIL ∼ 0.37 | — | 68 |
| M2HEAPr | 293.15–343.15 | u | [−1.23, −1.20] at xIL ∼ 0.4 | >0/(0–1) | 122 |
| BHEAC | 303.15–353.15 | u | [−1.23, −1.20] at xIL ∼ 0.32 | <0, T < 333.15; >0, T < 333.15/(0–1) | 123 |
| [CyN1,1PrSO3H][Tos] | 303.15–328.15 | 124 | |||
| Phosphonium-based ILs | |||||
| [aP4443][Ala] | 298.15–343.15 | — | (0.25–1) | 125 | |
| [aP4443][Val] | 298.15–343.15 | — | (0.25–1) | 125 | |
| [aP4443][Leu] | 298.15–343.15 | — | (0.25–1) | 125 | |
| [P2444][DEP] | 298.15–348.15 | [−0.781, −1.17] at xIL ∼ 0.2 | <0/(0–1) | 126 | |
| At elevated pressures | |||||
|---|---|---|---|---|---|
| DESs | T/K, P/MPa | Shape (VE–xIL/DES) | x DES = extreme position | (∂|VE|/∂P)T,x/range (xDES) | Ref. |
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–323.15, 0.1–50 | u | [−0.34, −0.24] at xDES ∼ 0.4 | <0/(0–1) | 76 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–323.15, 0.1–50 | u | [−0.16, −0.08] at xDES ∼ 0.3 | <0/(0–1) | 75 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–323.15, 0.1–50 | u | [−0.33, −0.24] at xDES ∼ 0.4 | <0/(0–1) | 77 |
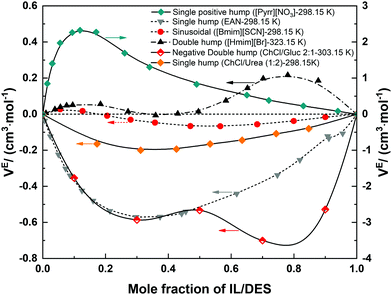
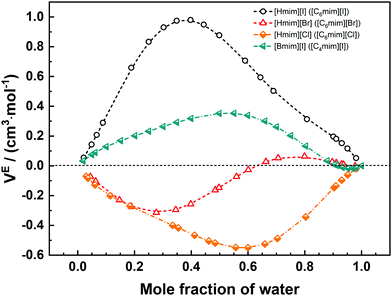
The characteristic features of VE within the whole compositional range (i.e. from 0 to 1) can be very different for different ILs/DESs. According to the reported experimental data from literatures, the VE plotted against the mole fraction for an IL/DES–H2O binary mixture can be positive,68 negative,69 or both,70 and the shapes of the curves (VE–x) can display a single hump,69,71 positive double humps,72 negative double humps73 or be sinusoidal (S-shape)70,74 as shown in Fig. 2.
Further analysis of the available experimental data shows that most of the densities were measured at atmospheric pressure. The magnitude, sign and shape of VE depend on the anion, cation and other physical parameters such as temperature. In general, the values of VE increase with increasing temperature for the studied systems, that is, with the increase of temperature, the absolute value of VE decreases when VE is negative, whereas it increases when VE is positive. However, for the ammonium-based ILs, the temperature only exhibits a weak effect on the overall VE, and the value of VE for some ammonium-based ILs, e.g. TMEAC, becomes more negative with the increase of temperature, which is opposite to most conventional ILs. By increasing the alkyl chain length of cation, VE also increases for the ILs with the same anion for all the studied systems. The type of the alkyl group attached to the cation only shows a slight effect on VE, whereas when the alkyl group is replaced with a polar or hydrophilic group, a more significant effect can be observed.35 The anion is dominant in determining the magnitude, shape and sign of VE–xIL based on the reported experimental data for the ILs with the cations being imidazolium, pyridinium, pyrrolidinium, piperidinium and ammonium.35 The ILs with the anion with many oxygen atoms result in negative VE values. The absolute value of VE for the ammonium-based ILs is much greater compared with those for other ILs. The typical maxima of VE are smaller than 2 cm3 mol−1, but there are some exceptions, such as those for [Pyrr][NO3], [Pyrr][HSO4] and 3HPATFAc (Table 1).
Based on the results listed in Table 1, we can see that, for all the studied DESs, the extreme positions of VE–xDES are almost the same. Their values of VE are always negative, and the absolute value decreases with increasing temperature ((∂|VE|/∂T)P,x < 0). Over the entire compositional range, it can be divided into two regions: the H2O-rich (xDES < 0.5) and DES-rich (xDES > 0.5) regions. Almost for all the studied DESs, there is an extreme point near the H2O-rich region.
Only very few research groups have investigated the volumetric properties (density or molar volume) at high pressures, and all of them are for DESs. For example, Leron et al.75–77 reported the volumetric properties of dry/aqueous DESs at pressures up to 50 MPa. From the results, we can find that pressure has a more significant influence on the magnitude of VE than temperature for the studied DESs, and the magnitude of VE increases with increasing pressure.
2.2 Viscosity η
Viscosity η (i.e. dynamic viscosity, also known as absolute viscosity) is an important transport property as it is directly related to the mass transfer rate.127 Among the factors affecting viscosity, temperature is an important one, and viscosity should be always given at the measured (or calculated) temperature to make any sense. For most fluids, the viscosity can vary non-linearly many orders of magnitude with changes in temperature. The explanation on the molecular level to the decrease of viscosity with increasing temperature in a fluid is that: the temperature of a substance is directly proportional to the average velocity of the molecules consisting the substance, and the molecules require more free space around them when their velocities increase. As a result, the fluid density decreases, and the intermolecular interactions become weaker due to the increase of mutual distances between the molecules.Concerning the viscosities of ILs, we should remember that ILs are often complicate to synthesize, and their purities can be rather poor even for the commercially purchased samples. For most ILs/DESs, the most serious problem is that they are hygroscopic and absorb moisture as soon as they are exposed to atmosphere in the experiments with a variety of viscometers. This problem has been recently highlighted by Diogo et al.128 Significantly different experimental values for viscosities were reported for the same pure IL/DES from different laboratories,71,78 in fact, deviating much more than the reported experimental uncertainties. These large discrepancies imply not only the difficulty in measuring the viscosity of ILs/DESs accurately but also the special nature of ILs/DESs.
The relatively high viscosity of ILs/DESs generally results in the difficulty in their practical applications for different industrial processes, especially in those with traditional mass transfer units. The addition of H2O can change the viscosity significantly, sometimes, even with orders of magnitude. To further illustrate the effect of water on the viscosity, excess viscosity ηE (i.e. viscosity deviation Δη) or excess logarithm viscosity (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E can be calculated from the measured experimental data of viscosity.78,85ηE, Δη, and (ln
η)E can be calculated from the measured experimental data of viscosity.78,85ηE, Δη, and (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E are calculated as following:
η)E are calculated as following:
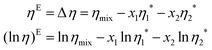 | (2) |
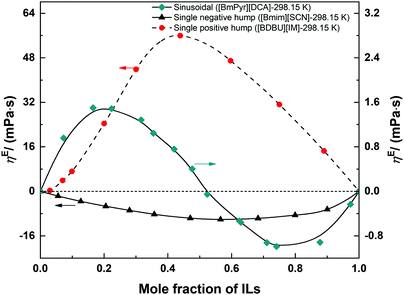
Similar to VE, the magnitude and sign of Δη or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E can be positive, negative, or both, and the shape of the curve (Δη–x or (ln
η)E can be positive, negative, or both, and the shape of the curve (Δη–x or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E–x) can be single hump or sinusoidal (S-shape),78,85,129 reflecting the non-ideal behaviour of these binary systems (Fig. 3). The extreme position shows the water content having the largest effect on the transport properties of these systems. Similar to density, the viscosity of IL/DES–H2O is also a subject with extensive research efforts. Until August 2018, more than 550 articles have been published reporting the viscosity of ILs/DESs with H2O. Most of them focus on the effect of water as a trace impurity130,131 or on the water-saturated ILs/DESs,65,132 and only around 60 articles studied the viscosity of IL/DES–H2O systems over a wide compositional range even up to the entire compositional range. The reported results show that the presence of H2O in ILs/DESs can decrease the viscosity greatly.71 No review articles have been published previously to summarize all these experimental viscosity results. In Table 2, we summarize all, up to date, available experimental data on the viscosity of IL/DES–H2O systems over a wide compositional range, the values of Δη or (ln
η)E–x) can be single hump or sinusoidal (S-shape),78,85,129 reflecting the non-ideal behaviour of these binary systems (Fig. 3). The extreme position shows the water content having the largest effect on the transport properties of these systems. Similar to density, the viscosity of IL/DES–H2O is also a subject with extensive research efforts. Until August 2018, more than 550 articles have been published reporting the viscosity of ILs/DESs with H2O. Most of them focus on the effect of water as a trace impurity130,131 or on the water-saturated ILs/DESs,65,132 and only around 60 articles studied the viscosity of IL/DES–H2O systems over a wide compositional range even up to the entire compositional range. The reported results show that the presence of H2O in ILs/DESs can decrease the viscosity greatly.71 No review articles have been published previously to summarize all these experimental viscosity results. In Table 2, we summarize all, up to date, available experimental data on the viscosity of IL/DES–H2O systems over a wide compositional range, the values of Δη or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E, the extreme positions and the shapes of the curve (Δη–x or (ln
η)E, the extreme positions and the shapes of the curve (Δη–x or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E–x), as well as the changes of Δη or (ln
η)E–x), as well as the changes of Δη or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E with temperature, i.e. (∂|YE|/∂T)P,x. Since the viscosity of IL/DES–H2O over a wide compositional range, even up to the entire compositional range, is the main focus here, the effects of possible impurities including a trace amount of water on the viscosity are not included.
η)E with temperature, i.e. (∂|YE|/∂T)P,x. Since the viscosity of IL/DES–H2O over a wide compositional range, even up to the entire compositional range, is the main focus here, the effects of possible impurities including a trace amount of water on the viscosity are not included.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E of IL/DES–H2O at atmospheric pressure
η)E of IL/DES–H2O at atmospheric pressure
| ILs & DESs | T/K | Range (xIL/DES) | Δη or ηE or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E (∂|YE|b/∂T)P,x η)E (∂|YE|b/∂T)P,x |
Shapea (YE–xIL/DES)/xIL/DES = extreme position | Ref. |
|---|---|---|---|---|---|
a ‘u’: negative single hump, ‘n’: positive single hump, and ‘s’: sinusoidal.
b
Y: Δη or ηE or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E. η)E.
|
|||||
| DESs | |||||
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
293.15–323.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xDES = 0.8 | 80 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–333.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xDES = 0.8 | 71 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
293.15–363.15 | 0–1 | (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E < 0/(∂|YE|/∂T)P,x < 0; (ln η)E < 0/(∂|YE|/∂T)P,x < 0; (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E > 0/(∂|YE|/∂T)P,x > 0 η)E > 0/(∂|YE|/∂T)P,x > 0 |
u/xDES = 0.6 (T < 343.15); s/xDES = 0.3 and 0.7 (T = 343.15); n/xDES = 0.4 (T > 343.15) | 78 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
283.15–363.15 | 0–1 | (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E > 0/(∂|YE|/∂T)P,x < 0 η)E > 0/(∂|YE|/∂T)P,x < 0 |
n/xDES = 0.4 | 85 |
DEAC/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–343.15 | 0–1 | — | u/xDES = 0.5 | 90 |
DEAC/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–343.15 | 0–1 | — | u/xDES = 0.3 | 90 |
| Imidazolium-based ILs | |||||
| [Mmim][DMP] | 298.15 | 0.252–1 | — | — | 93 |
| [Emim][DMP] | 298.15–333.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0; Δη < 0/Δη > 0 | u/xIL = 0.4 (T > 298.15); s/xIL = 0.3 and 0.8 (T = 298.15) | 102 |
| [Emim][EtSO4] | 298.15 | 0.4–1 | Δη < 0 | — | 133 |
| [Emim][EtSO4] | 288.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.6 | 134 |
| [Emim][EtSO4] | 278.15–348.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.6 | 96 |
| [Emim][MeSO4] | 303.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.5 | 135 |
| [Emim][Ac] | 298.15 | 0–1 | — | u/xIL = 0.65 | 136 |
| [Emim][Ac] | 298.15–353.15 | 0.665–0.955 | — | — | 33 |
| [Emim][Ac] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.6 (T ≤ 313.15); w/xIL = 0.2 and 0.9 (T = 328.15); s/xIL = 0.1 and 0.6 (T = 343.15) | 98 |
| [Emim][Ac] | 293.15–353.15 | 0.295–0.653 | — | — | 99 |
| [Emim][SCN] | 288.15–318.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.6 | 137 |
| [Emim][DCA] | 288.15–318.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.65 | 137 |
| [Emim][DCA] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.77 (T ≤ 343.15); n/xIL = 0.3 and 0.9 (T = 343.15); | 98 |
| [Emim][Cl] | 373 | 0.8–1 | — | — | 138 |
| [Emim][OTf] | 278.15–348.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 96 |
| [Emim][TCM] | 298.15 | 0–1 | Δη < 0 | u/xIL = 0.6 | 97 |
| [Emim][TFA] | 278.15–348.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0; Δη > 0/(∂|YE|/∂T)P,x > 0 | u/xIL = 0.5 (T = 278.15); s/xIL = 0.1 and 0.5 (T > 278.15); n/xIL = 0.25 (T > 328.15) | 96 |
| [Emim][NTf2] | 0.75–1 | (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E > 0 η)E > 0 |
— | 139 | |
| [Emim][NTf2] | 293 | 0.8–1 | — | — | 140 |
| [Emim][BF4] | 293.15 | 0–1 | Δη < 0 | u/xIL = 0.6 | 141 |
| [Bmim][HSO4] | 303.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.6–0.7 | 135 |
| [Bmim][MeSO3] | 298.15–343.15 | 0.025–0.441 | — | — | 142 |
| [Bmim][MeSO4] | 303.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.5–0.6 | 135 |
| [Bmim][PF6] | 0.75–1 | (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E > 0 η)E > 0 |
139 | ||
| [Bmim][DCA] | 278.15–363.15 | 0.52–1 | — | — | 143 |
| [Bmim][TCM] | 278.15–363.15 | 0.52–1 | — | — | 143 |
| [Bmim][Ac] | 298.15 | 0–1 | — | s/xIL = 0.75 and 0.64 | 136 |
| [Bmim][SCN] | 298.15–348.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.57 | 70 |
| [Bmim][BF4] | 303.15–353.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 144 |
| [Bmim][BF4] | 293.15 | 0–1 | Δη < 0 | u/xIL = 0.65 | 141 |
| [Bmim][BF4] | 298.15 | 0–1 | Δη < 0 | u/xIL = 0.7 | 145 |
| [Bmim][BF4] | 283.15–373.15 | 0.6–1 | (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E > 0 η)E > 0 |
— | 146 |
| [Bmim][NTf2] | 0.75–1 | (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E > 0 η)E > 0 |
— | 139 | |
| [Bmim][I] | 298.15 | 0.1–1 | Δη < 0 | u/xIL = 0.7 | 147 |
| [Bmim][DMP] | 298.15–333.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.4 | 102 |
| [Bdmim][BF4] | 298.15 | 0–1 | Δη < 0 | u/xIL = 0.7 | 145 |
| [Amim][Cl] | 293.15–333.15 | 0.2–1 | — | — | 148 |
| [Amim][Cl] | 298.15 | 0–1 | — | u/xIL = 0.5 | 107 |
| [Amim][Cl] | 298.15–353.15 | 0.665–0.955 | — | — | 33 |
| [Hmim][Cl] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.8 | 149 |
| [Hmim][Br] | 293.15–333.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 72 |
| [Omim][Cl] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 149 |
| [HDBU][IM] | 293.15–313.15 | 0–1 | Δη > 0/(∂|YE|/∂T)P,x < 0 | n/xIL = 0.45 | 105 |
| [BDBU][IM] | 293.15-313.15 | 0–1 | Δη > 0/(∂|YE|/∂T)P,x < 0 | n/xIL = 0.45 | 105 |
| [TMG][IM] | 293.15–313.15 | 0–1 | Δη > 0/(∂|YE|/∂T)P,x < 0 | n/xIL = 0.45 | 106 |
| Pyridinium-based ILs | |||||
| [B4mPy][SCN] | 298.15–348.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.54 | 70 |
| [PPy][BF4] | 293.15–323.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 150 |
| [BPy][BF4] | 293.15–323.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 150 |
| [BPy][BF4] | 283.15–348.15 | 0.144–0.89 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 151 |
| [OcPy][BF4] | 283.15–348.15 | 0.144–0.89 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 151 |
| Cholinium-based ILs | |||||
| [Cho][Glyc] | 293.15–323.15 | 0.063–0.634 | — | — | 152 |
| [Cho][Lac] | 293.15–323.15 | 0.059–0.9728 | — | — | 152 |
| Ammonium-based ILs | |||||
| TMEAC | 293.15–323.15 | 0–1 | (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E > 0/(∂|YE|/∂T)P,x < 0 η)E > 0/(∂|YE|/∂T)P,x < 0 |
n/xIL = 0.16 | 118 |
| [MTEOA][MeSO4] | 298.15 | 0–1 | Δη > 0 | n/xIL = 0.4 | 153 |
| [DEMA][MeSO4] | 293.15–353.15 | 0.295–0.653 | — | — | 99 |
| [BA][Ac] | 293.15–313.15 | 0.031–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.6 | 154 |
| [BA][NO3] | 293.15–313.15 | 0.1–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.65 | 154 |
| [EA][NO3] | 293.15–318.15 | 0–1 | η E < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.74 | 74 |
| Phosphonium-based ILs | |||||
| [aP4443][Ala] | 298.15–343.15 | 0.25–1 | — | — | 125 |
| [aP4443][Val] | 298.15–343.15 | 0.25–1 | — | — | 125 |
| [aP4443][Leu] | 298.15–343.15 | 0.25–1 | — | — | 125 |
| Piperidinium-based ILs | |||||
| [Bmpip][SCN] | 298.15–348.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.63 | 70 |
| [Bmpip][DCA] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.62 | 129 |
| [Empip][EtSO4] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 108 |
| Piperazinium-based ILs | |||||
| [(OH)2C2pi][C2CO2] | 293.15–323.15 | 0–0.12 | 36 | ||
| Guanidinium-based ILs | |||||
| [(C2)22(C1)2(C1)23gu][EtSO4] | 293.15–323.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.6 | 113 |
| [TMG][Lac] | 303.15–328.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.3 | 115 |
| [TMG][BEN] | 298.15 | 0–0.224 | — | — | 114 |
| [TMG][SAL] | 298.15 | 0–0.224 | — | — | 114 |
| Morpholinium-based ILs | |||||
| [Emmor][EtSO4] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 108 |
| [Emmor][Ac] | 338.15–348.15 | 0–0.7155 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 116 |
| [Emmor][Pro] | 343.15, 348.15 | 0–0.7436 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.7 | 116 |
| Pyrrolidinium-based ILs | |||||
| [Pyrr][C7CO2] | 293.15–313.15 | 0–1 | η E > 0/(∂|YE|/∂T)P,x < 0 | n/xIL = 0.4 | 155 |
| [P3myrr][Br] | 298.15–343.15 | 0–0.3535 | — | — | 156 |
| [Pe5myrr][Br] | 298.15–343.15 | 0–0.3254 | — | — | |
| [B4mPyr][Br] | 298.15–343.15 | 0–0.4450 | — | — | 156 |
| [BmPyr][SCN] | 298.15–348.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.47 | 70 |
| [BmPyr][DCA] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0; Δη > 0/(∂|YE|/∂T)P,x < 0 | s/xIL = 0.8; xIL = 0.2 | 129 |
| [Empyr][EtSO4] | 298.15–343.15 | 0–1 | Δη < 0/(∂|YE|/∂T)P,x < 0 | u/xIL = 0.5 | 108 |
All the available data was measured at atmospheric pressure, and no data was found available at the pressure higher than 1 bar. For most of the IL/DES–H2O systems, the shapes of the curves (Δη–x or (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E–x) show a single hump (positive or negative). Only 6 IL/DES–H2O systems with the ILs/DESs of ChCl/urea (1
η)E–x) show a single hump (positive or negative). Only 6 IL/DES–H2O systems with the ILs/DESs of ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), [Emim][Ac], [Bmim][Ac], [Emim][TFA], [Emim][DMP] and [BmPyr][DCA] show sinusoidal or S-shaped curve, and the S-shaped curve for the former 5 ILs/DESs can only be observed in a certain temperature range. Δη(ηE) is more widely used than (ln
2), [Emim][Ac], [Bmim][Ac], [Emim][TFA], [Emim][DMP] and [BmPyr][DCA] show sinusoidal or S-shaped curve, and the S-shaped curve for the former 5 ILs/DESs can only be observed in a certain temperature range. Δη(ηE) is more widely used than (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η)E for analyzing the viscosity of IL/DES–H2O binary systems. In general, the absolute value of Δη shown in Table 2 decreases with increasing temperature for the studied ILs/DESs except ChCl/urea (1
η)E for analyzing the viscosity of IL/DES–H2O binary systems. In general, the absolute value of Δη shown in Table 2 decreases with increasing temperature for the studied ILs/DESs except ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)78 and [Emim][TFA].96 Most of the reported data is for the imidazolium-based ILs. From the results listed in Table 2, the excess viscosity is negative for most of the studied ILs/DESs, and the corresponding extreme point is near the IL/DES-rich region (xIL/DES > 0.5). This phenomenon implies that the viscosity decreases rapidly in the IL/DES-rich region and thus the presence of water expands the volume of the fluids and favors the diffusion of molecules.
2)78 and [Emim][TFA].96 Most of the reported data is for the imidazolium-based ILs. From the results listed in Table 2, the excess viscosity is negative for most of the studied ILs/DESs, and the corresponding extreme point is near the IL/DES-rich region (xIL/DES > 0.5). This phenomenon implies that the viscosity decreases rapidly in the IL/DES-rich region and thus the presence of water expands the volume of the fluids and favors the diffusion of molecules.
As discussed in the previous section, a trace amount of water for the hygroscopic IL/DES and the other impurities of ILs will significantly affect the viscosity of IL/DES. This is essential for determining a reliable viscosity of pure ILs/DESs or ILs/DESs with a trace amount of water accurately, while such effect is not so crucial for studying the viscosity of IL/DES–H2O system over the wide/entire compositional range.
