Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents
Adam C.
Sedgwick
 ab,
Luling
Wu
ab,
Luling
Wu
 a,
Hai-Hao
Han
a,
Hai-Hao
Han
 c,
Steven D.
Bull
c,
Steven D.
Bull
 *a,
Xiao-Peng
He
*a,
Xiao-Peng
He
 *c,
Tony D.
James
*c,
Tony D.
James
 *ad,
Jonathan L.
Sessler
*ad,
Jonathan L.
Sessler
 *b,
Ben Zhong
Tang
*b,
Ben Zhong
Tang
 *e,
He
Tian
*e,
He
Tian
 *c and
Juyoung
Yoon
*c and
Juyoung
Yoon
 *f
*f
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: s.d.bull@bath.ac.uk; t.d.james@bath.ac.uk
bDepartment of Chemistry, University of Texas at Austin, 105 E 24th street A5300, Austin, TX 78712-1224, USA. E-mail: sessler@cm.utexas.edu
cKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Rd, Shanghai 200237, China. E-mail: xphe@ecust.edu.cn; tianhe@ecust.edu.cn
dDepartment of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554, Japan
eDepartment of Chemistry, The Hong Kong University of Science & Technology (HKUST), Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
fDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 120-750, Korea. E-mail: jyoon@ewha.ac.kr
First published on 26th October 2018
Abstract
In this review we will explore recent advances in the design and application of excited-state intramolecular proton-transfer (ESIPT) based fluorescent probes. Fluorescence based sensors and imaging agents (probes) are important in biology, physiology, pharmacology, and environmental science for the selective detection of biologically and/or environmentally important species. The development of ESIPT-based fluorescence probes is particularly attractive due to their unique properties, which include a large Stokes shift, environmental sensitivity and potential for ratiometric sensing.
Introduction
Fluorescence based sensors and imaging agents have been routinely used in biology, physiology, pharmacology, and environmental science for the selective detection of biologically and/or environmentally important species for around 150 years. The use of fluorescent probes has revolutionised our understanding of a range of physiological or pathological processes and have provided effective sensing protocols for the detection of harmful species that may have been released into the environment (aqueous and gaseous). Despite the significant progress made in this exciting field, a number of challenges, including ease-of-use, selectivity, and sensitivity, still remain. The challenges are compounded by the increasingly stringent requirements for detection that have been introduced by regulatory bodies, which means that current fluorescent probes may not be suitable for use in a real-world setting.1,2a,bFluorescent probes use either specific host–guest interactions, or a selective chemical reaction to produce a change in the fluorescence properties of the system. When the binding between the host and guest is non-covalent and reversible, then the fluorescent probe is known as a chemosensor. However, if the interaction between the host and guest produces an irreversible chemical reaction, then the fluorescent probe is referred to as a chemidosimeter.3–6 Over the years, these two definitions, as well as the term ‘fluorescent probe’, have been used interchangeably (and ambiguously), therefore for the purpose of this review both types of sensor will be referred to as fluorescent probes. Traditionally, three classic fluorescence mechanisms have been exploited to generate a fluorescence response, namely PET (Photoinduced Electron Transfer), FRET (Förster Resonance Energy Transfer) and ICT (Internal Charge Transfer). In this review, we will focus on probes that use excited-state intramolecular proton transfer (ESIPT), or a combination of fluorescence mechanisms (AIE/ESIPT, PET/ESIPT, ICT/ESIPT or FRET/ESIPT) for the detection of biologically and/or environmentally important species.