2.3 Electrical conductivity Λ
Electrical conductivity Λ is another important transport property, and it provides the information on the mobility of ions or charged molecules in systems. There are mainly two kinds of electrical conductivity data for IL/DES–H2O binary systems. One is the electrical conductivity Λ for the IL/DES–H2O binary systems over a wide compositional range, and the other is that for the dilute IL/DES solution. Analyzing the Λ of the dilute IL/DES solution can reflect the ionic association behaviour in water, i.e. can obtain the ion association constants kA and provide insight on the specific microstructures. Besides, some investigations on the Λ for the ILs/DESs with a trace amount of water have been conducted,157 but are excluded in this review. In Table 3, the data of the Λ over the wide compositional range and the reported ion association constants kA is summarized.| ILs & DESs | T/K | Range xIL/DES or ΦIL/DES or mIL/DESa | Λ/s m−1 (xIL/DES or ΦIL/DES or mIL/DES = extreme position) | Ref. |
|---|---|---|---|---|
| a x IL/DES, ΦIL/DES and mIL/DES are the mole fraction, volumetric fraction and molarity of IL/DES (mol m−3), respectively. b Unit: g L−1. | ||||
| Wide compositional range | ||||
| Imidazolium-based ILs | ||||
| [Bmim][PF6] | 288.15–308.15 | x IL = 0.2 | — | 160 |
| [Bmim][BF4] | 298.15 | Φ IL = 0–1 | ∼5 (ΦIL ∼ 0.4) max | 161 |
| [Bmim][BF4] | 298.15 | m IL = 0–5.1812 | ∼4.7 (mIL ∼ 2.45) max | 162 |
| [Bmim][BF4] | 293.15–323.15 | x IL = 0.0256–0.8986 | 4.41–8.710 (xIL ∼ 0.078) max | 163 |
| [Bmim][DCA] | 298.15 | Φ IL = 0–1 | ∼4.8 (ΦIL ∼ 0.6) max | 161 |
| [Bmim][MeSO4] | 298.15–343.14 | x IL = 0–0.5 | 4.059 (xIL ∼ 0.049) max | 142 |
| [Bmim][MeSO4] | 298.15 | x IL = 0–1 | 3.416 (xIL ∼ 0.0563) max | 164 |
| [Bmim][I] | — | x IL = 0–1 | 1.5 (xIL ∼ 0.05) max | 165 |
| [Bmim][I] | — | x IL = 0.1–1 | — | 147 |
| [Bmim][Br] | 298.15 | m IL = 0.019–5.396 | ∼7.07 (mIL ∼ 2.29) max | 162 |
| [Bmim][Cl] | 298.15 | m IL = 0.0–5.502 | ∼6.68 (mIL ∼ 2.345) max | 162 |
| [Bmim][Cl] | 298.15 | x IL = 0–0.5018 | 5.98 (xIL ∼ 0.0498) max | 166 |
| [Emim][Br] | 298.15 | m IL = 0.0195–6.903 | ∼8.86 (mIL ∼ 2.318) max | 162 |
| [Emim][BF4] | 298.15 | Φ IL = 0–1 | ∼9 (ΦIL ∼ 0.5) max | 161 |
| [Emim][BF4] | 298.15 | x IL = 0–1 | 8.52 (xIL ∼ 0.175) max | 167 |
| [Emim][MeSO4] | 298.15 | x IL = 0–1 | 6.232 (xIL ∼ 0.075) max | 164 |
| [Emim][EtSO4] | 298.15 | Φ IL = 0–1 | ∼4.5 (ΦIL ∼ 0.5) max | 161 |
| [Emim][EtSO4] | 298.15 | x IL = 0–1 | 4.11 (xIL ∼ 0.0554) max | 168 |
| [Emim][EtSO4] | 298.15 | x IL = 0–1 | 4.45 (xIL ∼ 0.0709) max | 167 |
| [Emim][BuSO4] | 298.15 | x IL = 0–1 | 3.09 (xIL ∼ 0.0773) max | 168 |
| [Emim][HeSO4] | 298.15 | x IL = 0–1 | 2.48 (xIL ∼ 0.0282) max | 168 |
| [Emim][NTf2] | 303.15 | x IL = 0.5–1 | — | 169 |
| [Ehim][NTf2] | 303.15 | x IL = 0.5–1 | — | 169 |
| [Emim][OTf] | 303.15 | x IL = 0.5–1 | — | 169 |
| [Ehim][OTf] | 303.15 | x IL = 0.5–1 | — | 169 |
| [Mmim][Cl] | 450 | x IL = 0.25–1 | — | 170 |
| [Amim][Cl] | 293.15–333.15 | x IL = 0.2–1 | — | 148 |
| [Amim][Cl] | 298.15 | x IL = 0.000171–1 | 7.922 (xIL ∼ 0.0665) max | 107 |
| [TMG][IM] | 293.15–313.15 | 0–1 | 4.0 (xIL ∼ 0.05) max | 106 |
| Phosphonium-based ILs | ||||
| [aP4443][Ala] | 298.15–343.15 | x IL = 0.25–1 | — | 125 |
| [aP4443][Val] | 298.15–343.15 | x IL = 0.25–1 | — | 125 |
| [aP4443][Leu] | 298.15–343.15 | x IL = 0.25–1 | — | 125 |
| Ammonium-based ILs | ||||
| CPL-TBAF | 293.15–333.15 | x IL = 0.00183–0.171 | 0.765–0.64 (xIL ∼ 0.0505) max | 171 |
| CPL-TBABr | 308.15–328.15 | x IL = 0.0505 | 0.78–0.64 (xIL ∼ 0.0505) max | 171 |
| CPL-TBACl | 293.15–333.15 | x IL = 0.00183–0.171 | 0.8–0.74 (xIL ∼ 0.0505) max | 171 |
| [BA][NO3] | 298.15 | x IL = 0–1 | 3.7 (xIL ∼ 0.109) max | 154 |
| [BA][Ac] | 298.15 | x IL = 0–1 | 1.727 (xIL ∼ 0.0579) max | 154 |
| Choline-based ILs | ||||
| [Cho][H2Prop] | 298.15–333.15 | x IL = 0.004–0.1657 | 2.834–3.356 (xIL ∼ 0.04) max | 172 |
| Pyridinium-based ILs | ||||
| [EPy][MeSO3] | 298.15–333.15 | x IL = 0.0039–0.383 | 4.672–4.995 (xIL ∼ 0.05) max | 172 |
| Piperazinium-based ILs | ||||
| [(OH)2C2pi][EtCOO] | 293.15–323.15 | x IL = 0–0.12 | 3.75 (xIL ∼ 0.0291) max | 36 |
| DESs | ||||
DEAC/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–343.15 | x DES = 0.1002–0.9018 | — | 173 |
DEAC/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15–343.15 | x DES = 0.1–0.9 | — | 173 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
293.15–363.15 | x DES = 0–1 | 5.97 (xDES ∼ 0.1) max | 79 |
| ILs | T/K | Range mIL/DES (mol m−3) | k A (dm3 mol−1) | Ref. |
|---|---|---|---|---|
| Dilute solutions of ILs | ||||
| Imidazolium-based ILs | ||||
| [Bmim][BF4] | 298.15 | 50–1000 | — | 174 |
| [Bmim][BF4] | 303.2–323.2 | 4.2–55.3 | — | 175 |
| [Bmim][BF4] | 298.15 | 0.1–1.4 | 105 | 158 |
| [Bmim][PF6] | 303.2–323.2 | 3–5.6 | — | 175 |
| [Bmim][PF6] | 298.15 | 0.1–1.4 | 113 | 158 |
| [Bmim][MeSO4] | 303.2–323.2 | 5–16.3 | — | 175 |
| [Bmim][OTf] | 303.2–323.2 | 4–21.1 | — | 175 |
| [Bmim][Br] | 298.15 | 0.1–1.4 | 66 | 158 |
| [Bmim][Cl] | 278.15–313.15 | 6 | 7.2–4.5 | 176 |
| [Bmim][Cl] | 298.15 | 0.4734–2.099 | 4.04 | 177 |
| [Bmim][Cl] | 298.15 | 0.4833–1.681 | 8.56 | 178 |
| [Bmim][HCOO] | 298.15 | 2.25 | 179 | |
| [Bmim][Ac] | 298.15 | 0.37 | 179 | |
| [Bmim][EtCOO] | 298.15 | –0.24 | 179 | |
| [Emim][EtSO4] | 278.15–313.15 | 0.25–5 | 0.014–0.7 | 180 |
| [Hmim][Cl] | 298.15 | 0.1239–1.927 | 5.36 | 177 |
| [Hmim][Cl] | 293.15–303.15 | 0.0009–0.0841 | 2.4–7.8 | 181 |
| [Hmim][Cl] | 298.15 | 0.2349–1.62 | 4.78 | 178 |
| [Hmim][Br] | 283.15–303.15 | 0.6–63.84 | 2.94–4.93 | 159 |
| [Hmim][Br] | 298.15 | 0.1–1.4 | 71 | 158 |
| [Omim][Cl] | 298.15 | 0.3264–1.863 | 2.51 | 177 |
| [Omim][Cl] | 298.15 | 0.2751–1.631 | 1.54 | 178 |
| [Omim][Br] | 298.15 | 0.1–1.4 | 79 | 158 |
| [C10mim][Cl] | 298.15 | 0.1226–1.530 | 4.53 | 177 |
| [C10mim][Cl] | 298.15 | 0.3586–1.878 | 25.86 | 178 |
| [C10mim][Br] | 298.15 | 0.1–1.4 | 84 | 158 |
| [C12mim][Br] | 298.15 | 0.1–1.4 | 96 | 158 |
| Others | ||||
| [C3C1pyr][PF6] | 288.15–318.15 | 0–1.4b | — | 182 |
| [C3C1pip][PF6] | 288.15–318.15 | 0–1.4b | — | 182 |
| [N4444][PF6] | 288.15–318.15 | 0–0.25b | — | 182 |
| [P4444][PF6] | 288.15–318.15 | 0–0.25b | — | 182 |
According to the results listed in Table 3, the most studied ILs are imidazolium-based. The electrical conductivity of IL/DES–H2O binary systems shows, in general, a maximum value at a relatively low IL/DES concentration (xIL/DES ∼ 0.03 – 0.1), which is unique and different when compared with the aqueous solutions with common electrolytes (e.g. NaCl, Na2SO4) or other solvents (e.g. CH3COOH). The further comparison of the electrical conductivity for the IL/DES–H2O binary systems, the aqueous electrolyte (i.e. NaCl, Na2SO4) solutions and the aqueous CH3COOH solution, depicted in Fig. 4, shows that Λ of aqueous electrolyte solutions increases linearly with the increase of solute concentration (i.e. electrolyte) in mole fraction. While for the IL/DES–H2O binary solutions, it initially increases whereas it thereafter decreases with increasing solute concentration (i.e. IL/DES) in mole fraction. Meanwhile, the values of Λ for the IL/DES–H2O binary systems are lower than those for aqueous solutions with strong electrolytes (i.e. NaCl, Na2SO4) but higher than those for aqueous solutions with CH3COOH. This implies that aqueous IL solutions are very different from other aqueous solutions.
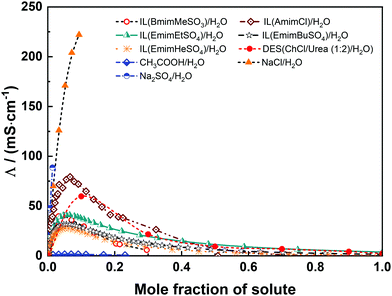 | ||
| Fig. 4 The electrical conductivity Λ of aqueous solutions with selected electrolytes, organic solvent and ILs/DESs. | ||
For the dilute IL solutions, the ILs with the [Bmim]+ were mostly investigated, and their kA values in H2O were reported. The values of kA obtained from different research groups show considerable discrepancies. For example, kA of [Hmim][Br] from Wang et al.158 is 71, while that from Shekaari et al.159 is only 4.9. These discrepancies may be caused by choosing the experimental data in different compositional ranges. Therefore, a reliable and standard method still needs to be developed to obtain reliable kA values.
Most of the reported kA values range from 0.014 to 113. There is only one exception for [Bmim][EtCOO] with a negative kA.158 The unreasonably negative kA was obtained when 3 parameters (i.e. kA, the limiting molar conductivity and the ion distance parameter) were iteratively fitted to a non-linear least square expression without considering the physical meaning of these parameters.
The anions affect the value of kA greatly. For the ILs with the same cation [Bmim]+, the values of kA follow the order: [PF6]− > [BF4]− > [Br]− > [Cl]− > [HCOO]− > [MeCOO]− > [EtCOO]−. Except the imidazolium-based ILs, only few results on Λ have been reported for the phosphonium-, ammonium-, and piperazinium-based ILs as well as DESs.
2.4 Excess/mixing enthalpy Hm
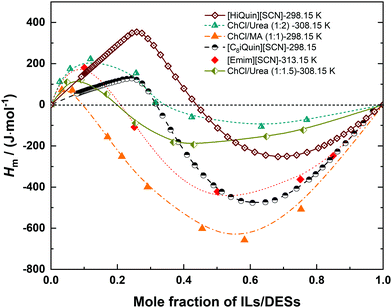
Energetic properties of IL/DES–H2O mixtures are relevant both in fundamental and application contexts.34 At the fundamental level, it is closely related to the nature of molecular or ionic interactions and to the microscopic structure of mixtures.34 In applications, it may concern the contributions from thermodynamics and thermochemistry to other scientific disciplines such as chemistry, materials science, chemical engineering, and so on.34Although the mixing enthalpy Hm is important, the reported experimental data for IL/DES–H2O binary systems is quite limited compared with other properties, such as density and viscosity. Podgorsek et al.34 has reviewed the Hm of binary mixtures containing ILs (IL-associating compounds (i.e. H2O and alcohols), IL-non-associative compounds (i.e. acetone, 1-butanone, and 3-pentanone) and IL-IL) until 2015. They state that the thermodynamic quantities of mixing could provide valuable insight into molecular interactions and microscopic structures in such mixtures. Combined with the new results after 2015, the updated experimental results are summarized in this work, and the available IL/DES–H2O binary systems over the wide compositional range are listed in Table 4. With the increase of IL/DES content, both the magnitude and sign of mixing enthalpy (Hm) are changed. The Hm can be positive or negative, and the shapes of Hm–xIL/DES for most of the ILs/DESs show a single hump. For [Emim][SCN], [HiQuin][SCN], [C8iQuin][SCN], ChCl/MA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ChCl/urea (1
1), ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ChCl/urea (1
2) and ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5), the values of Hm show an S-shaped curve as depicted in Fig. 5. For these 6 binary systems, the values of Hm are always positive in the H2O-rich region (0 < xIL/DES < 0.5) and then change to negative with an increase of the mole fraction of IL/DES. This observation implies that the mixing process is initially endothermic and then changes to exothermic with increasing the content of IL/DES. The turnover points depend on the type of ILs/DESs.
1.5), the values of Hm show an S-shaped curve as depicted in Fig. 5. For these 6 binary systems, the values of Hm are always positive in the H2O-rich region (0 < xIL/DES < 0.5) and then change to negative with an increase of the mole fraction of IL/DES. This observation implies that the mixing process is initially endothermic and then changes to exothermic with increasing the content of IL/DES. The turnover points depend on the type of ILs/DESs.
| ILs & DESs | T, K | x IL/DES/Hm,mina | x IL/DES/Hm,maxa | x IL/DES, Hm = 0 | Shape/Hma | (∂|Hm|/∂T)P,x | Ref. |
|---|---|---|---|---|---|---|---|
| a The unit for Hm is kJ mol−1. | |||||||
| DESs | |||||||
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15/308.15 | 0.4/[−1.08, −1.06] | — | — | u/<0 | >0 | 183 |
ChCl/MA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
298.15/308.15 | 0.4/∼−0.6 | 0.05/∼0.1 | 0.1 | s/(<0, >0) | >0, Hm < 0; >0, Hm > 0 | 183 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
298.15/308.15 | 0.4/∼−0.9 | — | — | u/<0 | >0 | 183 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
308.15/318.15 | 0.7/∼−0.07 | 0.14/∼0.2 | 0.3 | s/(<0, >0) | >0, Hm < 0; <0, Hm > 0 | 184 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
308.15/318.15 | 0.5/[−0.3, −0.2] | 0.05/∼0.15 | 0.2 | s/(<0, >0) | >0, Hm < 0; <0, Hm > 0 | 184 |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) 2.5) |
308.15/318.15 | 0.16/∼0.28 | — | n/>0 | <0 | 184 | |
| Imidazolium-based ILs | |||||||
| [Emim][SCN] | 313.14–348.13 | 0.61/∼−0.45 | 0.10/[0.2, 0.37] | 0.32 | s/(<0, >0) | <0, Hm < 0; >0, Hm > 0 | 185 |
| [Emim][MeSO3] | 313.14–348.14 | 0.25/∼−2.73 | — | — | u/<0 | >0 | 186 |
| [Emim][HSO4] | 313.14 | 0.5/∼−2.55 | — | — | u/<0 | — | 186 |
| [Emim][MeSO4] | 313.14 | 0.25/∼−1.14 | — | — | u/<0 | — | 187 |
| [Emim][DEP] | 313.13–348.12 | 0.38/∼−6 | — | — | u/<0 | <0 | 185 |
| [Emim][EtSO4] | 313.14–348.12 | 0.25/[−0.834, −0.975] | — | — | u/<0 | <0 | 188 |
| [Emim][EtSO4] | 298.15 | 0.2/−0.991 | — | — | u/<0 | — | 189 |
| [Emim][OTf] | 313.13–348.12 | — | 0.5/[1.127, 1.244] | n/>0 | >0 | 188 | |
| [Emim][TFA] | 313.04–348.06 | 0.5/[−2.14, −2.0] | — | — | u/<0 | <0 | 188 |
| [Emim][TCM] | 288.15–318.15 | — | 0.5/∼1.6 | — | n/>0 | — | 97 |
| [Emim][BF4] | 298.15 | — | 0.57/0.483 | — | n/>0 | — | 190 |
| [Emim][EtSO4] | 363.15 | 0.39/−0.814 | — | — | u/<0 | — | 191 |
| [Emim][EtSO4] | 303.15 | 0.2/−0.986 | — | — | u/<0 | — | 187 |
| [Emim][MeSO4] | 303.15 | 0.3/−0.537 | — | — | u/<0 | — | 187 |
| [Emim][OTf] | 303.15 | — | 0.40/1.071 | n/>0 | — | 187 | |
| [Emim][DMP] | 298.15 | 0.3/−6.25 | — | — | u/<0 | — | 192 |
| [Emim][DMP] | 298.15 | 0.3/−6.3 | — | — | u/<0 | — | 193 |
| [Bmim][BF4] | 278.15–333.15 | — | 0.33/[2.05–2.35] | — | u/>0 | >0 | 194 |
| [Bmim][Cl] | 303.15/318.15 | — | — | — | u/<0 | <0 | 195 |
| [Bmim][OTf] | 303.15 | — | 0.40/1.389 | — | n/>0 | — | 187 |
| [Hmim][OTf] | 313.12–347.85 | — | 0.38/[1.6, 2.1] | — | n/>0 | >0 | 185 |
| [Mmim][DMP] | 298.15 | 0.3/−6.5 | — | — | u/<0 | — | 93 |
| [OHemim][TFA] | 323.13–384.14 | 0.32/∼−1.25 | — | — | u/<0 | <0 | 186 |
| Phosphonium-based ILs | |||||||
| [P2444][DEP] | 313.13–348.12 | 0.3/[−2.5, −3] | — | — | u/<0 | <0 | 185 |
| [P2444][DEP] | 298.15 | 0.3/−1.76 | — | — | u/<0 | — | 1, 2, 126 and 196 |
| Pyridinium-based ILs | |||||||
| [B3mpy][BF4] | 298.15/318.15 | 0.30/[1.635–2.099] | n/>0 | >0 | 197 | ||
| [B4mpy][BF4] | 298.15/318.15 | 0.33/[1.81–2.245] | n/>0 | >0 | 198 | ||
| [BPy][BF4] | 298.15/318.15 | 0.31–0.36/[1.78–2.549] | n/>0 | >0 | 199 | ||
| [B2mpy][BF4] | 298.15/318.15 | 0.33–0.36/[1.49–2.13] | n/>0 | >0 | 200 | ||
| [B3mpy][BF4] | 303.15 | 0.40/2.319 | n/>0 | — | 187 | ||
| Choline-based ILs | |||||||
| [Cho][Glyc] | 303.15 | 0.20/−6 | — | u/<0 | — | 152 | |
| [Cho][Lac] | 303.15 | 0.19/−20 | — | u/<0 | — | 152 | |
| Ammonium-based ILs | |||||||
| [EA][NO3] | 298.15 | 0.378/0.6977 | n/>0 | — | 69 | ||
| [PA][NO3] | 298.15 | 0.34/0.8955 | n/>0 | — | 69 | ||
| Piperidinium-based ILs | |||||||
| [Empip][EtSO4] | 298.15 | 0.21/−1.255 | — | u/<0 | — | 201 | |
| [Bmpip][DCA] | 298.15 | 0.371/−0.774 | — | u/<0 | — | 129 | |
| Morpholinium-based ILs | |||||||
| [Emmor][EtSO4] | 298.15 | 0.2/−0.675 | — | u/<0 | — | 201 | |
| Pyrrolidinium-based ILs | |||||||
| [Bmpyr][DCA] | 298.15 | 0.3857/−0.953 | — | u/<0 | — | 129 | |
| [Empyr][EtSO4] | 298.15 | 0.24/−1.615 | — | u/<0 | — | 201 | |
| Quinolinium-based ILs | |||||||
| [HiQuin][SCN] | 298.15–308.15 | 0.68/[−0.29, −0.25] | 0.26/[0.33, 0.35] | 0.42 | s/(<0, >0) | >0, Hm < 0 | 202 |
| [C8iQuin][SCN] | 298.15–308.15 | 0.62/∼−0.5 | 0.26/[0.1, 0.3] | 0.31 | s/(<0, >0) | >0, Hm < 0; >0, Hm > 0 | 203 |
2.5 Other important properties – surface and interfacial tensions
Other properties such as surface and interfacial tensions are also of importance in industrial applications although they have not been investigated as widely as the other properties discussed above.62 The surface and interfacial tensions reflect the properties on the liquid–gas/vapor surface and liquid–liquid phase boundary, respectively. The surface tension is an important property in studies of physics and chemistry at the free surface because it affects the transfer rates of gas absorption, for example, where a gas–liquid interface exists.204 Although some work has been conducted on the surface tension of IL/DES–H2O binary systems, the experimental data over a whole compositional range at different temperatures is still scarce.62 For the interfacial tension,205 only few results were published.2.6 Summing-up
A large amount of experimental investigations has been carried out to study the physicochemical properties of IL/DES–H2O binary systems including density, viscosity, electrical conductivity, excess/mixing enthalpy and so on. Density and viscosity are the most widely studied properties. The electrical conductivity and excess/mixing enthalpy both have much attention but the number of investigated ILs is still small. Studies of surface and interfacial tensions over a whole compositional range at different temperatures are still scarce. The electrical conductivity of IL/DES–H2O binary systems over a wide compositional range generally shows a maximum value at a relatively low IL/DES concentration. The excess volume and viscosity and mixing enthalpy are often used as a measure of non-ideal behaviour of IL/DES–H2O binary system, and the shape depends on the cations and anions, as well as the physical conditions, i.e. temperature and pressure. This implies that the properties of the mixtures are truly unique and at the same time largely unpredictable. To understand the IL/DES–H2O binary systems deeper, more experiments of measuring the electrical conductivity and excess/mixing enthalpy should be carried out in order to reflect the non-ideal behavior directly.3. Mechanistic studies
The physicochemical properties of IL/DES–H2O binary systems are a macroscopic manifestation of the microscopic interactions of ion–ion and ion–solvent (H2O). Understanding how H2O interacts with ILs/DESs and how the properties of ILs/DESs are affected by the presence of H2O is highly important in both scientific research and practical applications. Therefore, in this section, a selection of mechanistic studies on the IL/DES–H2O systems are surveyed.Considering that as many as 1018 ILs/DESs could conceivably be synthesized by combining the possible cations with possible anions, it is crucial to develop theoretical models for the reliable prediction of the properties of these systems. However, it appears that most of the modelling work still empirically correlates the experimental data for a specific system with a specific property. The development of more general theoretical models remains limited. Moreover, there are currently no theoretical models describing the thermodynamic properties over the entire compositional range from pure ILs/DESs to infinitely dilute ILs/DESs solutions. The primary reason for this lack of models is the extremely complex molecular behaviour of ILs/DESs, both in their pure state and combined with water. Various analyses using X-ray and neutron scattering, NMR spectroscopy and other experimental methods have shown that ILs are highly heterogeneous with long-range structural correlations, while the structures of DESs are more complex. The development of reliable theoretical models for IL/DES–H2O binary systems will require investigations of the molecular behaviour based on mechanistic studies using both experimental and theoretical approaches.
To examine the molecular behaviour of IL/DES–H2O binary systems, much research work has been carried out using different computational methods, including molecular dynamics simulations, Monte Carlo simulations and quantum chemical calculations, and advanced experimental methods including infrared, Raman and NMR spectroscopy, X-ray scattering and neutron scattering as well as thermodynamic analyses. However, reviews that include all these mechanistic analyses are still scarce. Therefore, past investigations of IL/DES–H2O systems are summarized in this section, along with the work concerning aqueous electrolyte solutions to allow comparisons, because of the similarities of ILs/DESs to electrolytes.
3.1 Molecular dynamics or Monte Carlo simulations
Molecular Dynamics (MD) or Monte Carlo (MC) simulations are commonly used to study the microscopic structure and behaviour of molecules in condensed phases and interfaces. MC can provide structural and thermodynamic properties. MD can even be used to study the dynamical and time-dependent phenomena including transport properties. Imidazolium-based ILs with a halide anion (e.g. [Cl]−, [Br]− or [I]−) are those more frequently studied as we can see from the summary in Table 5.| Cation | Anion | MD or MC simulation | QC calculation |
|---|---|---|---|
| Imidazolium-based ILs | |||
| [Mim]+ | [Cl]− | — | 232 |
| [Mmim]+ | [Cl]− | 37 and 38 | 232 |
| [Mmim]+ | [PF6]− | — | — |
| [Mmim]+ | [DMP]− | 219 | — |
| [Emim]+ | [Cl]− | 206 | 227 and 232 |
| [Emim]+ | [Br]− | — | 227 |
| [Emim]+ | [BF4]− | — | 227 |
| [Emim]+ | [PF6]− | — | 227 |
| [Emim]+ | [EtSO4]− | 209 | — |
| [Emim]+ | [Ac]− | 219 | — |
| [Bmim]+ | [BF4]− | 210 and 218 | — |
| [Bmim]+ | [Cl]− | 207 and 219 | 231 |
| [Bmim]+ | [Br]− | — | 231 |
| [Bmim]+ | [I]− | 147 and 165 | |
| [Bmim]+ | [SCN]− | — | 228 and 231 |
| [Bmim]+ | [TFA]− | — | 231 |
| [Bmim]+ | [OTf]− | — | 231 |
| [Bmim]+ | [TOS]− | — | 231 |
| [Bmim]+ | [MeSO3]− | — | 231 |
| [Bmim]+ | [DMP]− | — | 231 |
| [Bmim]+ | [Ac]− | — | 231 |
| [Bmim]+ | [DCA]− | — | 228 |
| [Bmim]+ | [TCM]− | — | 228 |
| [Bmim]+ | [TCB]− | — | 228 |
| [Hmim]+ | [I]− | 208 | — |
| [Omim]+ | [NO3]− | 217 | — |
| [Omim]+ | [BF4]− | 218 | — |
| [Omim]+ | [Cl]− | 218 | — |
| [Pmim]+ | [Cl]− | — | 232 |
| [C4C1mim]+ | [Cl]− | — | 232 |
| [C7mim]+ | [Cl]− | — | 232 |
| [C10mim]+ | [Cl]− | — | 232 |
| [Amim]+ | [Cl]− | — | 232 |
| [C20H28N3O]+ | [NTf2]− | 211 | — |
| [C20H28N3O]+ | [NO3]− | 211 | — |
| Ammonium-based ILs | |||
| [Dema]+ | [OTf]− | 212 | |
| [Glyc]+, [Ala]+, [Val]+, [Leu]+ | [Cl]−, [Br]−, [BF4]−, [PF6]− | — | 233 |
| Phosphonium-based ILs | |||
| [P6,6,6,14]+ | [BOB]− | 220 and 221 | — |
| Pyrrolidinium-based ILs | |||
| [Bmpyr]+ | [Cl]− | — | 229 |
| Pyridinium-based ILs | |||
| [B2mPy]+ | [Cl]− | — | 229 |
| [B3mPy]+ | [Cl]− | — | 229 |
| [B4mPy]+ | [Cl]− | — | 229 |
| [BPy]+ | [Cl]− | — | 229 |
| [Bpy]+ | [BF4]− | — | 229 |
| Piperidinium-based ILs | |||
| [Bmpip]+ | [Cl]− | — | 229 |
| DESs | |||
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
79 and 213–215 | — | |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
213 and 216 | — | |
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
213 | — | |
Aqueous ILs were first investigated using MD simulations by Hanke et al.38 for [Mmim][Cl]. The simulations were carried out in the range of dilute IL solutions, and the results show a formation of the H2O–Cl complex (hydrated [Cl]−) and then a particular interaction between hydrated-[Cl]− and the imidazolium ion. After that, Spickermann et al.206 performed Car–Parrinello MD simulations for a single [Emim][Cl] ion-pair dissolved in 60 H2O molecules. Under their simulation conditions (IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, xIL = 0.0164), contact ion-pair still exists, and the hydration is not complete for both the anion and cation. The cation ([Emim]+) shows a stronger connection to the H2O-hydrated-[Cl]− compared with the H2O-hydrated-metal-ion, facilitating the formation of a joint hydration shell that stabilizes the [Emim][Cl] ion-pair. The MD simulation work conducted by Remsing et al.207 shows that the dissociated ions from [Bmim][Cl] are completely solvated at low concentrations as a typical electrolyte, but water cannot completely disrupt the interaction between ions as ion-pairs still exist even when the mole fraction of water is 0.999.
60, xIL = 0.0164), contact ion-pair still exists, and the hydration is not complete for both the anion and cation. The cation ([Emim]+) shows a stronger connection to the H2O-hydrated-[Cl]− compared with the H2O-hydrated-metal-ion, facilitating the formation of a joint hydration shell that stabilizes the [Emim][Cl] ion-pair. The MD simulation work conducted by Remsing et al.207 shows that the dissociated ions from [Bmim][Cl] are completely solvated at low concentrations as a typical electrolyte, but water cannot completely disrupt the interaction between ions as ion-pairs still exist even when the mole fraction of water is 0.999.
Recently, D’Angelo et al.208 studied the molecular structure of [Hmim][I]–H2O mixture with the molar ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (i.e. xIL = 0.005–0.5) with MD simulation. Their results show that the H2O molecules coordinate around the [I]− anion and the [I]−-hydration shell becomes more and more crowded with increasing H2O content. ILs exist mainly as ion-pairs when the H2O content is low, while no ion-pairs can be observed in the most diluted IL solutions. Sha et al.165 studied ion-association from a very dilute [Bmim][I] solution to pure IL. The simulation results show that the cation–anion association is completely replaced by the anion–water network in the dilute IL solution. When the H2O content is increased, the anions will be gradually hydrated with more than 7 H2O molecules in the first anion coordination shell for a very dilute IL solution. By analyzing the interaction energies of cation–cation, anion–H2O, cation–H2O and H2O–H2O as a function of IL mole fraction (xIL), they found that there is a strong anion hydration effect. When xIL is lower than 0.25, the cage structure of ions will be broken down, and the ion-hydration starts to dominate. The association structure of cation–anion vanishes in the lowest IL mole fraction of 0.003. For [Bmim][I], Nickerson et al.147 also found that at low concentrations of water, the H2O molecules get inserted into the ordered cation/anion structure. Hydrogen-bonding (H-bonding) between H2O and [I]− was found to be strong and important for the properties of mixtures, particularly leading to the dramatic increase in conductivity at high water concentrations.
200 (i.e. xIL = 0.005–0.5) with MD simulation. Their results show that the H2O molecules coordinate around the [I]− anion and the [I]−-hydration shell becomes more and more crowded with increasing H2O content. ILs exist mainly as ion-pairs when the H2O content is low, while no ion-pairs can be observed in the most diluted IL solutions. Sha et al.165 studied ion-association from a very dilute [Bmim][I] solution to pure IL. The simulation results show that the cation–anion association is completely replaced by the anion–water network in the dilute IL solution. When the H2O content is increased, the anions will be gradually hydrated with more than 7 H2O molecules in the first anion coordination shell for a very dilute IL solution. By analyzing the interaction energies of cation–cation, anion–H2O, cation–H2O and H2O–H2O as a function of IL mole fraction (xIL), they found that there is a strong anion hydration effect. When xIL is lower than 0.25, the cage structure of ions will be broken down, and the ion-hydration starts to dominate. The association structure of cation–anion vanishes in the lowest IL mole fraction of 0.003. For [Bmim][I], Nickerson et al.147 also found that at low concentrations of water, the H2O molecules get inserted into the ordered cation/anion structure. Hydrogen-bonding (H-bonding) between H2O and [I]− was found to be strong and important for the properties of mixtures, particularly leading to the dramatic increase in conductivity at high water concentrations.
Hanke and Lynden-Bell37 studied the local structure and dynamics for the mixtures of H2O and IL (either [Mmim][Cl] or [Mmim][PF6]). They found that the H2O molecules tend to be isolated from one another in the IL-rich mixtures, and a water percolating network is formed when the molar proportion of water reaches 75%. Bernardes et al.209 analyzed the structural feature of the [Emim][EtSO4]–H2O binary system in the entire compositional interval using MD simulations. They found that the size of IL-aggregates decreases with increasing water content. The chain-like IL network is broken down at the point with the mole fraction of water (xwater) at 0.95, and then small IL-aggregates or ‘icebergs’ start to form. For the dilute IL solutions (xwater = 0.996), only isolated ions and dimers were found and the probability of forming an isolated ion is almost 60%. Therefore, the IL is dissociated mostly under the infinitely dilute IL condition, and water clearly shields the electrostatic interaction energy between the cation and anion, but dimers still exist. Zhong et al.210 performed MD simulation for the [Bmim][BF4]–H2O binary system in the entire compositional range, and they proposed that the local structure is divided into 3 distinct regions (xwater < 0.2, 0.2 < xwater < 0.8, 0.8 < xwater). When xwater < 0.2, most of the H2O molecules are isolated with one another and experience a local environment in the polar network nearly the same as that in pure IL. The polar network of IL is maintained very well until xwater reaches 0.8. When xwater > 0.8, an aggregation is observed and reaches maximum at 0.9 < xwater < 0.95, which means that, in this compositional range, IL starts to dissolve in water and exists in the form of small clusters by the aggregation of cations and anions. Gutiérrez et al.141 carried out MD simulations for binary systems of [Emim][BF4] and [Bmim][BF4] with several molecular solvents (water, ethylene glycol and dimethylformamide) to obtain a microscopic view of structuring and intermolecular forces, H-bonding as well as their relationship with macroscopic behaviour. The results show that the molecular solvents interact preferentially with [BF4]− whereas the interaction between the cation and the molecular solvent is less important. When water is used as solvent, stronger interactions and hydration can be observed. The increase of the alkyl-chain length in the cations (i.e. from [Emim]+ to [Bmim]+) does not have a noticeable effect on the properties of mixtures compared with the change of anions.
Yuvaraj et al.211 studied the solvation mechanism of two TSILs containing a common L-histidine-derived-imidazolium cation and an anion of [Br]− or [NTf2]−, using classical MD simulations and density functional theory (DFT) calculations, and H2O was chosen as the solvent. On average, 4 H2O molecules are associated with each anion in these two ILs. The [Br]− is completely enclosed within the solvation shell, making the [Br]− fully hydrated. While for the [NTf2]−-based TSIL, [NTf2]− is only partially hydrated and the cation and anion remain coordinated interacting even in the presence of H2O, which implies that this TSIL may still exist as ion-pairs. Chang et al.212 studied the structure and dynamics of aqueous ammonium-based IL ([Dema][OTf]) using MD simulations. They observed that the H2O molecules form a distinct solvation shell around both the cation and anion and thus weaken their mutual association. At low water contents, the H2O molecules were observed to be isolated from one another while coordinated strongly to the ions. As the water content increases, the ions were found to have more H2O molecules in their coordination shells, which is more noticeable for the anions. When the water mole fraction is 0.8, the proportion of the anions without any hydrogen-bonds (H-bonds) with water is only 2%. Therefore, the [OTf]− has a strong affinity for water, and it is easily hydrated by water.
Shah et al.79 performed MD simulations to analyze the intermolecular interactions in a type-III DES, ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with the addition of water. The results show that the anion ([Cl]−) becomes preferentially hydrated by water compared with urea or cation, and the effects of water can be classified into 3 regimes based on the mass fraction of H2O (wH2O) (i.e. wH2O < 5%, 5% < wH2O < 25%, and wH2O > 25%). In the low water fraction zone (wH2O < 5%), the number of H-bonds shows a maximum at wH2O ≈ 2.5%. At the intermediate water fraction (5% < wH2O < 25%), the components of ChCl/urea (1
2) with the addition of water. The results show that the anion ([Cl]−) becomes preferentially hydrated by water compared with urea or cation, and the effects of water can be classified into 3 regimes based on the mass fraction of H2O (wH2O) (i.e. wH2O < 5%, 5% < wH2O < 25%, and wH2O > 25%). In the low water fraction zone (wH2O < 5%), the number of H-bonds shows a maximum at wH2O ≈ 2.5%. At the intermediate water fraction (5% < wH2O < 25%), the components of ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) are hydrated by H2O, and the anion and urea show a low diffusivity. At the high water fraction (wH2O > 25%), both anion and urea show high diffusivities. The simulation also shows that there is a strong urea–H2O interaction, but the [Cl]− interacts strongly with H2O compared with urea. Both urea and [Cl]− can be hydrated by water. Zhekenov et al.213 studied the formation of three type-III DESs (ChCl/urea (1
2) are hydrated by H2O, and the anion and urea show a low diffusivity. At the high water fraction (wH2O > 25%), both anion and urea show high diffusivities. The simulation also shows that there is a strong urea–H2O interaction, but the [Cl]− interacts strongly with H2O compared with urea. Both urea and [Cl]− can be hydrated by water. Zhekenov et al.213 studied the formation of three type-III DESs (ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), ChCl/Gly (1
2), ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ChCl/EG (1
2) and ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) and the effect of water on the intermolecular interactions by analyzing the number of H-bonds and the diffusion of the constituent species of DESs. A similar behavior was observed for different DESs. Specifically, when water is added in a small amounts (wH2O < 5%), water gets absorbed in the structure of DES by forming H-bonds with the ions and HBDs; beyond that water amount (wH2O > 5%), water also dampens the intramolecular interactions in the DES, leading to drastic changes of properties. Fetisov et al.214 investigated the behaviour of ChCl/urea (1
2)) and the effect of water on the intermolecular interactions by analyzing the number of H-bonds and the diffusion of the constituent species of DESs. A similar behavior was observed for different DESs. Specifically, when water is added in a small amounts (wH2O < 5%), water gets absorbed in the structure of DES by forming H-bonds with the ions and HBDs; beyond that water amount (wH2O > 5%), water also dampens the intramolecular interactions in the DES, leading to drastic changes of properties. Fetisov et al.214 investigated the behaviour of ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and its equimolar mixture with water (xH2O = 0.5 or wH2O = 17%) by performing first-principles MD simulation. Their results show that the addition of H2O breaks the strong H-bonds between the H atoms of urea and [Cl]− in the pure ChCl/urea (1
2) and its equimolar mixture with water (xH2O = 0.5 or wH2O = 17%) by performing first-principles MD simulation. Their results show that the addition of H2O breaks the strong H-bonds between the H atoms of urea and [Cl]− in the pure ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) by preferentially hydrating [Cl]− and forming urea–water H-bonds. Gao et al.215 performed MD simulations to study the properties of ChCl/urea (1
2) by preferentially hydrating [Cl]− and forming urea–water H-bonds. Gao et al.215 performed MD simulations to study the properties of ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)–H2O mixtures from the pure ChCl/urea (1
2)–H2O mixtures from the pure ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to infinitely dilute ChCl/urea (1
2) to infinitely dilute ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solutions (xH2O = 0.999 or wH2O = 99.5%). The simulation results show that, even at xH2O = 0.9, neither [Cl]− nor cation is saturated by water, which means that, at this composition, the ions are only partially hydrated. Moreover, at low water contents, the ion-pairing of cation and anion is dominant, whereas the hydration of ions turns out to be dominant with the increase of water content. The competition between these two interactions (i.e. ion-pairing and ions hydration) results in a non-ideal behaviour of properties observed from macroscale. For ChCl/Gly (1
2) solutions (xH2O = 0.999 or wH2O = 99.5%). The simulation results show that, even at xH2O = 0.9, neither [Cl]− nor cation is saturated by water, which means that, at this composition, the ions are only partially hydrated. Moreover, at low water contents, the ion-pairing of cation and anion is dominant, whereas the hydration of ions turns out to be dominant with the increase of water content. The competition between these two interactions (i.e. ion-pairing and ions hydration) results in a non-ideal behaviour of properties observed from macroscale. For ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)–H2O binary mixture, Ahmadi et al.216 performed MD simulations at xH2O = 0.9, three typical distributions for the DES constituents were observed, and ChCl prefers to form H-bonds with water instead of glycerol.
2)–H2O binary mixture, Ahmadi et al.216 performed MD simulations at xH2O = 0.9, three typical distributions for the DES constituents were observed, and ChCl prefers to form H-bonds with water instead of glycerol.
MD simulations have been conducted in order to find the structural regimes with different amounts of water. The simulation from Jiang et al.217 was performed for [Omim][NO3]–H2O mixture at various water concentrations. It reveals that there is a turnover point (xwater ≈ 0.75–0.8) for the structural organization of the water network (water–anion–water) and micelle (cation–cation) structure, and water can form liquid-like associated aggregates due to the planar symmetry and strong basicity of [NO3]−. Feng et al.218 investigated the effects of anion and alkyl chain length of cation on the structure and dynamic based on 3 IL–H2O binary systems ([Bmim][BF4]–H2O, [Omim][BF4]–H2O, and [Omim][Cl]–H2O) with the water content from 0.2 to 0.9615 in mole fraction. They found that by increasing the alkyl chain length of cation from [Bmim]+ to [Omim]+, the aggregation of the cations becomes stronger and the diffusion of the anions becomes slower. The H2O molecules are more likely to remain close to the [Cl]− than the [BF4]−. The diffusion coefficients of anions and water molecules show two recognizable regimes (xwater < 0.8 and xwater > 0.8). The system may be in a glassy state at xwater < 0.8. While when xwater > 0.8, the ion-dominated networks will be broken down and changed to water-dominated networks. The MD simulations performed by Niazi et al.219 were used to study the properties of 3 imidazolium-based ILs ([Bmim][Cl], [Emim][Ac] and [Mmim][DMP]) with water at 350 K, ranging from 0 to 99.95% of water in mole fraction (xwater), and it was found that a transition takes place from IL–H2O mixed solvents to an aqueous IL solution at around xwater = 0.75. Wang et al.220,221 performed atomistic MD simulations for the mixture of a phosphonium-based IL ([P6,6,6,14][BOB]) and water to study their volumetric and dynamic properties as well as their local microscopic structures. The results show that the microscopic liquid structure of the pure IL is a connected polar network composed of alkyl chains in the cation and isolated polar domains consisting of the central segments of [P6,6,6,14]+ in the cation and [BOB]−, i.e. the isolated polar domain is a direct contact ion-pair. When xwater is low, the water is embedded into cavities to form solvent-shared ion-pairs through a cation-water-anion triple complex, and no free water can be observed. 4 distinct structural regimes were proposed within the entire compositional range based on xwater (0 ≤ xwater < 0.50, 0.50 < xwater < 0.80, 0.8 < xwater < 0.95, 0.95 < xwater ≤ 1) concerning the evolution of microscopic liquid structure and the transition of the local ionic organization.
In summary, numerous MD and MC simulations have been conducted to find out the molecular behaviour of the IL/DES–H2O binary systems. The investigated ILs involve both conventional ILs and TSILs. In general, the interaction between the anion and water is stronger than that between cation and water, the anion can be fully hydrated or partially hydrated depending on its size and basicity, and the structural regimes change with the water contents.
It should be mentioned here that, due to the highly complex interaction landscape, ILs are highly challenging to study with molecular mechanical force field based simulations. If the standard force fields that were developed originally for the organic or biomolecular systems are used, it is common that the simulations give too low diffusion and too high viscosity.222–224 In Car–Parrinello simulations and quantum chemical DFT calculations of ILs, it has been observed that there is a certain degree of charge transfer taking place among the ion-pairs and thus diluting the atomic charges in the vicinity of the charge centers.225 For example, for the imidazolium-based ILs, the atomic charge gets reduced by a factor roughly 0.85.226 Scaling the charges down to obtain correct diffusion (and viscosity) has become praxis (although somewhat ad hoc) for studying ILs reliably.
3.2 Quantum chemical calculations
Quantum chemical (QC) calculations, including ab initio quantum chemistry and DFT, as well as semi-empirical methods, such as the conductor-like screening model for realistic solvents (COSMO-RS), have been used to give insight into the interactions between H2O and IL/DES molecules. Besides, QC calculations become necessary when the information on the electronic structure or molecular properties such as dipole moments, polarizabilities, etc. is needed. QC can also be used to obtain interaction energies and new force field parameters often missing for the new IL/DES systems.Several ILs have been studied as summarized in Table 5. Wang et al.227 performed DFT calculations to investigate the interaction between H2O and the imidazolium-based ILs ([Emim]+) with the anions of [Cl]−, [Br]−, [BF4]− and [PF6]−. The predicted geometries and interaction energies imply that H2O interacts with the anion ([Cl]−, [Br]− or [BF4]−) to form X−⋯W, 2X−⋯2W, [BF4]−⋯W, and W⋯[BF4]−⋯W complexes where X = Cl or Br and W = H2O. While for [PF6]−, a hydrophobic anion, no stable complex can be formed with water, but either cation or ion-pair can form a complex with the H2O molecules.