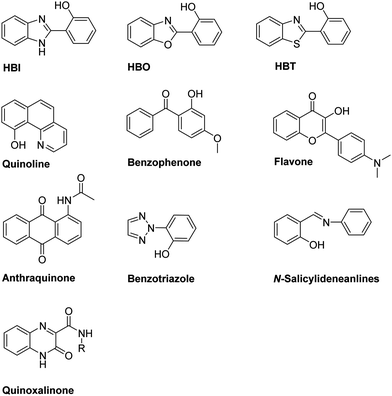
The process of excited-state intramolecular proton transfer (ESIPT) was first reported by Weller in the 1950s for salicylic acid.7 Since then, the photophysics of ESIPT has been extensively studied and applied to a range of applications.8–11 In general, molecules can exhibit ESIPT fluorescence if their structures incorporate an intramolecular hydrogen bonding interaction between a hydrogen bond donor (–OH and NH2) and a hydrogen bond acceptor (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and C
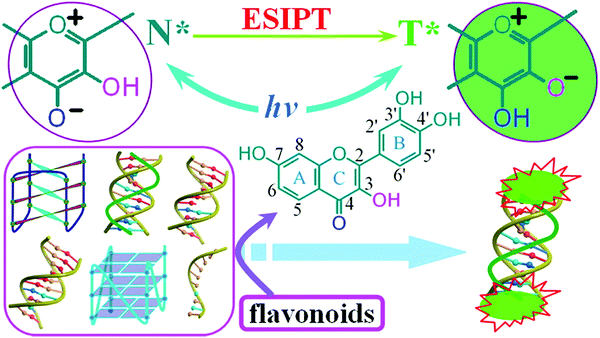
N– and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The most common ESIPT fluorophores are analogues of 2-(2′-hydroxyphenyl)benzimidazole (HBI), 2-(2′-hydroxyphenyl)benzoxazole (HBO) and 2-(2′-hydroxyphenyl)benzothiazole (HBT). Additional ESIPT fluorophores that have been reported include quinoline,12 benzophenones,13 flavones,14 anthraquinones,15 benzotriazoles,16N-salicylideneaniline,17 and quinoxalines,18 as illustrated in Fig. 1.
O). The most common ESIPT fluorophores are analogues of 2-(2′-hydroxyphenyl)benzimidazole (HBI), 2-(2′-hydroxyphenyl)benzoxazole (HBO) and 2-(2′-hydroxyphenyl)benzothiazole (HBT). Additional ESIPT fluorophores that have been reported include quinoline,12 benzophenones,13 flavones,14 anthraquinones,15 benzotriazoles,16N-salicylideneaniline,17 and quinoxalines,18 as illustrated in Fig. 1.
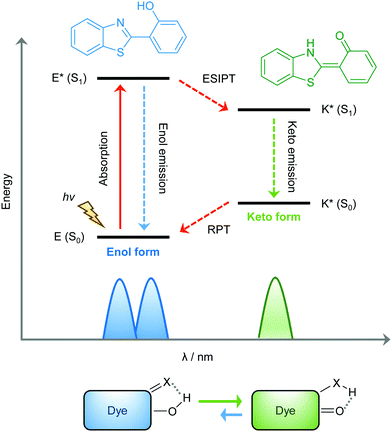
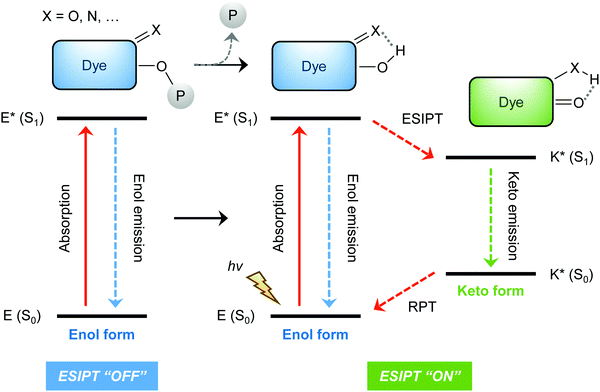
ESIPT is a unique four-level photochemical process, with the electronic ground state of ESIPT fluorophores typically existing in an enol (E) form. Upon photoexcitation, the electronic charge of such molecules can be redistributed, resulting in greater acidity for the hydrogen bond donor group and increased basicity for the hydrogen bond acceptor within the E form. As a result, an extremely fast enol to keto phototautomerization (kESIPT > 1012 s−1) event takes place, with the excited state enol form (E*) rapidly converting to its excited keto form (K*). After decaying radiatively back to its electronic ground state, a reverse proton transfer (RPT) takes place to produce the original E form (see Scheme 1).