Batista et al.228 studied the H2O–IL interaction for the imidazolium-based ILs ([Bmim]+) with the anions of [SCN]−, [DCA]−, [TCM]− and [TCB]− with experimental and computational (COSMO-RS) techniques. The [SCN]−- and [DCA]−-based ILs are favorable to interact with the H2O molecules, while the other two ILs indicate weak interactions with H2O. Based on the sigma (screening charge density) profile generated from COSMO-RS, the electronegativity of the anions plays an important role in their interaction with H2O.
In the work by Khan et al.,229 the aqueous solutions with the imidazolium-, pyridinium-, pyrrolidinium- and piperidinium-based ILs or cholinium-based ILs were studied. For the aqueous cholinium-based ILs, the results based on the COSMO-RS method show that the H-bonding between H2O and IL–anion is the dominant interaction, while the electrostatic-misfit interaction and van der Waals forces have minor contributions to the total excess enthalpies.230 For [Bmim][Cl], the turnover point of the curve Hm–xwater is at around xwater = 0.75 according to COSMO-RS calculation, which implies that [Cl]− forms “a complex” with 3 H2O molecules.231 The aromaticity of the cation weakens the IL–H2O interaction.229 The H2O–IL interaction is weakened by increasing the alkyl chain length on the imidazolium ring. The excess enthalpies, calculated from COMSO-RS, suggest the formation of complexes between 3 H2O molecules and one IL molecule for the alkyl-methyl-imidazolium chloride–H2O binary system.232
Zhu et al.233 conducted a systematic investigation on the interactions of the H2O molecule with a series of amino acid cations ([Glyc]+, [Ala]+, [Val]+, and [Leu]+), halogen anions ([Cl]−, [Br]−, [BF4]−, and [PF6]−), and clusters (GlyCl)n (n = 1–5) at the B3LYP-DFT and MP2 levels of theory. Unlike the conventional ILs, the novel GlyCl-type amino acid ILs (AAILs) favour the interaction of its cationic part, rather than its anionic part, with the surrounding H2O molecules.
Wagle et al.234 elucidated the molecular interactions, charge transfer interactions, and the thermodynamics associated with three pure DESs by QC calculations. Studies of the effect of water on the DES systems, based on QC calculations are not reported.
3.3 Advanced experimental methods
Experimental methods, such as IR, Raman, NMR, X-ray, UV and neutron scattering, have been carried out to explore the molecular structure and behaviour of IL/DES–H2O binary systems (Table 6). Again, a major part of the research is on the conventional imidazolium- and pyridinium-based ILs, and some work is on TSILs and DESs.| Cation | Anion | Methods | Ref. |
|---|---|---|---|
| ILs | |||
| [Emim]+ | [BF4]− | 2-D vibrational spectroscopy | 235 |
| [Emim]+ | [Cl]− | Small-angle neutron scattering | 238 |
| [Bmim]+ | [BF4]−, [Br]− | UV absorption spectrophotometry | 239 |
| [Omim]+ | [Br]− | UV absorption spectrophotometry | 239 |
| [Bmim]+ | [BF4]− | NMR spectroscopy | 236 |
| [Bmim]+ | [BF4]− | Small-angle neutron scattering | 237 |
| [Bmim]+ | [Cl]−, [Br]−, [I]−, [BF4]− | Raman Spectroscopy | 244 |
| [Bmim]+ | [PF6]−, [SbF6]−[ClO4]−, [(CF3SO2)2N]−, [NO3]−, [CF3CO2]− | ATR-IR spectroscopy | 240 |
| [Emim]+ | [NTf2]− and [EtSO4]− | FTIR spectroscopy and DFT | 243 |
| [Bmim]+ | [NTf2]− | PGSE NMR | 242 |
| [Bpy]+ | [BF4]− | Infrared spectroscopy and DFT | 245 |
| [Cho]+ | [Lac]−, [Prop]− and [Mal]− | 1H NMR | 246 |
| [TEA]+ | [MeSO3]− | FIR spectroscopy and DFT | 247 |
| [N0002]+ | [C3CO2]− | X-ray scattering | 248 |
| [Bmim]+,[Emim]+ | [MeSO4]− | NMR spectroscopy | 164 |
| [DEMA]+ | [MeSO3]− | NMR, Raman, Infrared spectroscopy | 259 |
| DESs | |||
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), ChCl/EG (1 2), ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), ChCl/urea (1 2), ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
FTIR absorbance and Raman spectroscopy | 249 | |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Neutron scattering | 252 | |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), ChCl/EG (1 2), ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), ChCl/urea (1 2), ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
PFG-NMR | 251 | |
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
FTIR and Raman spectroscopy | 216 | |
Zhang et al.235 applied two-dimensional (2D) IR spectroscopy to investigate the dilution process of [Emim][BF4] in H2O. It shows that, with the addition of H2O, the cohesion between cation and anion becomes weak. During the dilution process, the three-dimensional (3D) network structure of pure ILs is gradually destroyed into ion-clusters, the ion-cluster is then further dissociated into ion-pairs surrounded by H2O, and the ion-pairs surrounded by H2O become dominant with a relatively high amount of H2O. Ultimately, the ion-pairs surrounded by H2O may be dissociated into fully hydrated and separated ions. However, within the investigated compositional range (xwater from 0.02 to 0.9), the IL has not been completely dissociated into ions. Mele et al.236 studied the structure of [Bmim][BF4] with/without trace amount of water by NMR spectroscopy through intermolecular nuclear overhauser enhancements to present a direct experimental evidence of the interactions (i.e. cation–cation, cation–H2O, and cation–anion interactions). The water content investigated in their work is from 0 to 0.52 in the mole fraction of water. The results show that the presence of water makes the cation–cation association looser and water can act as HBD towards [BF4]−. The IL structure is appreciably changed with the increase of water content, and the IL still exists as tight ion-pairs with small amounts of water. For [Bmim][BF4]–H2O system, Almásy et al.237 investigated the structure by small-angle neutron scattering. They strongly suggested that the system is close to phase separation at xIL ≈ 0.075. For [Emim][Cl]–H2O system, the investigation by using small-angle neutron scattering technique shows that [Emim][Cl] is homogenously dissolved in water, which is mainly caused by a strong hydration of [Cl]− by water.238 Zhang et al.239 investigated the aggregation of the imidazolium-based ILs ([Bmim][BF4], [Bmim][Br] and [Omim][Br]) in water by UV absorption spectrophotometry with xIL from 5.62 × 10−7 to 0.062 (i.e xwater > 0.94). Three transformations (α, β and γ) at different IL contents occur within the investigated compositional range. In the very dilute IL solutions (xIL from 0 to α), the anions and imidazolium cations are almost “free”, and H-bonds are dominant among the hydrated ions. After that, a floating hydrogen-bonded network with 3D structure starts to form, and the association among anions and cations occurs to form ion-pairs. When reaching another transformation point (β), the coulombic and aliphatic interactions become dominant to form IL aggregation. The longer the alkyl-chain length and the smaller the anion, the easier the formation of IL-aggregates in the second transformation region. With a further increase of the IL content, the number of the IL-aggregates increases and, finally, an IL quasi-structure is formed at the γ transformation point.
Using the attenuated total reflection and transmission infra-red (ATR-IR) spectroscopy, Cammarata et al.240 investigated the behaviour of H2O in the ILs with [Bmim]+ and the anions of [PF6]−, [SbF6]−, [BF4]−, [ClO4]−, [(CF3SO2)2N]−, [NO3]− and [CF3CO2]−. They concluded that the anion is responsible for the interaction between IL and H2O. Besides, for [NO3]− and [CF3CO2]−, H2O forms aggregates or a kind of associations in the IL–H2O system, similar to hydrated ions in the aqueous electrolyte solutions.241 Rollet et al.242 studied the self-diffusion of [Bmim][NTf2] in H2O ranging from 0.03 to 0.3 (saturation) in mole fraction of water using the pulsed gradient spin-echo NMR (PGSE NMR) method. It was found that the anion and cation show comparable self-diffusion coefficients, indicating that the anion and cation are associated with one another strongly and move together largely. The shielding effect of H2O on the electrostatic interactions leads to a decrease of IL cohesion, resulting in a decrease of the viscosity.
Combining FTIR spectroscopy and DFT calculations, Köddermann et al.243 found that, for [Emim][NTf2] and [Emim][EtSO4], when the mass concentration of water is below 1 wt%, single H2O molecules can be embedded into the IL environment and no H2O clusters exist. This means that the H2O molecules are isolated from one another, interacting only with the IL in the IL-rich region. Singh et al.244 investigated the ILs with [Bmim]+ and different anions ([Cl]−, [Br]−, [I]−, or [BF4]−) as well as their aqueous mixtures (xwater from 0 to 1) with Raman spectroscopy and dispersion-corrected DFT. It was found that, for the ILs with the anions [Cl]−, [Br]−, or [I]−, the structure changes significantly with the increase of water content, while for [Bmim][BF4], no significant change in the structure was observed due to the strong cation–anion interaction. At low IL concentrations, a complete dissociation of IL into cation and anion by the water disruption is indicated. Wang et al.245 elucidated the H-bonding between H2O and pyridinium-based IL ([BPy][BF4]) using the IR spectroscopy and DFT calculations, and a strong anion–H2O–anion complex and a new H-bond with the aromatic C–H on [BPy]+ can be formed. While for the imidazolium-based ILs, the strong anion–cation interaction and a steric hindrance from the alkyl chains prevent the H2O molecules forming H-bonds with the aromatic C–H on the cation. The study from Khan et al.232 indicates that the increase of the molar volume, i.e. increase the alkyl chain length on the cation of ILs, can lead to a decrease of their ability to form H-bonds with H2O based on the experimental observation on the increase of the blue shift in the IR spectra for the alkyl-methyl-imidazolium chloride–H2O binary systems.
For the hydrophobic ILs, for example, [Cnmim][NTf2], the water solubility is only up to 1.8 wt%. At the water concentrations below 1 wt%, the H2O molecules are embedded in the IL environment and no water clusters exist.243 According to the self-diffusion coefficients, Rollet et al.242 proposed that the hydrophobicity of [NTf2]− prevents H2O being incorporated into the ILs and then dissociating the ILs into ion-pairs. Meanwhile, H2O is not homogeneously mixed with the IL and thus forms small aggregates (i.e. [Bmim][NTf2] + several H2O molecules or H2O + several [Bmim][NTf2] molecules), and the size of the H2O cluster increases with increasing H2O content.
For the TSILs, Patinha et al.246 investigated the solvation scheme for 3 cholinium carboxylate IL–H2O binary systems ([Cho][Lac]–H2O, [Cho][Prop]–H2O, [Cho][Mal]–H2O) by performing 1H NMR studies. Both the cation and anion interact with H2O, but the anion does more strongly. For the IL–H2O binary systems with the cholinium cation and the small carboxylate anion, it is feasible to form the domains of hydrophobic clusters. Combining FIR spectroscopy and DFT calculations, Peter et al.247 studied the ion speciation for [TEA][MeSO3] in water. The results show a significant decrease of contact ion-pairs in the mixture with increasing water concentration. A minimum of 4 water molecules are needed to initiate the transition process from contact ion-pairs to solvent-separated ion-pairs. Salma et al.248 investigated the effect of water on the nano-structural organization of [N0002][C3CO2] (i.e. so-called EAB) using a small- and wide-angle X-ray scattering where the water is from 0 to 0.91 in mole fraction. There are two peaks, i.e. pre-peak and principle peak, for pure EAB. By adding water, the pre-peak moves towards lower transferred-momentum (Q) values, while the principal peak approaches larger Q values. The pre-peak shifting to the lower Q values comes from the strong H-bond interaction between water and the polar ends of IL-cation and -anion, and it indicates that the H-bond interaction between water and EAB ions (i.e. [N0002]+ and [C3CO2]−) leads to a progressive disruption of the cation–anion coulombic interaction.
Mechanistic study on DES–H2O system has also been conducted by Pandey et al.249 who studied the effects of temperature and the addition of H2O on the solvatochromic probe behaviour using the FTIR absorbance and Raman spectroscopy. Compared with the conventional ILs, as more components are involved in DESs, the interactions are more complex. For pure DES, a 3D intermolecular H-bonding network has been confirmed.250 According to the results of Pandey et al.,249 for ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) or so-called reline, the addition of H2O definitely helps to form inter-species H-bonding. However, with the addition of H2O, the overall H-bonding landscape will not alter much. For the different DESs, i.e. ChCl/Gly (1
2) or so-called reline, the addition of H2O definitely helps to form inter-species H-bonding. However, with the addition of H2O, the overall H-bonding landscape will not alter much. For the different DESs, i.e. ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) or so-called glyceline, ChCl/EG (1
2) or so-called glyceline, ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) or so-called ethaline, and reline, the H-bonding between H2O and ethaline or glyceline is more pertinent compared with that between H2O and reline. For the ethaline- and glyceline-based DESs, the H-bonding among inter-species appears to be important in their aqueous solutions. While for reline, the interstitial accommodation of H2O appears to be dominant. D’Agostino et al.251 used the pulsed field gradient (PFG) NMR to probe self-diffusion of molecular and ionic species in the choline chloride (ChCl)-based DESs–H2O binary systems. The self-diffusion of water in the studied DES–H2O binary systems is considerably higher than that in the homogeneous bulk liquid water, which strongly suggests that the studied DES–H2O solutions are not homogeneous but contain distinct microscopic water-rich phases after a significant amount of water is added. In addition, it was also found that when water is added to the solutions, their viscosities decrease but a discontinuity is observed for all the studied systems at the point corresponding to 1
2) or so-called ethaline, and reline, the H-bonding between H2O and ethaline or glyceline is more pertinent compared with that between H2O and reline. For the ethaline- and glyceline-based DESs, the H-bonding among inter-species appears to be important in their aqueous solutions. While for reline, the interstitial accommodation of H2O appears to be dominant. D’Agostino et al.251 used the pulsed field gradient (PFG) NMR to probe self-diffusion of molecular and ionic species in the choline chloride (ChCl)-based DESs–H2O binary systems. The self-diffusion of water in the studied DES–H2O binary systems is considerably higher than that in the homogeneous bulk liquid water, which strongly suggests that the studied DES–H2O solutions are not homogeneous but contain distinct microscopic water-rich phases after a significant amount of water is added. In addition, it was also found that when water is added to the solutions, their viscosities decrease but a discontinuity is observed for all the studied systems at the point corresponding to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole equivalent of H2O
1 mole equivalent of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cl]−. Hammond et al.252 studied the effect of water on the nanostructure of ChCl/urea (1
[Cl]−. Hammond et al.252 studied the effect of water on the nanostructure of ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with neutron scattering and empirical potential structure refinement (EPSR). The results show that the interactions in this DES are weakened by the addition of water, but the solution still remains the nature of pure-DES even at high wH2O (up to 40.95%). When wH2O reaches the overcrowding point (50.98%), i.e. xwater = 0.833, the DES component will be fully solvated by water, and the system changes to an aqueous solution. For a DES–H2O binary mixture, Ahmadi et al.216 investigated the character of the ChCl/Gly (1
2) with neutron scattering and empirical potential structure refinement (EPSR). The results show that the interactions in this DES are weakened by the addition of water, but the solution still remains the nature of pure-DES even at high wH2O (up to 40.95%). When wH2O reaches the overcrowding point (50.98%), i.e. xwater = 0.833, the DES component will be fully solvated by water, and the system changes to an aqueous solution. For a DES–H2O binary mixture, Ahmadi et al.216 investigated the character of the ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) system in the presence of water by combining FTIR and Raman spectroscopy. They found that for this DES, the association of water with DES reaches a maximum at xwater ≈ 0.9, proposing that the DES–H2O interaction is dominant and over the DES–DES interaction. During the hydration process, the nature of DES is preserved instead of the nature of the constituent (i.e. glycerol), which also implies that the DES–H2O mixture is unique compared with the organic solvent mixtures.
2) system in the presence of water by combining FTIR and Raman spectroscopy. They found that for this DES, the association of water with DES reaches a maximum at xwater ≈ 0.9, proposing that the DES–H2O interaction is dominant and over the DES–DES interaction. During the hydration process, the nature of DES is preserved instead of the nature of the constituent (i.e. glycerol), which also implies that the DES–H2O mixture is unique compared with the organic solvent mixtures.
Several investigations have found that pure ILs can exhibit a nanoscale spatial heterogeneity, i.e. nano-phase separation on the nanoscopic level due to the separation of polar/non-polar domains,253,254 and such polar/non-polar nano-phase separation can also occur in IL–H2O mixtures which can be reflected by the observation of the structural regimes with different amounts of water using MD simulation as we mentioned above.217–221 Based on the MD simulation results, some research groups studied the glass transition for the IL–H2O systems by analyzing the Differential Thermal Analysis (DTA) trace and Raman spectra.255–258 For [DEME][BF4]–H2O system,255 the glass-transition temperature (Tg) varies with the water content (xwater), which implies that this homogeneous solution may separate into a polar-rich phase and a nonpolar-rich phase. Based on the features in the glassy state, it has presumed that the presence of water may enhance the nano-phase separation and the ‘nearly-free state’ of H2O molecules may trigger the transition. The turning point of phase transition for this [DEME][BF4]–H2O solution is around xwater = 0.96. Moreover, the glass transition behaviour has also been demonstrated for the [DEME][I]–H2O256 and [Bmim][BF4]–H2O.257 It shows that the glass-formation region depends on the cation and anion of ILs, for example, for [DEME][I]–H2O,256 the region is xwater < 0.95, while it is xwater < 0.9 for [Bmim][BF4]–H2O.257 Therefore, as a special solvent, the IL–H2O homogeneous solutions may behave as different nano-phases on nanoscopic level. The presence of H2O molecules may in this case enhance the micro-heterogeneity in the IL–H2O mixtures.
Based on these experimental methods, the molecular structure and behaviour of IL/DES–H2O binary systems can be explored. In addition, these techniques can also be used to evaluate “ionicity”, which is an effective fraction of ions available to participate in conduction and an evaluation of the fraction of free charges at a specific temperature. Andanson et al.164 studied the ionic association and interactions in ILs ([Bmim][MeSO4] and [Emim][MeSO4]) with the addition of water. In this work, based on the diffusion coefficients measured by NMR spectroscopy, the molar conductivity was obtained with the Nernst–Einstein equation. The ionicity was quantitatively obtained by calculating the ratio between the molar conductivity based on the results from NMR and those directly measured experimentally. It shows that for these two ILs, their ionicity is around 0.4–0.6, and there is no obvious increase in ionicity with an increase of water content until xwater > 0.8. From these observations, they concluded that when xwater < 0.8, there is no major modification of the structure and the ionic association occurs despite of the strong decrease of the viscosity occurs. Yaghini et al.259 studied how the added water affects the transport properties, the local coordination, and the thermal behaviour of [DEMA][MeSO3] by NMR, Raman and infrared spectroscopy. The results show that water interacts primarily with the anion and slightly affects the ionicity of IL. The ionicity at xwater ≈ 0–0.57 in their work was measured with the same method as Andanson et al.164 The changes on the ionicity with the addition of water are limited to 0.55–0.65, and only a slight increase in the ionicity is observed by adding water. In addition, the ionicity can also be assessed qualitatively with the Walden plot of log (molar conductivity) versus log (reciprocal viscosity) by comparing the lines of IL–H2O mixtures with that of 0.01 M aqueous KCl solution.166
3.4 Thermodynamic analysis
Thermodynamic analysis can also be performed to study the interaction in the IL–H2O binary systems indirectly. Katayangi et al.260 studied the mixing schemes for IL–H2O systems and compared with the NaCl–H2O system by calorimetric analysis at 298.15 K. It was found that at the infinitely dilute solution of IL, the IL molecules break away from their pure environment and settle in the H2O environment presumably as separated ions. A complete dissociation of ion-pairs only takes place when the content of IL is extremely low, for example, 0.015 (xIL) for [Bmim][BF4] and 0.013 (xIL) for [Bmim][I]. The anion [I]− in [Bmim][I] is hydrated by H2O although the hydration is much weaker compared with aqueous NaCl solutions. However, the [BF4]− in [Bmim][BF4] may not be hydrated by H2O at all or only partially. With increasing the IL concentration, the IL-ions begin to organize themselves, directly or in an H2O-mediated manner. When the xIL is high, such as xIL = 0.5–0.6, IL exists as clusters similar to pure ILs. Using the similar method, Miki et al.261 studied 1-propanol-[Bmim][Cl]–H2O system. They find that the effect of [Bmim]+ is similar when the temperature is increased, which means that the effect of [Bmim]+ is to reduce the global average of the H-bond probability of bulk H2O. They provided circumstantial evidence that the IL is dissociated below a certain threshold. When the IL concentration is higher than this threshold, the IL-ions start to attract one another. They suggested that, in the H2O-rich region, the hydration “structure” is similar to the structure of D-fructose. The net effect of this hydration “structure” is to reduce the probability of forming H-bonds in the bulk H2O away from solutes.Seddon et al.262 studied the effects of impurities and additives (e.g. H2O, [Cl]− and other co-solvents) on the properties of [Cnmim]+-based ILs. The [Cl]− impurities present in ILs can be from the unreacted materials, i.e. dissolved NaCl or AgCl (metathesis), or reactant, i.e. HCl to form acid–base reaction. The results of volumetric properties show that the same pattern is observed at different temperatures although the volumetric expansion from the ideal behaviour increases with increasing temperature. They found that the anion has a primary effect on the H2O miscibility and the cation has a secondary effect. Those so-called hydrophobic ILs are in fact hygroscopic. The interaction between H2O and [Bmim][BF4] was further investigated. It was suggested that H2O is accommodated inside the IL structure in the IL-rich region (0.5 < xIL < 1) by forming H-bonds with both cation and anion, leading to a decrease of electrostatic attraction between the anion and cation and thereby a decrease of viscosity. Further addition of H2O (xIL < 0.5) may result in completely solvated ions and the appearance of the H2O molecules without H-bonds to the other species (i.e. the so-called “free H2O”263).
Gardas et al.264 measured the density, the speed of sound and the osmotic coefficients for ILs ([Emim][Br], [Emim][Cl]) in the dilute concentration at different temperatures. The volumetric analysis shows that the changes of the apparent molar volume with concentration are different from those for an aqueous solution of alkali halides, but similar to those for aqueous solutions of tetraalkylammonium salts. This implies that the cation has a great effect on the properties of the solutions. The values of the limiting apparent molar volume appear to increase linearly with increasing temperature, indicating the weakening of ion-solvent interactions.265 By analyzing osmotic and activity data, the hydration numbers for [Emim][Br] and [Emim][Cl] with the values of 1.78 and 2.3, respectively, were obtained, which are smaller compared with the values for alkali halides. They assumed that the decreased hydration number of anions was because the cations do not get hydrated. The estimated excess partial molar entropies within the studied compositional range (xIL = 0–0.004) indicate that ion-pair formation is favored over the hydration of ions.
Rumyantsev et al.266 investigated the peculiarities in the structural organization of DESs based on carboxybetaine (betaine) as a function of water at temperatures from 243 to 360 K by using the activation thermodynamics, IR spectroscopic measurements and QC calculations. The viscosity at different temperatures was used to obtain the activation thermodynamic parameters, such as entropy (ΔSA), enthalpy (ΔHA), and free energy (ΔGA) based on the Frenkel–Eyring–Kobeko relation. The results show that the values of T·ΔSA do not change significantly with temperature for the investigated DESs, i.e. the betaine- and choline-based DESs. However, for the betaine-based DES, T·ΔSA drops sharply with the increase of wH2O from 5 to 20% and increases sharply with the increase of wH2O from 30 to 40%. This observation is peculiar compared with the linear change with wH2O for the choline-based DES (ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)). Combining the QC calculation and IR spectroscopy reveals a formation of strong H-bond complexes between betaine and water.
2)). Combining the QC calculation and IR spectroscopy reveals a formation of strong H-bond complexes between betaine and water.
Reid et al.267 used the Kirkwood–Buff theory of solutions to quantify the interaction for 6 IL–H2O solutions. The studied ILs are protic ILs (PILs): [DMEtA][Pr] and [DMEtA][NTf2] and aprotic ILs (AILs): [Emim][OTf], [Emim][BF4], [Bmim][BF4], and [Cho][NTf2], and the concentrations of water are from 0 to 0.5 in mole fraction. It shows that at these concentrations, the strength of ion–ion interaction is not significantly affected by the presence of water for the PIL. Although the strong H2O–H2O interactions were observed in the PILs, the solution remains stable as a homogeneous phase other than phase split.
Wang et al.158 measured the electrical conductivity of 8 ILs in various solvents including H2O and studied their ion association and solvation, and the measurement was performed in the successively diluted solutions. They claimed that the association of [Cnmim][Br] in H2O relates to not only the electrostatic interactions between the cation and anion but also the hydration strength of cation. The weak hydration of the cation leads to an enhanced association between the cation and anion (larger kA). They compared the results for the ILs with the anions of [Br]−, [BF4]− and [PF6]−, finding that the association constant kA in H2O is lowest for the ILs with [Br]− due to the strong hydration of [Br]−. While for the ILs with [BF4]− and [PF6]−, their association constants kA in H2O are similar under the vacuum conditions because of the weak or non-existing hydration for these two anions.
To infer the atomistic/molecular properties, the molecular thermodynamic models such as the perturbed chain-statistical associating fluid theory (PC-SAFT) can be used, and the conceptual framework will be useful in order to extend the molecular thermodynamic models to the IL/DES–H2O binary systems. In the next section (3.5), the observed mechanisms from our analysis of the current literature are summarized and used for a new conceptual framework for further modelling work at molecular level.
3.5 Summing-up
Many investigations have been conducted both theoretically and experimentally to study the molecular behaviour of IL/DES–H2O systems, however, most of the work is focused on the conventional ILs. For the DESs and novel ILs, such as TSILs and AAILs, the work is still limited. It shows that the mechanisms for DESs and novel ILs are much more complicated due to the highly-complex and heterogeneous liquid structures themselves. More research needs to be carried out to obtain a molecular level picture to rationalize the structure that is formed and the driving forces behind. It is found that, except AAILs, water usually connects more tightly with anions than cations, and the hydration of anions frequently takes place. Usually, small anions (e.g. halide ions) are fully hydrated at the infinitely dilute IL/DES solution, large anions (e.g. [BF4]−) are only partially hydrated, and hydrophobic anions (e.g. [NTf2]−) interact with water but do not behave like a normal hydration. For some particular ILs, glass transition has been demonstrated, which means that there is a special phase change in these IL–H2O binary systems on a nanoscopic level.Based on the results on conventional IL–H2O and typical DES–H2O systems, the observed mechanisms can be summarized in a simplified way as shown in Fig. 6a and b. The general schematic mechanisms of NaCl–H2O system are depicted in Fig. 6c for comparison. Note that our intention is to illustrate the mechanisms upon the addition of water, and in Fig. 6, both the cation and anion for NaCl are represented as spherical particles with different sizes, while, for IL, each cation is represented as an asymmetric particle concerning their large size and asymmetry, but each anion is still represented as a spherical particle.
As shown in Fig. 6a, the pure IL is a supercluster with three-dimensional structure network (I). Upon the addition of H2O,
(1) When the amount of H2O is low, the H2O molecules mix well with IL with strong interactions (van der Waals, H-bonds, electrostatic, etc.), and H2O is embedded inside and at the periphery of the IL without any H2O clusters formed (Fig. 6a, region II). This is similar to the mixed solvents.
(2) With a further addition of H2O, the IL clusters start to dissociate into individual ions or ion-pairs and the dissociated ions are gradually hydrated (Fig. 6a, region III).
(3) When the amount of H2O becomes very high, the IL structures are completely dissociated as ions (Fig. 6a, region IV). Depending on the size of the IL-ions, some of them are fully hydrated, while some are partially hydrated. This is partially similar to the aqueous electrolyte solutions (e.g. aqueous NaCl solution), in which all the ions in a dilute solution are fully hydrated as described in Fig. 6c (region III).
Comparing the conventional IL–H2O systems with aqueous electrolyte (e.g. NaCl) solutions, the main differences are on:
(a) For IL–H2O systems, IL itself can be a solvent with specific properties, for example, the dielectric constants can be as high as 86,268 and the content of IL can be in the range of 0–100%; for aqueous NaCl solutions, no pure NaCl solution can exist at all under the same condition, and the concentration of NaCl can only go up to a certain range (saturated solubility xsat.), i.e. the content of NaCl can be in the range of 0–xsat..
(b) For IL–H2O systems, the size of the ions are regularly much larger compared with Na+ or Cl−. Even in a very dilute solution, some large IL-ions can only be partially hydrated (Fig. 6a, region IV); for aqueous NaCl solutions, in a dilute solution, all the ions are fully hydrated (Fig. 6c, region III).
(c) For IL–H2O systems, in general, the hydration of the IL–anions is more pronounced compared with the IL-cations; for aqueous NaCl solutions, the hydration of the cation is more pronounced compared with the anion.
(d) For IL–H2O systems, the association is dominant even the concentration of IL is not so high; for aqueous NaCl solutions, NaCl is dissociated completely.
Even though DES is widely identified as either a new class of ILs or IL analogues, IL and DES are distinctly different as a solvent system. ILs are usually described as a general formula of CA. While for DES, the general formula is CA·nY, where Y and n refer to the HBD and the number of Y molecules interacting with the anion, respectively. As we can see from Fig. 6b, for the DES–H2O system, the mechanism is similar to that for IL–H2O (Fig. 6a), while it becomes more complex due to the presence of a neutral compound (Y or HBD), besides cation and anion for DES itself. In addition, the comparison of the differences between water solutions of DES and individual components from DES with water is an interesting topic. However, more work needs to be carried out to collect sufficient information for a through and comprehensive review in this topic.
4. Further analysis of IL/DES–H2O binary systems
There have been many studies based on determining the physicochemical properties of IL/DES–H2O binary systems and the mechanistic analyses summarized in Sections 2 and 3. The conventional ILs, TSILs and DESs were primarily studied and the focus is on physicochemical properties while detailed mechanistic studies over varied concentration intervals are limited. Based on the summary of the experimental data (Section 2) and mechanistic studies (Section 3), six typical and widely-studied IL/DES–H2O binary systems ([Bmim][I]–H2O, [Bmim][BF4]–H2O, [Emim][BF4]–H2O, [Emim][EtSO4])–H2O, ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)–H2O, and ChCl/Gly (1
2)–H2O, and ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)–H2O were chosen to map the key experimental observations to the corresponding results obtained from mechanistic studies (Table 7).
2)–H2O were chosen to map the key experimental observations to the corresponding results obtained from mechanistic studies (Table 7).
| ILs & DESs | Experimental data | Mechanistic study | |||||
|---|---|---|---|---|---|---|---|
| V E | η E | Λ | H m | MD/MC | QC | Advanced experiments | |
| [Bmim][BF4] | 144 | 145 | 161 | 194 and 197 | 210 | — | 237 and 244 |
| [Bmim][I] | 269 | 147 | 147 and 165 | — | 147 and 165 | — | 244 |
| [Emim][BF4] | 270 | — | 161, 167, 271 and 272 | 190 | — | 227 and 228 | 235 and 273 |
| [Emim][EtSO4] | 134 and 274 | 134 | 161 | 187 and 188 | 209 | — | — |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
71 | 71 | 79 | 184 | 79 | — | 249, 251 and 252 |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
77 and 85 | 85 | — | 183 | — | — | 249 and 251 |
Fig. 7 shows the comparison of the macroscopic-properties of these 6 IL–H2O binary systems. For [Bmim][I], some research work has shown a complete hydration of [I]− in the infinitely dilute IL solution.147,165,260 MD simulations show that when the mole fraction of water is above 0.25, the ion–H2O interactions are stronger than those between cation–anion. This can be reflected by the rapid increase of Λ (Fig. 7a). When the mole fraction of IL is 0.12, the association degree is only 0.075, which corresponds to the peak of the electrical conductivity as we can see from Fig. 7a. [Emim][BF4] or [Bmim][BF4] is a typical type of IL with large anions, and it cannot be completely dissociated even when the mole fraction of water is up to 0.9. This can be seen from Fig. 7b that the peak of Λ is located at around 0.95. This observation agrees well with the results from the simulation and thermodynamic analysis that [BF4]− is not hydrated or partially hydrated in the dilute IL solutions.210 For the [BF4]-based ILs, when the cation is changed from [Bmim]+ to [Emim]+, the peak of Λ will be shifted to left (IL-rich region), which means that the maximum Λ appears at a lower mole fraction of water. This implies that the cation will also affect the association of IL. This observation agrees with the results from the QC calculations that an increase of the molar volume of the ions may result in a decrease of the ability to form H-bonds.232 In addition, the maximum Λ of [Emim][BF4] is found in the solution at a low mole fraction of water around 0.86, and this is in line with the results from the mechanistic analysis that the intruding water cannot completely dissociate ILs into ions even when the mole fraction of water is up to 0.9. Based on the above analysis, we can conclude that Λ can establish a link between the macroscopic-properties and the microstructure of IL–H2O systems.
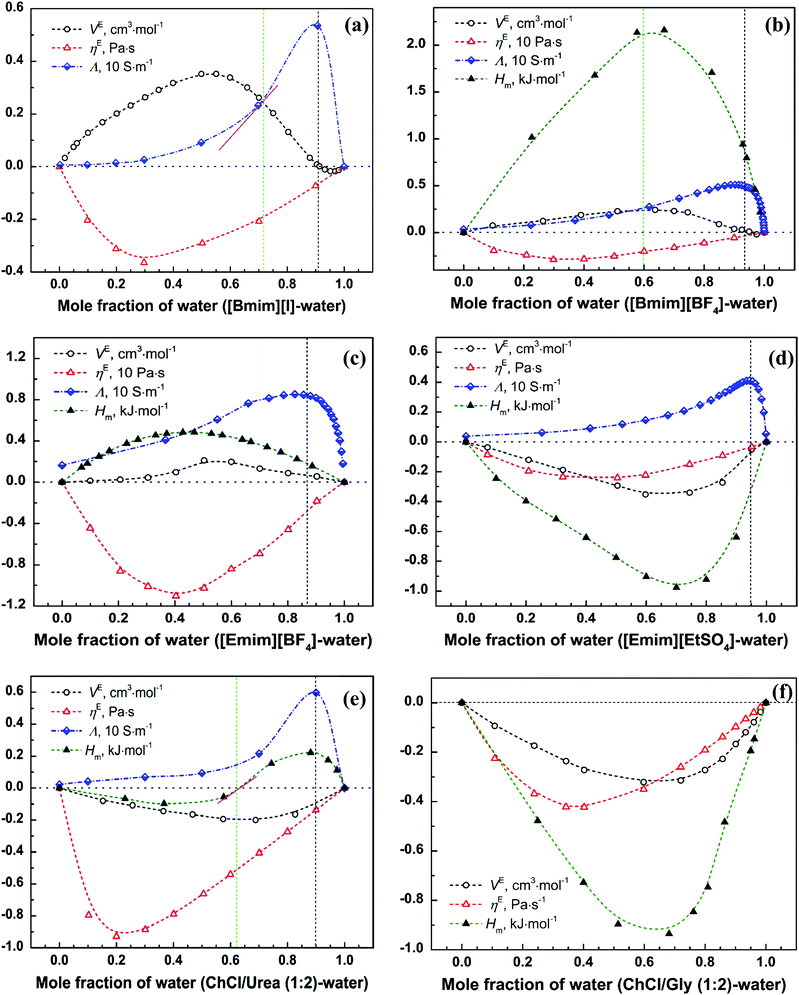 | ||
Fig. 7 The physicochemical properties of 6 typical IL–H2O binary systems. (a) [Bmim][I]; (b) [Bmim][BF4]; (c) [Emim][BF4]; (d) [Emim][EtSO4]; (e) ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); (f) ChCl/Gly (1 2); (f) ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
We have also compared other properties of these 6 systems. For [Bmim][BF4]–H2O and [Bmim][I]–H2O, the shapes and values of VE do not vary much. This is because the effective molar volume of [I]− (48.7 cm3 mol−1) is almost the same as that of [BF4]− (53.4 cm3 mol−1),275 while there is a negative region for [Bmim][I]–H2O when the mole fraction of water is between 0.9 to 1. This phenomenon may be the reflection from the hydration of [I]−. Meanwhile, for the ILs with a same anion (e.g. [BF4]−), when the cation is changed from [Bmim]+ to [Emim]+, the values of ηE, VE and Hm also change greatly. This implies that the type of cation also affects the properties of IL–H2O binary systems because of the relative competition between cation–H2O and cation–anion interactions.
MD simulations show that for a very dilute IL solution (xwater = 0.996), 60% [Emim][EtSO4] molecules can be dissociated into isolated ions, and water clearly decreases the electrostatic interaction energy between the cation and anion by shielding. Therefore, we can conclude that when the IL is infinitely diluted, the dissociated ions will be hydrated. The MD simulations also proposed two percolation limits: 0.8 and 0.95 of water in mole fraction,209 and these two percolation limits can reflect the minimum points of VE and Hm, respectively.
For the two chosen DESs, both of them contain [Cl]− which is usually hydrated in aqueous solutions. The simulations show that at a low water fraction (mass fraction of 0.1 or mole fraction of 0.33), [Cl]− prefers H2O more than urea, and thus urea molecules tend to associate together. The typical macroscopic-property, i.e. the enthalpy of mixing, also shows a maximum energy release at the mole fraction of water around 0.35. In addition, the mass fraction of water at around 0.25 (mole fraction around 0.6) is a turnover point, where the DES (i.e. ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) is sufficiently dissociated and hydrated, and the enthalpy of mixing Hm changes from exothermic to endothermic. When urea is replaced by glycerol, the H-bonding between water and DES changes, which is reflected by the values of VE, ηE and Hm. However, the mole fraction of water at around 0.6 is still a typical turnover point at where the mixture behaviour of DESs in water changes. Based on the mechanistic study from Hammond et al.,252 they concluded that when xwater = 0.833, the DES components are preferable to be fully hydrated, and the nanostructure of this system changes from mixed-solvents to aqueous “electrolyte” solutions. This turning point may reflect the maximum points of Λ and Hm.
2)) is sufficiently dissociated and hydrated, and the enthalpy of mixing Hm changes from exothermic to endothermic. When urea is replaced by glycerol, the H-bonding between water and DES changes, which is reflected by the values of VE, ηE and Hm. However, the mole fraction of water at around 0.6 is still a typical turnover point at where the mixture behaviour of DESs in water changes. Based on the mechanistic study from Hammond et al.,252 they concluded that when xwater = 0.833, the DES components are preferable to be fully hydrated, and the nanostructure of this system changes from mixed-solvents to aqueous “electrolyte” solutions. This turning point may reflect the maximum points of Λ and Hm.
As we can see from Fig. 7, for each IL or DES, the excess or mixing properties are quite complex, and their shapes and values show that these binary systems are far different from ordinary binary solvent mixtures. For different properties, the dominant factors may be different, and thus the minimum or maximum positions in composition are quite different. For example, the minimum or maximum position in composition for excess viscosity is in the IL/DES-rich region, while that for excess volume is in the water-rich region. Some researchers interpreted that the positive excess volume is due to the breaking of the cation–anion network structure by the anion-hydration effect.165 This explanation may be somewhat unreasonable. For example, the VE values for [Bmim][BF4] and [Emim][BF4] are positive, however, the anions in these two ILs cannot be hydrated or can only be partially hydrated at the dilute solutions, therefore, the anion-hydration is only one of the reasons, while the structure and size of the anion can also be the reasons to affect the volumetric properties. If we further compare the experimental VE in Fig. 8 for the ILs with the anions of [I]−, [Br]− and [Cl]−, it follows the order: [I]− > [Br]− > [Cl]−. Since all these anions will be hydrated, it is reasonable to conclude that the anion-hydration has a negative effect on the value of VE. In addition, the size of the cation also has an effect on the value of VE, the larger the size, the higher the VE value, as we can see from the VE values of [Bmim][I] and [Hmim][I] (Fig. 8).
The electrical conductivity Λ can reflect both the dissociation and association of ILs/DESs. According to the experimental results, we can find that the maximum Λ is always for the solutions with water at around 0.9 to 1 (mole fraction). In this water-rich region, IL/DES is highly dissociated, and isolated ions are formed. Compared the ILs that can be fully hydrated (e.g. [Bmim][I]) with those ILs cannot completely hydrated (e.g. [Bmim][BF4]), the curves of electrical conductivity Λ do not have a noticeable difference. This means that, based on the electrical conductivity Λ, we can only get an indication of possible association/dissociation, while it is difficult to tell that the dissociated ions will be fully or partially hydrated or show no hydration at all. In this case, molecular simulations are needed in order to obtain the threshold and hydration numbers.
In summary, to some extent, ηE, VE, Hm, and Λ can reflect the complex interaction in IL/DES–H2O binary systems, however, in order to develop a model to describe the dissociation and hydration of IL in water, Λ is more important. To get the threshold of complete dissociation and the hydration numbers of IL in water, MD or MC simulations are needed.
5. Modelling of IL–H2O binary systems based on molecular thermodynamics
Thermodynamic models have been developed to describe the properties of pure ILs, treating the ILs as either neutral molecules or completely dissociated ions.276–280 In the case of aqueous electrolyte solutions, two types of models have typically been developed: the excess Gibbs energy (GE) and equation of state (EoS) models. The GE models are used to describe the excess properties of mixtures in the liquid phase, and seldom consider pressure. The EoS approach can be used to describe the properties of pure components as well as the excess properties of mixtures in both vapor (or gas) and liquid phases, and does take pressure into consideration.IL/DES–H2O binary systems resemble aqueous electrolyte solutions, however, there has been minimal thermodynamic modelling of such systems because of their complexity. Most of the models developed to date are for aqueous IL solutions (i.e. the concentration of IL is from infinitely dilute to a certain concentration) and are primarily based on EoS models. Typical examples include the perturbed chain-statistical associating fluid theory (PC-SAFT), the binding mean spherical approximation (BiMSA), hetero-segmented SAFT and non-random hydrogen-bonding (NRHB). Only one work, conducted by Simoni et al.,281 was based on the electrolyte non-random two-liquid (eNRTL) approach, which is a GE model.
5.1 Aqueous ILs
The model developed by Mahato et al.282 is based on PC-SAFT, in which the IL was modelled as a molecule without ionic interactions, and the model parameters were fitted to the density of the pure ILs and the vapor pressure of IL–H2O mixtures. The studied ILs are [Cnmim][NTf2] (n = 2 to 8). In the model developed by Papaiconomou et al.283 based on the BiMSA, the IL was described as being weakly associated, the association constant was set as an adjustable parameter, and the osmotic coefficient was used in parameter fitting. The studied ILs are [Cnmim]+ with the anions of [Cl]−, [Br]−, [MeSO4]− and [EtSO4]− and n is from 2 to 8. The hetero-segmented SAFT,279,284,285 in which the IL was completely dissociated into cations and anions, was extended to describe the properties of aqueous ILs by using temperature-dependent segment energy of the cation head. The studied ILs are [Cnmim][Cl] and [Cnmim][Br]. Based on PC-SAFT, Shahriari et al.286 studied the thermodynamic properties of aqueous ILs involving ions of [Cnmim]+, [(CH3)4N]+, [(C2H5)4N]+, [Cl]−, [Br]−, [MeSO4]−, [EtSO4]− and [BF4]−. In modelling, the IL was completely dissociated into cations and anions, and the mean spherical approximation (MSA) was used to describe the long-range ion–ion interactions. The association interaction was used to account for the formation of ion-pairs. In the square-well chain fluid with variable range (SWCF-VR) model proposed by Li et al.,287 it was assumed that ion-pairs as well as free cations and anions can coexist in the solution, and both the short-range and long-range interactions were considered in the model. The long-range electrostatic interactions between ions were described by MSA model. The model parameters were fitted to the properties of aqueous ILs, rather than those for pure ILs. A non-electrolyte equation of state model, the NRHB model, was used to describe the phase behaviour of [Cnmim][NTf2]–H2O system, in which the IL was modeled as a neutral molecule without long-range ionic interactions.2885.2 IL–H2O with a wide compositional range
Models have been developed to study the properties of IL–H2O binary system in its whole compositional range. One is based on eNRTL conducted by Simoni et al.281 In their modelling, IL was completely dissociated into cation and anion, and the studied ILs include [Emim][EtSO4], [Emim][OTf], and [Emim][TFA]. eNRTL belongs to GE model, and the properties of pure ILs cannot be obtained. The other model is based on the COSMO model where the IL was modelled as both in the ionic state and in the ion-pair state with a degree of dissociation parameter α.289 The properties of vapor pressure, activity coefficients, and osmotic coefficients for the IL–H2O binary systems based on the ILs with [Cnmim]+ were studied, but not for other thermodynamic properties, such as mixing enthalpy, excess volume, etc.5.3 New framework for modelling IL/DES–H2O binary systems
IL/DES–H2O binary systems are unique compared with either mixed solvents or aqueous electrolyte solutions. This makes it essential to develop a new model for IL/DES–H2O systems instead of using the model for aqueous electrolyte solutions or for the common mixed solvents directly. The incorporation of association and hydration is important in a new model development. Based on the mechanistic studies, we propose a framework to model the IL/DES–H2O systems (Fig. 9).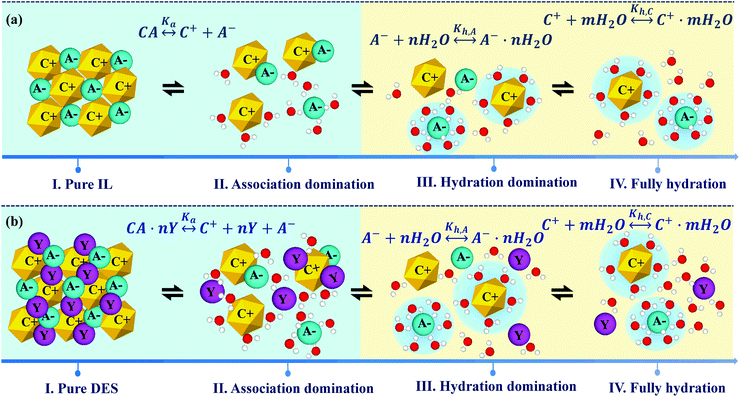 | ||
| Fig. 9 Conceptual scheme and framework to model (a) the IL–H2O system (b) the DES–H2O system. Note: the yellow, blue, red and purple ones represent cation, anion, H2O and HBD, respectively. | ||
Both the association equilibrium and hydration equilibrium exist in IL–H2O (eqn (3a)) and DES–H2O (eqn (3b)) systems
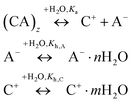 | (3a) |
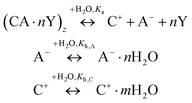 | (3b) |
The framework to model the IL/DES–H2O system includes 4 regions: pure IL/DES (I), association domination (II), hydration domination (III) and fully hydration (IV).