Because of this rapid four-level photochemical process, ESIPT fluorophores are characterized by several particularly attractive features that make them useful as fluorescent probes and imaging agents. For instance, ESIPT fluorophores have an unusually large Stokes shift (∼200 nm) when compared to traditional fluorophores (fluorescein, rhodamine, etc.). This helps avoid unwanted self-reabsorption and inner-filter effects. Moreover, due to the transient nature of the four-level photochemical process, the ESIPT emission is highly sensitive to its local surroundings. Consequently, the presence of polar and hydrogen bond donating solvents can result in inhibition of the ESIPT process with the keto (K*) emission often not being observed. Therefore, many ESIPT fluorescent probes require a large ratio of organic solvent, or cetyltrimethylammonium bromide (CTAB), to create a sufficiently hydrophobic micellar environment to produce a fluorescence response.19,20 Due to the importance of the environment in ESIPT fluorescence, in this review we have reported the measurement conditions in the text and for all figures and schemes. Most ESIPT fluorophores can be used for ratiometric sensing because they exhibit dual-emission spectra arising from the excited state enol (E*) and the excited state keto (K*) emissions. This is particularly useful for fluorescence detection of biologically and/or environmentally important species, because ratiometric probes provide direct information about the concentration of the target analyte without the need for calibration.21
The general strategy for the development of ESIPT-based fluorescent probes is based on a design that involves blocking the hydrogen bond donor of the ESIPT fluorophore with a reactive unit specific that prevents the ESIPT process. As a result, only enol emission is observed, because no exchangeable protons are available. However, exposure of a reactive unit in the probe to a specific analyte allows access to the keto form which results in the ESIPT process being turned on (Scheme 2).
 | ||
| Scheme 2 Diagrammatic representation of the most common strategy underlying the design of ESIPT-based fluorescent probes. | ||
ESIPT fluorescence-based sensors for cations
Proton (H+)
The proton (H+) is the smallest but most important cationic species involved in physiological and pathological processes. Damage to transporters that maintain pH homeostasis is known to result in cellular dysfunction and metabolic disruption that can lead to disease.22,23 Therefore, monitoring changes in the pH of biological systems can provide important insight into the development of different disease states.Sekar et al. combined HBI and HBT with fluorescein to afford ESIPT-fluorescein pH-based fluorescent probes (Fig. 2),24,25 that were able to detect pH values between 6–13. Fluorescein has a remarkably small Stokes shift of 18 nm; however, HBT-fluorescein and HBI-fluorescein displayed a remarkably large K* Stokes shift of 248 and 267 nm, respectively, at pH 7, thus demonstrating the benefit of the ESIPT fluorescent probe approach.
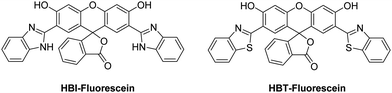 | ||
| Fig. 2 HBI-fluorescein and HBT-fluorescein for the use as pH fluorescent probes. Measurement conditions: 100% aqueous solution. | ||
Shuang et al. developed a series of oxime-appended spirobenzopyrans as fluorescent pH ratiometric probes 1-R that work in HEPES/ACN (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) buffer solutions – Scheme 3. Transformation of the acidochromic spirobenzopyran (closed) form to the merocyanine (open) form results in the ESIPT process taking place to provide a dual emission spectrum. Spirobenzopyran (R = tBu) provided a 68-fold increase in ratiometric intensities (I645/I522) when the pH decreased from 8.0 to 4.0 (pKa = 5.90).26
2) buffer solutions – Scheme 3. Transformation of the acidochromic spirobenzopyran (closed) form to the merocyanine (open) form results in the ESIPT process taking place to provide a dual emission spectrum. Spirobenzopyran (R = tBu) provided a 68-fold increase in ratiometric intensities (I645/I522) when the pH decreased from 8.0 to 4.0 (pKa = 5.90).26
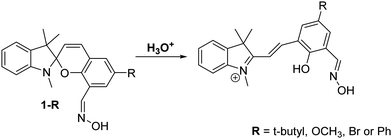 | ||
Scheme 3 Oxime-appended spirobenzopyrans 1-R for the use as pH fluorescent probes. Measurement conditions: HEPES/ACN (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) buffer solution. 2) buffer solution. | ||
Lysosomes are membrane-bound organelles with internal acidic environments (pH = 5), which enable a range of digestive enzymes to function. However, small changes to lysosomal pH can result in adverse effects that are associated with a number of diseases.27–29
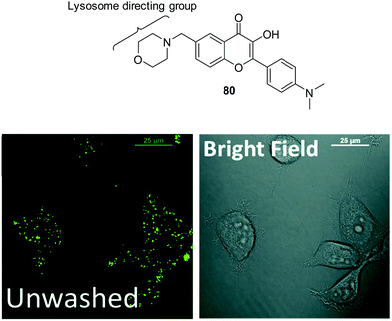
Zhang et al. have developed a new lysosome-targeted ratiometric fluorescent probe 2