In region II, there is either a neutral CA or CA·nY in the system, and some of the dissociated ions are hydrated. Both the association and hydration equilibria exist. However, association is dominating.
In region III, almost all the ILs/DESs are completely dissociated and the dissociated ions are completely or partially hydrated. Both association and hydration equilibria exist. However, the hydration is dominating.
In region IV, almost all the cations and anions are hydrated. There are only hydration equilibria. In addition, the hydration of cation may be ignored according to the mechanistic study.
For clarity, only a few IL/DES and water molecules are shown in Fig. 9 to introduce the concept. As we mentioned above, each cation is represented as an asymmetric particle concerning the large size and asymmetry, but each anion is represented as a spherical particle (Fig. 9). For a DES–H2O system, the framework is similar to that for IL–H2O, while the model is more complex due to the existence of another neutral compound besides cation and anion for DES itself.
6. Conclusions and perspectives
A comprehensive review of IL/DES–H2O binary systems has been presented. The available experimental data of physicochemical properties, including density, viscosity, electrical conductivity and enthalpy of mixing, was compiled, analyzed and discussed. Density and viscosity are the most widely measured properties. The electrical conductivity and excess/mixing enthalpy have also been attracted a lot attention but the number of investigated ILs is still limited. Surface and interfacial tensions over a whole compositional range at different temperatures are still scarce. The shapes and magnitudes of the excess volume, excess viscosity and enthalpy of mixing used to study the non-ideal behaviour have been quantitatively summarized. These characteristic shapes can be qualitatively described as single hump, positive double hump, negative double hump or S-shape depending on the interaction landscape and changes in solution structures upon varying the water concentration. The shapes of these excess properties for IL/DES–H2O binary systems and the magnitudes (amplitudes) of these shapes are modified with variations in the size and type of cation or anion, and with changes in physical parameters, i.e. temperature and pressure. This implies that the properties of the mixtures are unique and at the same time largely unpredictable. In addition, the electrical conductivity values of IL/DES–H2O binary systems over a wide compositional range generally exhibit a maximum value at a relatively low IL concentration, which is unique, compared with other solutions, such as aqueous electrolyte solutions and mixed organic solvents.Mechanistic studies of IL/DES–H2O systems based on MD/MC simulations, QC calculations, advanced experimental techniques and thermodynamic analyses were also surveyed, to better understand the manner in which H2O interacts with ILs/DESs. Various structure-forming mechanisms were identified on this basis. Specifically, pure ILs are evidently composed of heterogeneous super-clusters having 3D network structures. The addition of H2O drastically modifies this microstructure. Both dissociation and hydration should be considered when modelling such systems and, in the case of an infinitely dilute IL/DES solution, the IL/DES can be assumed to be completely dissociated. According to the sizes of the IL/DES ions, they may be fully or partially hydrated.
Six typical IL/DES–H2O systems were chosen to further study the link between the macro-properties (as indicated by experimental results) and the microstructures of molecules (as determined by mechanistic studies). This examination demonstrates that the values of ηE, VE, Hm and Λ all reflect the complex interactions in IL/DES–H2O systems to some extent, and that Λ is the most important variable in terms of developing a reliable model considering the dissociation and hydration of such systems. In addition, MD/MC simulations are required to obtain parameters for the complete dissociation thresholds and the hydration numbers of IL/DES-ions in water.
Thermodynamic models, developed to describe the properties of IL/DES–H2O systems, were also reviewed. An IL/DES is assumed to be a “neutral molecule” or to be composed of completely dissociated ions in the currently developed thermodynamic models. The majority of these models are for aqueous IL/DES solutions (i.e. the concentration of IL/DES from infinitely dilute to a certain concentration) and are typically based on an equation of state approach. The dissociation of the IL/DES is sometimes considered, but ion hydration is seldom taken into account. Based on the mechanistic studies reviewed herein, we have suggested a new conceptual framework including the dissociation of the IL/DES and ion hydration for the modeling of IL/DES–H2O systems ranging from a pure IL/DES to infinitely dilute IL/DES solutions.
In the future, the thermodynamic modelling based on this proposed conceptual framework by combining the molecular interaction from MD/MC simulations with experimental data including density, viscosity, electrical conductivity and enthalpy of mixing, etc. will be of great interest and meaningful to the many practical applications of IL/DES-based technology. Compared with IL–H2O binary system, the DES–H2O binary systems are more complex and more work needs to be carried out to reveal the differences between water solutions of DES and individual components from DES with water.
Abbreviations
ILs & DESs
ChCl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (1 ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
ChCl/Gluc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (2 glucose (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
ChCl/LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1 lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
ChCl/MA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malonic acid (1 malonic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
ChCl/TG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triethylene glycol (1 triethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
CrCl3/ChCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | Chromium(III) chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Choline chloride (1 Choline chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
CrCl3/ChCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) 2.5) | Chromium(III) chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Choline chloride (1 Choline chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) 2.5) |
DEAC/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
N,N-Diethylethanol ammonium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (1 ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
DEAC/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
N,N-Diethylethanol ammonium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
ATPPB/DEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) | Allyltriphenyl phosphonium bromide-diethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
ATPPB/DEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) | Allyltriphenyl phosphonium bromide-diethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
ATPPB/DEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16) 16) | Allyltriphenyl phosphonium bromide-diethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16) 16) |
| [Amim][Cl] | 1-Allyl-3-methyl-imidazolium chloride |
| [BDBU][IM] | 8-Butyl-1,8-diazabicyclo[5,4,0]-undec-7-enium imidazolide |
| [Bdmim][BF4] | 1-Butyl-2,3-dimethylimidazolium tetrafluoroborate |
| [Bmim][Ac] | 1-Butyl-3-methylimidazolium acetate |
| [Bmim][BF4] | 1-Butyl-3-methylimidazolium tetrafluoroborate |
| [Bmim][Br] | 1-Butyl-3-methylimidazolium bromide |
| [Bmim][Cl] | 1-Butyl-3-methylimidazolium chloride |
| [Bmim][DCA] | 1-Butyl-3-methylimidazolium dicyanamide |
| [Bmim][DMP] | 1-Butyl-3-methylimidazolium dimethylphosphate |
| [Bmim][Ac] | 1-Butyl-3-methylimidazolium acetate |
| [Bmim][HCOO] | 1-Butyl-3-methylimidazolium formate |
| [Bmim][HSO4] | 1-Butyl-3-methylimidazolium hydrogensulfate |
| [Bmim][I] | 1-Butyl-3-methylimidazolium iodide |
| [Bmim][L-lactate] | 1-Butyl-3-methylimidazolium L-lactate |
| [Bmim][EtCOO] | 1-Butyl-3-methylimidazolium propionate |
| [Bmim][MeSO3] | 1-Butyl-3-methylimidazolium methanesulfonate |
| [Bmim][MeSO4] | 1-Butyl-3-methylimidazolium methylsulphate |
| [Bmim][TCM] | 1-Butyl-3-methylimidazolium tricyanomethanide |
| [Bmim][OTf] | 1-Butyl-3-methylimidazolium trifluoromethanesulfonate |
| [Bmim][TOS] | 1-Butyl-3-methylimidazolium tosylate |
| [Bmim][PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate |
| [Bmim][SCN] | 1-Butyl-3-methylimidazolium thiocyanate |
| [Bmim][NTf2] | 1-Butyl-3-methylimidazolium bis(trifluoromethane sulfonyl)imide |
| [Bmim][TCB] | 1-Butyl-3-methylimidazolium tetracyanoborate |
| [C4C2im][Br] | 1-Butyl-3-ethylimidazolium bromide |
| [C4C1mim][Cl] | 1-Butyl-2,3-dimethylimidazolium chloride |
| [Mim][Cl] | 1-Methylimidazolium chloride |
| [Emim][Ac] | 1-Ethyl-3-methylimidazolium acetate |
| [Emim][BF4] | 1-Ethyl-3-methyl imidazolium tetrafluoroborate |
| [Emim][BuSO4] | 1-Ethyl-3-methylimidazolium butylsulphate |
| [Emim][DCA] | 1-Ethyl-3-methylimidazolium dicyanamide |
| [Emim][DEP] | 1-Ethyl-3-methylimidazolium diethylphosphate |
| [Emim][DMP] | 1-Ethyl-3-methylimidazolium dimethylphosphate |
| [Emim][EtSO4] | 1-Ethyl-3-methylimidazolium ethylsulphate |
| [Emim][HexSO4] | 1-Ethyl-3-methylimidazolium hexylsulphate |
| [Emim][HSO4] | 1-Ethyl-3-methylimidazolium hydrogensulfate |
| [Emim][L-lactate] | 1-Ethyl-3-methylimidazolium L-lactate |
| [Emim][MeSO3] | 1-Ethyl-3-methylimidazolium methanesulfonate |
| [Emim][MeSO4] | 1-Ethyl-3-methylimidazolium methylsulphate |
| [Emim][OcSO4] | 1-Ethyl-3-methylimidazolium octylsulphate |
| [Emim][OTf] | 1-Ethyl-3-methylimidazolium trifluoromethanesulfonate |
| [Emim][PF6] | 1-Ethyl-3-methylimidazolium hexafluorophosphate |
| [Emim][SCN] | 1-Ethyl-3-methylimidazolium thiocyanate |
| [Emim][TCM] | 1-Ethyl-3-methylimidazolium tricyanomethanide |
| [Emim][NTf2] | 1-Ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [Emim][TFA] | 1-Ethyl-3-methylimidazolium trifluoroacetate |
| [Emim][Glyci] | 1-Ethyl-3-methylimidazolium,glycinate |
| [Emim][Et2PO4] | 1-Ethyl-3-methylimidazolium diethylphosphate |
| [Emim][Br] | 1-Ethyl-3-methylimidazolium bromide |
| [Emim][Cl] | 1-Ethyl-3-methylimidazolium chloride |
| [HDBU][IM] | 8-Hydrogen-1,8-diazabicyclo[5,4,0]-undec-7-enium imidazolide |
| [Hmim][Br] | 1-Hexyl-3-methyl imidazolium bromide |
| [Hmim][BF4] | 1-Hexyl-3-methyl imidazolium tetrafluoroborate |
| [Hmim][Br] | 1-Hexyl-3-methyl imidazolium bromide |
| [Hmim][Cl] | 1-Hexyl-3-methyl imidazolium chloride |
| [Hmim][I] | 1-Hexyl-3-methyl imidazolium iodide |
| [Hmim][OTf] | 1-Hexyl-3-methyl imidazolium trifluoromethanesulfonate |
| [Mmim][Cl] | 1,3-Dimethylimidazolium chloride |
| [Mmim][DMP] | 1,3-Dimethylimidazolium dimethylphosphate |
| [Mmim][MeSO4] | 1,3-Dimethylimidazolium methylsulphate |
| [Mmim][PF6] | 1,3-Dimethylimidazolium hexafluorophosphate |
| [OHemim][TFA] | 1-(2-Hydroxyethyl)-3-methylimidazolium trifluoroacetate |
| [Omim][BF4] | 1-Octyl-3-methyl imidazolium tetrafluoroborate |
| [Omim][Br] | 1-Octyl-3-methylimidazolium bromide |
| [Omim][Cl] | 1-Octyl-3-methylimidazolium chloride |
| [Omim][NO3] | 1-Octyl-3-methylimidazolium nitrate |
| [Pmim][Cl] | 1-Pentyl-3-methylimidazolium chloride |
| [C7mim][Cl] | 1-Heptyl-3-methylimidazolium chloride |
| [C10mim][Br] | 1-Decyl-3-methylimidazolium bromide |
| [C10mim][Cl] | 1-Decyl-3-methylimidazolium chloride |
| [C12mim][Br] | 1-Dodecyl-3-methylimidazolium bromide |
| [TMG][IM] | 1,1,3,3-Tetramethylguanidine imidazolide |
| [C20H28N3O3][Br] | L-Histidine derived imidazolium bromide |
| [C20H28N3O3][NTf2] | L-Histidine derived imidazolium bis(trifluoromethanesulfonyl)imide |
| [B2mPy][BF4] | 1-Butyl-2-methylpyridinium tetrafluoroborate |
| [B2mPy][Cl] | 1-Butyl-2-methylpyridinium chloride |
| [B3mPy][BF4] | 1-Butyl-3-methylpyridinium tetrafluoroborate |
| [B3mPy][Cl] | 1-Butyl-3-methylpyridinium chloride |
| [B4mPy][BF4] | 1-Butyl-4-methylpyridinium tetrafluoroborate |
| [B4mPy][Cl] | 1-Butyl-4-methylpyridinium chloride |
| [B4mPy][SCN] | 1-Butyl-4-methylpyridinium thiocyanate |
| [BPy][BF4] | 1-Butylpyridinium tetrafluoroborate |
| [BPy][Cl] | 1-Butylpyridinium chloride |
| [OcPy][BF4] | 1-Octylpyridinium tetrafluoroborate |
| [PPy][BF4] | 1-Propylpyridinium tetrafluoroborate |
| [EPy][MeSO3] | 1-Ethylpyridinium methanesulfonate |
| [BmPyr][DCA] | 1-Butyl-1-methylpyrrolidinium dicyanamide |
| [Bmpyr][Cl] | 1-Butyl-1-methylpyrrolidinium chloride |
| [BmPyr][SCN] | 1-Butyl-1-methylpyrrolidinium thiocyanate |
| [B4mPyr][Br] | 1-Butyl-4-methylpyrrolidinium bromide |
| [Empyr][EtSO4] | 1-Ethyl-1-methylpyrrolidinium ethylsulfate |
| [Empyr][Ac] | 1-Ethyl-1-methylpyrrolidinium acetate |
| [Empyr][Glyc] | 1-Ethyl-1-methylpyrrolidinium glycolate |
| [Epmpyr][Cl] | N-Methyl-N-(2′,3′-epoxypropyl)-2-oxopyrrolidinium chloride |
| [Epmpyr][Ac] | N-Methyl-N-(2′,3′-epoxypropyl)-2-oxopyrrolidinium acetate |
| [Epmpyr][SAL] | N-(2′,3′-Epoxypropyl)-N-methyl-2-oxopyrrolidinium salicylate |
| [Pe5myrr][Br] | 1-Pentyl-5-methylpyrrolidinium bromide |
| [Pyrr][C7CO2] | Pyrrolidinium octanoate |
| [Pyrr][HCOO] | Pyrrolidinium formate |
| [Pyrr][HSO4] | Pyrrolidinium hydrogensulfate |
| [Pyrr][NO3] | Pyrrolidinium nitrate |
| [P3myrr][Br] | 1-Propyl-3-methylpyrrolidinium bromide |
| [C3C1pyr][PF6] | 1-Methyl-1-propylpyrrolidinium hexafluorophosphate |
| [Empip][EtSO4] | 1-Ethyl-1-methyl-piperidiniumethylsulfate |
| [Bmpip][Cl] | 1-Butyl-1-methylpiperidinium chloride |
| [Bmpip][SCN] | 1-Butyl-1-methylpiperidinium thiocyanate |
| [Bmpip][DCA] | 1-Butyl-1-methylpiperidinium dicyanamide |
| [C3C1pyr][PF6] | 1-Methyl-1-propylpiperidinium Hexafluorophosphate |
| [(OH)2C2pi][EtCOO] | N-(2-Hydroxyethyl)piperazinium Propionate |
| [MP][Glyci] | Methyl-piperazinium glycinate |
| [(C2)22(C1)2(C1)23gu][EtSO4] | 2,2-diethyl-1,1,3,3-tetramethylguanidinium ethylsulfate |
| [TMG][BEN] | Tetramethylguanidinium benzoate |
| [TMG][SAL] | Tetramethylguanidinium salicylate |
| [TMG][Lac] | 1,1,3,3-Tetramethylguanidinium Lactate |
| [Emmor][EtSO4] | 1-Ethyl-1-methylmorpholinium ethylsulfate |
| [Emmor][Ac] | 1-Ethyl-1-methyl-morpholinium acetate |
| [Emmor][Pro] | 1-Ethyl-1-methylmorpholinium propionate |
| [Emmor][Glyc] | 1-Ethyl-1-methylmorpholinium glycolate |
| [Col][HCOO] | Collidinium formate |
| [Cho][Lac] | Choline lactate |
| [Cho][Prop] | Choline propanoate |
| [Cho][Mal] | Choline malonate |
| [Cho][Glyc] | Choline glycolate |
| [Cho][NTf2] | Cholinium bis(trifluoromethanesulfonyl)imide |
| [Cho][Glyci] | Cholinium glycinate |
| [Cho][H2Prop] | Choline dihydrogen phosphate |
| [BA][Ac] | N-Butylammonium acetate |
| [BA][NO3] | N-Butylammonium nitrate |
| [DEMA][MeSO3] | Diethylmethylammonium methanesulfonate |
| [DEMA][OTf] | Diethylmethylammonium trifluoromethanesulfonate |
| [TEA][MeSO3] | Triethylammonium methanesulfonate |
| [DIPEA][HCOO] | Diisopropyl-ethylammonium formate |
| [DEME][BF4] | N,N-Diethyl-N-methyl-N-(2-methoxyethyl) ammonium tetrafluoroborate |
| [DEME][I] | N,N-Diethyl-N-methyl-N-(2-methoxyethyl) ammonium iodide |
| [EA][NO3] | Ethylammonium nitrate |
| [MTEOA][MeSO4] | Tris(2-hydroxyethyl) methylammonium methylsulfate |
| [PA][NO3] | Propylammonium nitrate |
| EAB/[N0002][C3CO2] | Ethylammonium butanoate |
| 2-HDEAH | 2-Hydroxy diethylammonium hexanoate |
| 2-HEAB | 2-Hydroxy ethylammonium butanoate |
| 2-HEAH | 2-Hydroxy ethylammonium hexanoate |
| 2-HEAP | 2-Hydroxy ethylammonium pentanoate |
| 3HPAAc | 3-Hydroxypropylammonium acetate |
| 3HPAF | 3-Hydroxypropylammonium formate |
| 3HPATFAc | 3-Hydroxypropylammonium trifluoroacetate |
| BHEAC | Bis(2-hydroxyethyl)ammonium acetate |
| CPL-TBABr | Caprolactam tetrabutyl ammonium bromide |
| CPL-TBACl | Caprolactam tetrabutyl ammonium chloride |
| CPL-TBAF | Caprolactam tetrabutyl ammonium fluorine |
| M2HEAPr | N-Methyl-2-hydroxyethylammonium propionate |
| PAAc | Propylammonium acetate |
| PAF | Propylammonium fromate |
| TMEAC | Tris(monoethanolamine) citrate |
| [N4444][PF6] | Tetrabutylammonium hexafluorophosphate |
| [DMEtA][Pr] | N,N-Dimethylethanolammonium propionate |
| [DMEtA][NTf2] | N,N-Dimethylethanolammonium bis(trifluoromethanesulfonyl)imide |
| [CyN1,1PrSO3H][Tos] | N,N-Dimethyl-N-(3-sulfopropyl)cyclohexylammonium tosylate |
| [aP4443][Ala] | (3-Aminopropyl) tributylphosphonium L-α-aminopropionic acid salt |
| [aP4443][Val] | (3-Aminopropyl) tributylphosphonium L-α-aminoisovaleric acid salt |
| [aP4443][Leu] | (3-Aminopropyl) tributylphosphonium L-α-amino-4-methylvaleric acid salt |
| [P6,6,6,14][BOB] | Trihexyltetradecylphosphonium bis(oxalato)-borate |
| [P2444][DEP] | Ethyl(tributyl)phosphonium diethylphosphate |
| [P4444][PF6] | Tetrabutylphosphonium hexafluorophosphate |
| [HiQuin][SCN] | N-Hexylisoquinolinium thiocyanate |
| [C8iQuin][SCN] | N-Octylisoquinolinium Thiocyanate |
Advanced experimental methods
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| FTIR | Fourier-transform infrared spectroscopy |
| ATR-IR | Attenuated total reflection and transmission infra-red |
| PGSE | Pulsed gradient spin-echo |
| FIR | Far-infrared |
| UV | Ultraviolet |
| PFG | Pulsed field gradient |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the financial support from the Swedish Energy Agency (P40548-1). XJ thanks to financial support from Joint Research Fund for Overseas Chinese Scholars and Scholars in Hong Kong and Macao Young Scholars (No. 21729601). AL and XJ wish to acknowledge financial support from the Swedish Science Council (VR, 2016-04023). C. Liu and X. Lu thank the financial support from the National Natural Science Foundation of China (No. 21838004). AL thanks for a partial support by a grant of Ministry of Research and Innovation, CNCS – UEFISCDI, project number PN-III-P4-ID-PCCF-2016-0050, within PNCDI III.References
- K. Binnemans, Chem. Rev., 2007, 107, 2592–2614 CrossRef CAS PubMed.
- Z. Lei, B. Chen, Y.-M. Koo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 399, 28–29 CrossRef.
- X. Zhang, X. Zhang, H. Dong, Z. Zhao, S. Zhang and Y. Huang, Energy Environ. Sci., 2012, 5, 6668–6681 RSC.
- X. Zhang, L. Bai, S. Zeng, H. Gao, S. Zhang and M. Fan, in Energy Efficient Solvents for CO2 Capture by Gas-Liquid Absorption: Compounds, Blends and Advanced Solvent Systems, ed. W. M. Budzianowski, Springer International Publishing, Cham, 2017, pp. 153–176 Search PubMed.
- C. Ye, W. Liu, Y. Chen and L. Yu, Chem. Commun., 2001, 2244–2245 RSC.
- I. Minami, Molecules, 2009, 14, 2286 CrossRef CAS PubMed.
- F. Zhou, Y. Liang and W. Liu, Chem. Soc. Rev., 2009, 38, 2590–2599 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- S. P. Magalhaes da Silva, A. M. da Costa Lopes, L. B. Roseiro and R. Bogel-Lukasik, RSC Adv., 2013, 3, 16040–16050 RSC.
- J. A. Sirviö, M. Visanko and H. Liimatainen, Biomacromolecules, 2016, 17, 3025–3032 CrossRef PubMed.
- M. Pan, G. Zhao, C. Ding, B. Wu, Z. Lian and H. Lian, Carbohydr. Polym., 2017, 176, 307–314 CrossRef CAS PubMed.
- Y.-L. Loow, T. Y. Wu, G. H. Yang, L. Y. Ang, E. K. New, L. F. Siow, J. Md. Jahim, A. W. Mohammad and W. H. Teoh, Bioresour. Technol., 2018, 249, 818–825 CrossRef CAS PubMed.
- W. Xing, G. Xu, J. Dong, R. Han and Y. Ni, Chem. Eng. J., 2018, 333, 712–720 CrossRef CAS.
- D. J. G. P. van Osch, L. J. B. M. Kollau, A. van den Bruinhorst, S. Asikainen, M. A. A. Rocha and M. C. Kroon, Phys. Chem. Chem. Phys., 2017, 19, 2636–2665 RSC.
- X. Zhang and D. Hu, Appl. Therm. Eng., 2012, 37, 129–135 CrossRef CAS.
- E.-S. Abumandour, F. Mutelet and D. Alonso, Appl. Therm. Eng., 2016, 94, 579–589 CrossRef CAS.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed.
- G. Cui, J. Wang and S. Zhang, Chem. Soc. Rev., 2016, 45, 4307–4339 RSC.
- X. Li, M. Hou, B. Han, X. Wang and L. Zou, J. Chem. Eng. Data, 2008, 53, 548–550 CrossRef CAS.
- S. Sarmad, J.-P. Mikkola and X. Ji, ChemSusChem, 2017, 10, 324–352 CrossRef CAS PubMed.
- S. Sarmad, Y. Xie, J.-P. Mikkola and X. Ji, New J. Chem., 2017, 41, 290–301 RSC.
- E. I. Ahmed, A. P. Abbott and K. S. Ryder, AIP Conf. Proc., 2017, 1888, 020006 CrossRef.
- A. P. Abbott, E. I. Ahmed, R. C. Harris and K. S. Ryder, Green Chem., 2014, 16, 4156–4161 RSC.
- K.-S. Kim, B.-K. Shin, H. Lee and F. Ziegler, Fluid Phase Equilib., 2004, 218, 215–220 CrossRef CAS.
- D. Zheng, L. Dong, W. Huang, X. Wu and N. Nie, Renewable Sustainable Energy Rev., 2014, 37, 47–68 CrossRef CAS.
- R. Abedin, S. Heidarian, J. C. Flake and F. R. Hung, Langmuir, 2017, 33, 11611–11625 CrossRef CAS PubMed.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098–3106 RSC.
- M. C. C. Ribeiro, J. Phys. Chem. B, 2012, 116, 7281–7290 CrossRef CAS PubMed.
- P. Cozma, W. Wukovits, I. Mămăligă, A. Friedl and M. Gavrilescu, Clean Technol. Environ. Policy, 2015, 17, 373–391 CrossRef CAS.
- S. Xia, G. A. Baker, H. Li, S. Ravula and H. Zhao, RSC Adv., 2014, 4, 10586–10596 RSC.
- C. J. de la Parra, J. R. Zambrano, M. D. Bermejo, A. Martin, J. J. Segovia and M. J. Cocero, J. Chem. Thermodyn., 2015, 91, 8–16 CrossRef.
- A. Podgoršek, J. Jacquemin, A. Pádua and M. Costa Gomes, Chem. Rev., 2016, 116, 6075–6106 CrossRef PubMed.
- I. Bahadur, T. M. Letcher, S. Singh, G. G. Redhi, P. Venkatesu and D. Ramjugernath, J. Chem. Thermodyn., 2015, 82, 34–46 CrossRef CAS.
- Q. Cao, X. X. Lu, X. Wu, Y. S. Guo, L. Xu and W. J. Fang, J. Chem. Eng. Data, 2015, 60, 455–463 CrossRef CAS.
- C. G. Hanke and R. M. Lynden-Bell, J. Phys. Chem. B, 2003, 107, 10873–10878 CrossRef CAS.
- C. G. Hanke, N. A. Atamas and R. M. Lynden-Bell, Green Chem., 2002, 4, 107–111 RSC.
- A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed.
- C. Dai, J. Zhang, C. Huang and Z. Lei, Chem. Rev., 2017, 117, 6929–6983 CrossRef CAS PubMed.
- K. Dong, X. Liu, H. Dong, X. Zhang and S. Zhang, Chem. Rev., 2017, 117, 6636–6695 CrossRef CAS PubMed.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- P. Hapiot and C. Lagrost, Chem. Rev., 2008, 108, 2238–2264 CrossRef CAS PubMed.
- Z. Lei, C. Dai and B. Chen, Chem. Rev., 2014, 114, 1289–1326 CrossRef CAS PubMed.
- V. H. Paschoal, L. F. O. Faria and M. C. C. Ribeiro, Chem. Rev., 2017, 117, 7053–7112 CrossRef CAS PubMed.
- A. Podgoršek, J. Jacquemin, A. A. H. Pádua and M. F. Costa Gomes, Chem. Rev., 2016, 116, 6075–6106 CrossRef PubMed.
- B. Wang, L. Qin, T. Mu, Z. Xue and G. Gao, Chem. Rev., 2017, 117, 7113–7131 CrossRef CAS PubMed.
- S. Zeng, X. Zhang, L. Bai, X. Zhang, H. Wang, J. Wang, D. Bao, M. Li, X. Liu and S. Zhang, Chem. Rev., 2017, 117, 9625–9673 CrossRef CAS PubMed.
- Q. Zhang and J. N. M. Shreeve, Chem. Rev., 2014, 114, 10527–10574 CrossRef CAS PubMed.
- S. Zhang, J. Zhang, Y. Zhang and Y. Deng, Chem. Rev., 2017, 117, 6755–6833 CrossRef CAS PubMed.
- D. Carriazo, M. Concepcion Serrano, M. Concepcion Gutierrez, M. Luisa Ferrer and F. del Monte, Chem. Soc. Rev., 2012, 41, 4996–5014 RSC.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, J. Nat. Prod., 2013, 76, 2162–2173 CrossRef CAS PubMed.
- E. Durand, J. Lecomte and P. Villeneuve, Eur. J. Lipid Sci. Technol., 2013, 115, 379–385 CrossRef CAS.
- B. Tang and K. H. Row, Monatsh. Chem., 2013, 144, 1427–1454 CrossRef CAS.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- D. V. Wagle, H. Zhao and G. A. Baker, Acc. Chem. Res., 2014, 47, 2299–2308 CrossRef CAS PubMed.
- D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramon, Eur. J. Org. Chem., 2016, 612–632 CrossRef CAS.
- S. Sarmad, Y. Xie, J.-P. Mikkola and X. Ji, New J. Chem., 2017, 41, 290–301 RSC.
- M. Tariq, M. G. Freire, B. Saramago, J. A. P. Coutinho, J. N. C. Lopes and L. P. N. Rebelo, Chem. Soc. Rev., 2012, 41, 829–868 RSC.
- M. Tariq, F. Moscoso, F. J. Deive, A. Rodriguez, M. A. Sanromán, J. M. S. S. Esperança, J. N. Canongia Lopes and L. P. N. Rebelo, J. Chem. Thermodyn., 2013, 59, 43–48 CrossRef CAS.
- T. Singh and A. Kumar, J. Chem. Thermodyn., 2011, 43, 958–965 CrossRef CAS.
- J. Jacquemin, P. Husson, A. A. H. Padua and V. Majer, Green Chem., 2006, 8, 172–180 RSC.
- J. Skowronek, M. Geppert-Rybczyńska, J. Jacquemin, P. Goodrich, J. A. Vicente, M. Chorążewski, S. Jężak, M. Zorębski, E. Zorębski, M. Żarska, W. Kaca, P. Berdyczko and M. Dzida, J. Solution Chem., 2015, 44, 824–837 CrossRef CAS.
- K. Oster, P. Goodrich, J. Jacquemin, C. Hardacre, A. P. C. Ribeiro and A. Elsinawi, J. Chem. Thermodyn., 2018, 121, 97–111 CrossRef CAS.
- M. Anouti, M. Caillon-Caravanier, Y. Dridi, J. Jacquemin, C. Hardacre and D. Lemordant, J. Chem. Thermodyn., 2009, 41, 799–808 CrossRef CAS.
- S. Porcedda, B. Marongiu, M. Schirru, D. Falconieri and A. Piras, J. Therm. Anal. Calorim., 2011, 103, 29–33 CrossRef CAS.
- U. Dománska and M. Królikowska, J. Solution Chem., 2012, 41, 1422–1445 CrossRef PubMed.
- Y. Xie, H. Dong, S. Zhang, X. Lu and X. Ji, J. Chem. Eng. Data, 2014, 59, 3344–3352 CrossRef CAS.
- J. G. Li, Y. F. Hu, S. F. Sun, Y. S. Liu and Z. C. Liu, J. Chem. Thermodyn., 2010, 42, 904–908 CrossRef CAS.
- F. S. Mjalli and O. Ahmad, Thermochim. Acta, 2017, 647, 8–14 CrossRef CAS.
- R. Zarrougui, M. Dhahbi and D. Lemordant, J. Solution Chem., 2015, 44, 686–702 CrossRef CAS.
- R. B. Leron and M.-H. Li, J. Chem. Thermodyn., 2012, 54, 293–301 CrossRef CAS.
- R. B. Leron and M.-H. Li, Thermochim. Acta, 2012, 546, 54–60 CrossRef CAS.
- R. B. Leron, D. S. H. Wong and M.-H. Li, Fluid Phase Equilib., 2012, 335, 32–38 CrossRef CAS.
- A. Yadav and S. Pandey, J. Chem. Eng. Data, 2014, 59, 2221–2229 CrossRef CAS.
- D. Shah and F. S. Mjalli, Phys. Chem. Chem. Phys., 2014, 16, 23900–23907 RSC.
- H. Shekaari, M. T. Zafarani-Moattar and B. Mohammadi, J. Mol. Liq., 2017, 243, 451–461 CrossRef CAS.
- A. Yadav, J. R. Kar, M. Verma, S. Naqvi and S. Pandey, Thermochim. Acta, 2015, 600, 95–101 CrossRef CAS.
- R. B. Leron, A. N. Soriano and M.-H. Li, J. Taiwan Inst. Chem. Eng., 2012, 43, 551–557 CrossRef CAS.
- Y. P. Hsieh, R. B. Leron, A. N. Soriano, A. R. Caparanga and M. H. Li, J. Chem. Eng. Jpn., 2012, 45, 939–947 CrossRef CAS.
- C. M. Lin, R. B. Leron, A. R. Caparanga and M. H. Li, J. Chem. Thermodyn., 2014, 68, 216–220 CrossRef CAS.
- A. Yadav, S. Trivedi, R. Rai and S. Pandey, Fluid Phase Equilib., 2014, 367, 135–142 CrossRef CAS.
- K.-S. Kim and B. H. Park, J. Mol. Liq., 2018, 254, 272–279 CrossRef CAS.
- M. Francisco, A. van den Bruinhorst, L. F. Zubeir, C. J. Peters and M. C. Kroon, Fluid Phase Equilib., 2013, 340, 77–84 CrossRef CAS.
- V. S. Protsenko, L. S. Bobrova and F. I. Danilov, Ionics, 2017, 23, 637–643 CrossRef CAS.
- L. S. Bobrova, F. I. Danilov and V. S. Protsenko, J. Mol. Liq., 2016, 223, 48–53 CrossRef CAS.
- K. R. Siongco, R. B. Leron and M. H. Li, J. Chem. Thermodyn., 2013, 65, 65–72 CrossRef CAS.
- H. Ghaedi, M. Ayoub, S. Sufian, A. M. Shariff, G. Murshid, S. M. Hailegiorgis and S. N. Khan, J. Mol. Liq., 2017, 248, 378–390 CrossRef CAS.
- S. Dožić, N. Zec, A. Tot, S. Papović, K. Pavlović, S. Gadžurić and M. Vraneš, J. Chem. Thermodyn., 2016, 93, 52–59 CrossRef.
- Z. He, Z. Zhao, X. Zhang and H. Feng, Fluid Phase Equilib., 2010, 298, 83–91 CrossRef CAS.
- E. Gómez, B. González, N. Calvar and Á. Domínguez, J. Chem. Thermodyn., 2008, 40, 1208–1216 CrossRef.
- E. Rilo, L. M. Varea and O. Cabeza, J. Chem. Eng. Data, 2012, 57, 2136–2142 CrossRef CAS.
- H. Rodríguez and J. F. Brennecke, J. Chem. Eng. Data, 2006, 51, 2145–2155 CrossRef.
- E. Vataščin and V. Dohnal, J. Chem. Thermodyn., 2015, 89, 169–176 CrossRef.
- E. Quijada-Maldonado, S. van der Boogaart, J. H. Lijbers, G. W. Meindersma and A. B. de Haan, J. Chem. Thermodyn., 2012, 51, 51–58 CrossRef CAS.
- C. Romich, N. C. Merkel, A. Valbonesi, K. Schaber, S. Sauer and T. J. S. Schubert, J. Chem. Eng. Data, 2012, 57, 2258–2264 CrossRef.
- J.-y. Wang, H.-c. Jiang, Y.-m. Liu and Y.-q. Hu, J. Chem. Thermodyn., 2011, 43, 800–804 CrossRef CAS.
- C. Herrera, L. T. Costa, M. Atilhan and S. Aparicio, J. Mol. Liq., 2017, 236, 81–92 CrossRef CAS.
- Y. H. Gong, C. Shen, Y. Z. Lu, H. Meng and C. X. Li, J. Chem. Eng. Data, 2012, 57, 33–39 CrossRef CAS.
- L. de Pablo, J. J. Segovia Puras, C. Martín and M. D. Bermejo, J. Chem. Eng. Data, 2018, 63, 1053–1064 CrossRef CAS.
- H. C. Jiang, Y. Zhao, J. Y. Wang, F. Y. Zhao, R. J. Liu and Y. Q. Hu, J. Chem. Thermodyn., 2013, 64, 1–13 CrossRef CAS.
- L. X. Chen, J. Y. Chen, Z. H. Song, G. K. Cui, Y. J. Xu, X. H. Wang and J. Liu, J. Chem. Thermodyn., 2015, 91, 292–300 CrossRef CAS.
- F. Ye, J. Zhu, K. Yu, R. Zhu, Y. Xu, J. Chen and L. Chen, J. Chem. Thermodyn., 2016, 97, 39–47 CrossRef CAS.
- D. Wu, B. Wu, Y. M. Zhang and H. P. Wang, J. Chem. Eng. Data, 2010, 55, 621–624 CrossRef CAS.
- M. Krolikowska, P. Lipinski and D. Maik, Thermochim. Acta, 2014, 582, 1–9 CrossRef CAS.
- A. Vasanthakumar, I. Bahadur, G. G. Redhi, R. M. Gengan and K. Anand, J. Mol. Liq., 2016, 219, 685–693 CrossRef CAS.
- V. Arumugam, G. G. Redhi and R. M. Gengan, J. Mol. Liq., 2017, 242, 1215–1227 CrossRef CAS.
- A. Vasanthakumar, I. Bahadur, G. Redhi and R. M. Gengan, RSC Adv., 2016, 6, 61566–61575 RSC.
- M. Królikowska, M. Zawadzki, U. Domańska, D. Ramjugernath and P. Naidoo, J. Chem. Thermodyn., 2017, 109, 71–81 CrossRef.
- L. F. Zhang, X. X. Lu, D. F. Ye, Y. S. Guo and W. J. Fang, J. Chem. Eng. Data, 2016, 61, 1023–1031 CrossRef CAS.
- A. P. Singh, R. L. Gardas and S. Senapati, Phys. Chem. Chem. Phys., 2015, 17, 25037–25048 RSC.
- S. Tian, S. Ren, Y. Hou, W. Wu and W. Peng, J. Chem. Eng. Data, 2013, 58, 1885–1892 CrossRef CAS.
- M. Krolikowska and M. Zawadzki, J. Mol. Liq., 2018, 251, 358–368 CrossRef CAS.
- D. J. S. Patinha, L. C. Tome, H. Garcia, R. Ferreira, C. S. Pereira, L. P. N. Rebelo and I. M. Marrucho, J. Chem. Thermodyn., 2015, 84, 93–100 CrossRef CAS.
- H. Zhang, C.-T. Lu, J.-G. Lu, L. Gao, G.-P. Hai, Y.-Q. Tang, L.-X. Chen, A.-C. Hua and L.-J. Wang, J. Solution Chem., 2014, 43, 2117–2132 CrossRef CAS.
- N. C. Merkel, C. Römich, R. Bernewitz, H. Künemund, M. Gleiß, S. Sauer, T. J. S. Schubert, G. Guthausen and K. Schaber, J. Chem. Eng. Data, 2014, 59, 560–570 CrossRef CAS.
- P. K. Chhotaray, S. Jella and R. L. Gardas, J. Mol. Liq., 2016, 219, 829–844 CrossRef CAS.
- R. R. Pinto, D. Santos, S. Mattedi and M. Aznar, Braz. J. Chem. Eng., 2015, 32, 671–682 CrossRef CAS.
- Y. Li, E. J. P. Figueiredo, M. Santos, J. B. Santos, N. M. C. Talavera-Prieto, P. J. Carvalho, A. G. M. Ferreira and S. Mattedi, J. Chem. Thermodyn., 2015, 88, 44–60 CrossRef CAS.
- M. M. Taib and T. Murugesan, J. Chem. Eng. Data, 2010, 55, 5910–5913 CrossRef CAS.
- D. Cai, J. Yang, H. Da, L. Li, H. Wang and T. Qiu, J. Mol. Liq., 2017, 229, 389–395 CrossRef CAS.
- Z. X. Wang, L. Fu, H. Xu, Y. Shang, L. Zhang and J. M. Zhang, J. Chem. Eng. Data, 2012, 57, 1057–1063 CrossRef CAS.
- M. Krolikowska and M. Zawadzki, J. Mol. Liq., 2018, 249, 153–159 CrossRef CAS.
- L. Labík, T. Moucha, R. Petříček, J. F. Rejl, L. Valenz and J. Haidl, Chem. Eng. Sci., 2017, 170, 451–463 CrossRef.
- J. C. F. Diogo, F. J. P. Caetano, J. M. N. A. Fareleira and W. A. Wakeham, Int. J. Thermophys., 2014, 35, 1615–1635 CrossRef CAS.
- M. Królikowska, M. Zawadzki and M. Królikowski, J. Chem. Thermodyn., 2014, 70, 127–137 CrossRef.
- B. Yoo, W. Afzal and J. M. Prausnitz, Z. Phys. Chem., 2013, 227, 157–165 CrossRef CAS.
- M. Krannich, F. Heym and A. Jess, J. Chem. Eng. Data, 2016, 61, 1162–1176 CrossRef CAS.
- M. A. R. Martins, C. Neves, K. A. Kurnia, P. J. Carvalho, M. A. A. Rocha, L. Santos, S. P. Pinho and M. G. Freire, Fluid Phase Equilib., 2016, 407, 188–196 CrossRef CAS PubMed.
- J. S. Torrecilla, T. Rafione, J. Garcia and F. Rodriguez, J. Chem. Eng. Data, 2008, 53, 923–928 CrossRef CAS.
- E. Gómez, B. González, N. Calvar, E. Tojo and Á. Domínguez, J. Chem. Eng. Data, 2006, 51, 2096–2102 CrossRef.
- A. Bhattacharjee, C. Varanda, M. G. Freire, S. Matted, L. M. N. B. F. Santos, I. M. Marrucho and J. A. P. Coutinho, J. Chem. Eng. Data, 2012, 57, 3473–3482 CrossRef CAS.
- S. Fendt, S. Padmanabhan, H. W. Blanch and J. M. Prausnitz, J. Chem. Eng. Data, 2011, 56, 31–34 CrossRef CAS.
- E. Vataščin and V. Dohnal, J. Chem. Thermodyn., 2017, 106, 262–275 CrossRef.
- T. Chen, M. Chidambaram, Z. P. Liu, B. Smit and A. T. Bell, J. Phys. Chem. B, 2010, 114, 5790–5794 CrossRef CAS PubMed.
- S. Pandey, K. A. Fletcher, S. N. Baker and G. A. Baker, Analyst, 2004, 129, 569–573 RSC.
- M. S. Kelkar and E. J. Maginn, J. Phys. Chem. B, 2007, 111, 4867–4876 CrossRef CAS PubMed.
- A. Gutiérrez, M. Atilhan, R. Alcalde, J. L. Trenzado and S. Aparicio, J. Mol. Liq., 2018, 255, 199–207 CrossRef.
- M. M. Hoffmann, E. D. Sylvester and J. W. Russo, J. Mol. Liq., 2014, 199, 175–183 CrossRef CAS.
- P. J. Carvalho, T. Regueira, L. Santos, J. Fernandez and J. A. P. Coutinho, J. Chem. Eng. Data, 2010, 55, 645–652 CrossRef CAS.
- Q. Zhou, L.-S. Wang and H.-P. Chen, J. Chem. Eng. Data, 2006, 51, 905–908 CrossRef CAS.
- I. B. Malham and M. Turmine, J. Chem. Thermodyn., 2008, 40, 718–723 CrossRef.
- J. S. Gao and N. J. Wagner, J. Mol. Liq., 2016, 223, 678–686 CrossRef CAS.
- S. D. Nickerson, E. M. Nofen, H. B. Chen, M. Ngan, B. Shindel, H. Y. Yu and L. L. Dai, J. Phys. Chem. B, 2015, 119, 8764–8772 CrossRef CAS PubMed.
- H. T. Xu, D. C. Zhao, P. Xu, F. Q. Liu and G. Gao, J. Chem. Eng. Data, 2005, 50, 133–135 CrossRef CAS.
- E. Gómez, B. González, Á. Domínguez, E. Tojo and J. Tojo, J. Chem. Eng. Data, 2006, 51, 696–701 CrossRef.
- V. Antón, H. Artigas, J. Muñoz-Embid, M. Artal and C. Lafuente, J. Chem. Thermodyn., 2016, 99, 116–123 CrossRef.
- B. Mokhtarani, A. Sharifi, H. R. Mortaheb, M. Mirzaei, M. Mafi and F. Sadeghian, J. Chem. Thermodyn., 2009, 41, 323–329 CrossRef CAS.
- D. Constantinescu, K. Schaber, F. Agel, M. H. Klingele and T. J. Schubert, J. Chem. Eng. Data, 2007, 52, 1280–1285 CrossRef CAS.
- A. Arce, A. Soto, J. Ortega and G. Sabater, J. Chem. Eng. Data, 2008, 53, 770–775 CrossRef CAS.
- Y. Xu, J. Chem. Thermodyn., 2013, 64, 126–133 CrossRef CAS.
- M. Anouti, A. Vigeant, J. Jacquemin, C. Brigouleix and D. Lemordant, J. Chem. Thermodyn., 2010, 42, 834–845 CrossRef CAS.
- M. Zawadzki, M. Królikowska and P. Lipiński, Thermochim. Acta, 2014, 589, 148–157 CrossRef CAS.
- J. A. Widegren, E. M. Saurer, K. N. Marsh and J. W. Magee, J. Chem. Thermodyn., 2005, 37, 569–575 CrossRef CAS.
- H. Wang, J. Wang, S. Zhang, Y. Pei and K. Zhuo, ChemPhysChem, 2009, 10, 2516–2523 CrossRef CAS PubMed.
- H. Shekaari, Y. Mansoori and R. Sadeghi, J. Chem. Thermodyn., 2008, 40, 852–859 CrossRef CAS.
- J. M. Zhang, W. Z. Wu, T. Jiang, H. X. Gao, Z. M. Liu, J. He and B. X. Han, J. Chem. Eng. Data, 2003, 48, 1315–1317 CrossRef CAS.
- C. E. Woodward and K. R. Harris, Phys. Chem. Chem. Phys., 2010, 12, 1172–1176 RSC.
- W. Liu, L. Cheng, Y. Zhang, H. Wang and M. Yu, J. Mol. Liq., 2008, 140, 68–72 CrossRef CAS.
- G. R. Chaudhary, S. Bansal, S. K. Mehta and A. S. Ahluwalia, J. Solution Chem., 2014, 43, 340–359 CrossRef CAS.
- J. M. Andanson, M. Traikia and P. Husson, J. Chem. Thermodyn., 2014, 77, 214–221 CrossRef CAS.
- M. Sha, H. Dong, F. Luo, Z. Tang, G. Zhu and G. Wu, J. Phys. Chem. Lett., 2015, 6, 3713–3720 CrossRef CAS PubMed.
- Q. W. Yang, K. Yu, H. B. Xing, B. G. Su, Z. B. Bao, Y. W. Yang and Q. L. Ren, J. Ind. Eng. Chem., 2013, 19, 1708–1714 CrossRef CAS.
- J. Vila, P. Gines, E. Rilo, O. Cabeza and L. M. Varela, Fluid Phase Equilib., 2006, 247, 32–39 CrossRef CAS.
- E. Rilo, J. Vila, S. Garcia-Garabal, L. M. Varela and O. Cabeza, J. Phys. Chem. B, 2013, 117, 1411–1418 CrossRef CAS PubMed.
- N. Yaghini, L. Nordstierna and A. Martinelli, Phys. Chem. Chem. Phys., 2014, 16, 9266–9275 RSC.
- N. J. English, D. A. Mooney and S. W. O'Brien, J. Mol. Liq., 2010, 157, 163–167 CrossRef CAS.
- E. H. Duan, Y. N. Guan, B. Guo, M. M. Zhang, D. Yang and K. Yang, J. Mol. Liq., 2013, 178, 1–4 CrossRef CAS.
- P. B. Sanchez, J. Garcia, J. Salgado and E. Gonzalez-Romero, ACS Sustainable Chem. Eng., 2016, 4, 5068–5077 CrossRef CAS.
- K. R. Siongco, R. B. Leron, A. R. Caparanga and M.-H. Li, Thermochim. Acta, 2013, 566, 50–56 CrossRef CAS.
- B. Wu, Y. M. Zhang and H. P. Wang, J. Chem. Eng. Data, 2009, 54, 1430–1434 CrossRef CAS.
- C. L. Wong, A. N. Soriano and M. H. Li, J. Taiwan Inst. Chem. Eng., 2009, 40, 77–83 CrossRef CAS.
- M. Bester-Rogac, A. Stoppa, J. Hunger, G. Hefter and R. Buchner, Phys. Chem. Chem. Phys., 2011, 13, 17588–17598 RSC.
- Y. J. Chen, X. P. Xuan, H. H. Zhang and K. L. Zhuo, Fluid Phase Equilib., 2012, 316, 164–171 CrossRef CAS.
- Y. J. Chen, M. Y. Fang, G. Y. Bai, K. L. Zhuo and C. L. Yan, J. Chem. Eng. Data, 2016, 61, 3779–3787 CrossRef CAS.
- K. L. Zhuo, Y. J. Chen, J. Chen, G. Y. Bai and J. J. Wang, Phys. Chem. Chem. Phys., 2011, 13, 14542–14549 RSC.
- M. Bester-Rogac, J. Hunger, A. Stoppa and R. Buchner, J. Chem. Eng. Data, 2011, 56, 1261–1267 CrossRef CAS.
- R. Sadeghi and N. Ebrahimi, J. Phys. Chem. B, 2011, 115, 13227–13240 CrossRef CAS PubMed.
- C. Neves, A. R. Rodrigues, K. A. Kurnia, J. Esperanca, M. G. Freire and J. A. P. Coutinho, Fluid Phase Equilib., 2013, 358, 50–55 CrossRef CAS.
- C. Ma, Y. Guo, D. Li, J. Zong, X. Ji and C. Liu, J. Chem. Thermodyn., 2017, 105, 30–36 CrossRef CAS.
- C. Ma, Y. Guo, D. Li, J. Zong, X. Ji, C. Liu and X. Lu, J. Chem. Eng. Data, 2016, 61, 4172–4177 CrossRef CAS.
- L. E. Ficke, R. R. Novak and J. F. Brennecke, J. Chem. Eng. Data, 2010, 55, 4946–4950 CrossRef CAS.
- L. E. Ficke and J. F. Brennecke, J. Phys. Chem. B, 2010, 114, 10496–10501 CrossRef CAS PubMed.
- G. García-Miaja, J. Troncoso and L. Romaní, J. Chem. Thermodyn., 2009, 41, 161–166 CrossRef.
- L. E. Ficke, H. c. Rodríguez and J. F. Brennecke, J. Chem. Eng. Data, 2008, 53, 2112–2119 CrossRef CAS.
- M. Leskiv, C. E. S. Bernardes, M. E. Minas da Piedade and J. N. Canongia Lopes, J. Phys. Chem. B, 2010, 114, 13179–13188 CrossRef CAS PubMed.
- V. K. Sharma, S. Bhagour, S. Solanki, Sheetal and S. K. Jangra, J. Chem. Thermodyn., 2014, 68, 235–243 CrossRef CAS.
- T. Hector and J. Gmehling, Fluid Phase Equilib., 2014, 371, 82–92 CrossRef CAS.
- J. Ren, Z. Zhao and X. Zhang, J. Chem. Thermodyn., 2011, 43, 576–583 CrossRef CAS.
- X. Zhang, D. Hu and Z. Zhao, J. Chem. Eng. Data, 2014, 59, 205–211 CrossRef CAS.
- L. P. N. Rebelo, V. Najdanovic-Visak, Z. P. Visak, M. Nunes da Ponte, J. Szydlowski, C. A. Cerdeirina, J. Troncoso, L. Romani, J. M. S. S. Esperanca, H. J. R. Guedes and H. C. de Sousa, Green Chem., 2004, 6, 369–381 RSC.
- M. Teodorescu and V. T. Popa, Rev. Roum. Chim., 2016, 61, 525–530 Search PubMed.
- W. Hermann, Angew. Chem., Int. Ed., 2008, 47, 654–670 CrossRef PubMed.
- J. Ortega, R. Vreekamp, E. Marrero and E. Penco, J. Chem. Eng. Data, 2007, 52, 2269–2276 CrossRef CAS.
- J. Ortega, R. Vreekamp, E. Penco and E. Marrero, J. Chem. Thermodyn., 2008, 40, 1087–1094 CrossRef CAS.
- R. Vreekamp, D. Castellano, J. Palomar, J. Ortega, F. Espiau, L. Fernández and E. Penco, J. Phys. Chem. B, 2011, 115, 8763–8774 CrossRef CAS PubMed.
- A. Navas, J. Ortega, R. Vreekamp, E. Marrero and J. Palomar, Ind. Eng. Chem. Res., 2009, 48, 2678–2690 CrossRef CAS.
- M. Królikowska, K. Paduszyński, M. Królikowski, P. Lipiński and J. Antonowicz, Ind. Eng. Chem. Res., 2014, 53, 258–262 CrossRef.
- M. Królikowska, K. Paduszyński, T. Hofman and J. Antonowicz, J. Chem. Thermodyn., 2012, 55, 144–150 CrossRef.
- M. Królikowska, K. Paduszyński and M. Zawadzki, J. Chem. Eng. Data, 2013, 58, 285–293 CrossRef.
- O. Cabeza, in Ionic Liquids in Separation Technology, ed. F. J. H. Fernández, Elsevier, Amsterdam, 2014, pp. 1–93 Search PubMed.
- J.-Y. Wang, X.-J. Zhang, Y.-M. Liu and Y.-Q. Hu, J. Chem. Eng. Data, 2011, 56, 3734–3737 CrossRef CAS.
- C. Spickermann, J. Thar, S. B. C. Lehmann, S. Zahn, J. Hunger, R. Buchner, P. A. Hunt, T. Welton and B. Kirchner, J. Chem. Phys., 2008, 129, 104505 CrossRef CAS PubMed.
- R. C. Remsing, Z. W. Liu, I. Sergeyev and G. Moyna, J. Phys. Chem. B, 2008, 112, 7363–7369 CrossRef CAS PubMed.
- P. D'Angelo, A. Serva, G. Aquilanti, S. Pascarelli and V. Migliorati, J. Phys. Chem. B, 2015, 119, 14515–14526 CrossRef PubMed.
- C. E. S. Bernardes, M. E. M. da Piedade and J. N. C. Lopes, J. Phys. Chem. B, 2011, 115, 2067–2074 CrossRef CAS PubMed.
- X. J. Zhong, Z. Fan, Z. P. Liu and D. P. Cao, J. Phys. Chem. B, 2012, 116, 3249–3263 CrossRef CAS PubMed.
- S. V. J. Yuvaraj, R. K. Zhdanov, R. V. Belosludov, V. R. Belosludov, O. S. Subbotin, K. Kanie, K. Funaki, A. Muramatsu, T. Nakamura and Y. Kawazoe, J. Phys. Chem. B, 2015, 119, 12894–12904 CrossRef CAS PubMed.
- T. M. Chang, L. X. Dang, R. Devanathan and M. Dupuis, J. Phys. Chem. A, 2010, 114, 12764–12774 CrossRef CAS PubMed.
- T. Zhekenov, N. Toksanbayev, Z. Kazakbayeva, D. Shah and F. S. Mjalli, Fluid Phase Equilib., 2017, 441, 43–48 CrossRef CAS.
- E. O. Fetisov, D. B. Harwood, I. F. W. Kuo, S. E. E. Warrag, M. C. Kroon, C. J. Peters and J. I. Siepmann, J. Phys. Chem. B, 2018, 122, 1245–1254 CrossRef CAS PubMed.
- Q. Gao, Y. Zhu, X. Ji, W. Zhu, L. Lu and X. Lu, Fluid Phase Equilib., 2018, 470, 134–139 CrossRef CAS.
- R. Ahmadi, B. Hemmateenejad, A. Safavi, Z. Shojaeifard, A. Shahsavar, A. Mohajeri, M. Heydari Dokoohaki and A. R. Zolghadr, Phys. Chem. Chem. Phys., 2018, 20, 18463–18473 RSC.
- W. Jiang, Y. T. Wang and G. A. Voth, J. Phys. Chem. B, 2007, 111, 4812–4818 CrossRef CAS PubMed.
- S. Feng and G. A. Voth, Fluid Phase Equilib., 2010, 294, 148–156 CrossRef CAS.
- A. A. Niazi, B. D. Rabideau and A. E. Ismail, J. Phys. Chem. B, 2013, 117, 1378–1388 CrossRef CAS PubMed.
- Y. L. Wang, M. R. Shimpi, S. Sarman, O. N. Antzutkin, S. Glavatskih, L. Kloo and A. Laaksonen, J. Phys. Chem. B, 2016, 120, 7446–7455 CrossRef CAS PubMed.
- Y.-L. Wang, S. Sarman, S. Glavatskih, O. N. Antzutkin, M. W. Rutland and A. Laaksonen, J. Phys. Chem. B, 2015, 119, 5251–5264 CrossRef CAS PubMed.
- X. Wu, Z. Liu, S. Huang and W. Wang, Phys. Chem. Chem. Phys., 2005, 7, 2771–2779 RSC.
- M. S. Kelkar, W. Shi and E. J. Maginn, Ind. Eng. Chem. Res., 2008, 47, 9115–9126 CrossRef CAS.
- S. L. Perkins, P. Painter and C. M. Colina, J. Phys. Chem. B, 2013, 117, 10250–10260 CrossRef CAS PubMed.
- T. G. A. Youngs and C. Hardacre, ChemPhysChem, 2008, 9, 1548–1558 CrossRef CAS PubMed.
- R. Ishizuka and N. Matubayasi, J. Chem. Theory Comput., 2016, 12, 804–811 CrossRef CAS PubMed.
- Y. Wang, H. Li and S. Han, J. Phys. Chem. B, 2006, 110, 24646–24651 CrossRef CAS PubMed.
- M. L. S. Batista, K. A. Kurnia, S. P. Pinho, J. R. B. Gomes and J. A. P. Coutinho, J. Phys. Chem. B, 2015, 119, 1567–1578 CrossRef CAS PubMed.
- I. Khan, M. Taha, S. P. Pinho and J. A. P. Coutinho, Fluid Phase Equilib., 2016, 414, 93–100 CrossRef CAS.
- I. Khan, K. A. Kurnia, T. E. Sintra, J. A. Saraiva, S. P. Pinho and J. A. P. Coutinho, Fluid Phase Equilib., 2014, 361, 16–22 CrossRef CAS.
- I. Khan, K. A. Kurnia, F. Mutelet, S. P. Pinho and J. A. P. Coutinho, J. Phys. Chem. B, 2014, 118, 1848–1860 CrossRef CAS PubMed.
- I. Khan, M. Taha, P. Ribeiro-Claro, S. P. Pinho and J. A. P. Coutinho, J. Phys. Chem. B, 2014, 118, 10503–10514 CrossRef CAS PubMed.
- X. Y. Zhu and H. Q. Ai, J. Mol. Model., 2016, 22 CAS.
- D. V. Wagle, C. A. Deakyne and G. A. Baker, J. Phys. Chem. B, 2016, 120, 6739–6746 CrossRef CAS PubMed.
- L. Zhang, Z. Xu, Y. Wang and H. Li, J. Phys. Chem. B, 2008, 112, 6411–6419 CrossRef CAS PubMed.
- A. Mele, C. D. Tran and S. H. De Paoli Lacerda, Angew. Chem., Int. Ed., 2003, 115, 4500–4502 CrossRef.
- L. Almásy, M. Turmine and A. Perera, J. Phys. Chem. B, 2008, 112, 2382–2387 CrossRef PubMed.
- T. Toshiyuki, H. Yusuke, F. Kenta and K. Shigeharu, Anal. Sci., 2008, 24, 1285–1290 CrossRef.
- H. Zhang, H. Liang, J. Wang and K. Li, Z. Phys. Chem., 2007, 221, 1061 CrossRef CAS.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC.
- P. A. Bergstroem, J. Lindgren and O. Kristiansson, J. Phys. Chem., 1991, 95, 8575–8580 CrossRef CAS.
- A.-L. Rollet, P. Porion, M. Vaultier, I. Billard, M. Deschamps, C. Bessada and L. Jouvensal, J. Phys. Chem. B, 2007, 111, 11888–11891 CrossRef CAS PubMed.
- T. Köddermann, C. Wertz, A. Heintz and R. Ludwig, Angew. Chem., Int. Ed., 2006, 45, 3697–3702 CrossRef PubMed.
- D. K. Singh, S. Cha, D. Nam, H. Cheong, S. W. Joo and D. Kim, ChemPhysChem, 2016, 17, 3040–3046 CrossRef CAS PubMed.
- N.-N. Wang, Q.-G. Zhang, F.-G. Wu, Q.-Z. Li and Z.-W. Yu, J. Phys. Chem. B, 2010, 114, 8689–8700 CrossRef CAS PubMed.
- D. J. S. Patinha, L. C. Tomé, H. Garcia, R. Ferreira, C. S. Pereira, L. P. N. Rebelo and I. M. Marrucho, J. Chem. Thermodyn., 2015, 84, 93–100 CrossRef CAS.
- S. Peter, F. Koichi and L. Ralf, Angew. Chem., Int. Ed., 2013, 52, 2990–2994 CrossRef PubMed.
- U. Salma, N. V. Plechkova, R. Caminiti and L. Gontrani, J. Phys. Chem. B, 2017, 121, 6399–6407 CrossRef CAS PubMed.
- A. Pandey and S. Pandey, J. Phys. Chem. B, 2014, 118, 14652–14661 CrossRef CAS PubMed.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Green Chem., 2016, 18, 2736–2744 RSC.
- C. D'Agostino, L. F. Gladden, M. D. Mantle, A. P. Abbott, E. I. Ahmed, A. Y. M. Al-Murshedi and R. C. Harris, Phys. Chem. Chem. Phys., 2015, 17, 15297–15304 RSC.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS PubMed.
- D. Xiao, J. R. Rajian, A. Cady, S. Li, R. A. Bartsch and E. L. Quitevis, J. Phys. Chem. B, 2007, 111, 4669–4677 CrossRef CAS PubMed.
- G. Raabe and J. Köhler, J. Chem. Phys., 2008, 128, 154509 CrossRef PubMed.
- Y. Imai, H. Abe, T. Miyashita, T. Goto, H. Matsumoto and Y. Yoshimura, Chem. Phys. Lett., 2010, 486, 37–39 CrossRef CAS.
- Y. Imai, H. Abe, H. Matsumoto, O. Shimada, T. Hanasaki and Y. Yoshimura, J. Chem. Thermodyn., 2011, 43, 319–322 CrossRef CAS.
- Y. Yoshimura, H. Kimura, C. Okamoto, T. Miyashita, Y. Imai and H. Abe, J. Chem. Thermodyn., 2011, 43, 410–412 CrossRef CAS.
- P. Sippel, V. Dietrich, D. Reuter, M. Aumüller, P. Lunkenheimer, A. Loidl and S. Krohns, J. Mol. Liq., 2016, 223, 635–642 CrossRef CAS.
- N. Yaghini, M. N. Garaga and A. Martinelli, Fuel Cells, 2016, 16, 46–54 CrossRef CAS.
- H. Katayanagi, K. Nishikawa, H. Shimozaki, K. Miki, P. Westh and Y. Koga, J. Phys. Chem. B, 2004, 108, 19451–19457 CrossRef CAS.
- K. Miki, P. Westh, K. Nishikawa and Y. Koga, J. Phys. Chem. B, 2005, 109, 9014–9019 CrossRef CAS PubMed.
- K. R. Seddon, A. Stark and M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275–2287 CAS.
- M. Abraham and M.-C. Abraham, Electrochim. Acta, 1986, 31, 821–829 CrossRef CAS.
- R. L. Gardas, D. H. Dagade, J. A. P. Coutinho and K. J. Patil, J. Phys. Chem. B, 2008, 112, 3380–3389 CrossRef CAS PubMed.
- S. Panda, V. Singh, N. Islam and R. L. Gardas, Fluid Phase Equilib., 2017, 445, 35–44 CrossRef CAS.
- M. Rumyantsev, S. Rumyantsev and I. Y. Kalagaev, J. Phys. Chem. B, 2018, 122, 5951–5960 CrossRef CAS PubMed.
- J. E. S. J. Reid, R. J. Gammons, J. M. Slattery, A. J. Walker and S. Shimizu, J. Phys. Chem. B, 2017, 121, 599–609 CrossRef CAS PubMed.
- M.-M. Huang, Y. Jiang, P. Sasisanker, G. W. Driver and H. Weingärtner, J. Chem. Eng. Data, 2011, 56, 1494–1499 CrossRef CAS.
- N. V. Sastry, N. M. Vaghela and P. M. Macwan, J. Mol. Liq., 2013, 180, 12–18 CrossRef CAS.
- S. Zhang, X. Li, H. Chen, J. Wang, J. Zhang and M. Zhang, J. Chem. Eng. Data, 2004, 49, 760–764 CrossRef CAS.
- E. Rilo, J. Vila, J. Pico, S. García-Garabal, L. Segade, L. M. Varela and O. Cabeza, J. Chem. Eng. Data, 2010, 55, 639–644 CrossRef CAS.
- E. Rilo, J. Vila, M. García, L. M. Varela and O. Cabeza, J. Chem. Eng. Data, 2010, 55, 5156–5163 CrossRef CAS.
- L. Q. Zhang, Z. Xu, Y. Wang and H. R. Li, J. Phys. Chem. B, 2008, 112, 6411–6419 CrossRef CAS PubMed.
- J. Lehmann, M. H. Rausch, A. Leipertz and A. P. Fröba, J. Chem. Eng. Data, 2010, 55, 4068–4074 CrossRef CAS.
- B. Lal, M. Sahin and E. Ayranci, J. Chem. Thermodyn., 2012, 54, 142–147 CrossRef CAS.
- U. Domańska and K. Paduszyński, J. Phys. Chem. B, 2008, 112, 11054–11059 CrossRef PubMed.
- S. Nebig and J. Gmehling, Fluid Phase Equilib., 2011, 302, 220–225 CrossRef CAS.
- K. Paduszyński and U. Domańska, J. Phys. Chem. B, 2012, 116, 5002–5018 CrossRef PubMed.
- G. Shen, C. Held, X. Lu and X. Ji, Fluid Phase Equilib., 2015, 405, 73–82 CrossRef CAS.
- X. Xu, C. Peng, H. Liu and Y. Hu, Ind. Eng. Chem. Res., 2009, 48, 11189–11201 CrossRef CAS.
- L. D. Simoni, L. E. Ficke, C. A. Lambert, M. A. Stadtherr and J. F. Brennecke, Ind. Eng. Chem. Res., 2010, 49, 3893–3901 CrossRef CAS.
- S. Mahato, A. Kumar and T. Banerjee, J. Taiwan Inst. Chem. Eng., 2016, 59, 69–78 CrossRef CAS.
- N. Papaiconomou, J.-P. Simonin and O. Bernard, J. Chem. Thermodyn., 2015, 91, 445–451 CrossRef CAS.
- X. Ji and C. Held, Fluid Phase Equilib., 2016, 410, 9–22 CrossRef CAS.
- G. Shen, C. Held, X. Lu and X. Ji, Mol. Phys., 2016, 114, 2492–2499 CrossRef CAS.
- R. Shahriari, M. R. Dehghani and B. Behzadi, Ind. Eng. Chem. Res., 2012, 51, 10274–10282 CrossRef CAS.
- J. Li, C. He, C. Peng, H. Liu, Y. Hu and P. Paricaud, Ind. Eng. Chem. Res., 2011, 50, 7027–7040 CrossRef CAS.
- C. Tsioptsias, I. Tsivintzelis and C. Panayiotou, Phys. Chem. Chem. Phys., 2010, 12, 4843–4851 RSC.
- B.-S. Lee and S.-T. Lin, Fluid Phase Equilib., 2013, 356, 309–320 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |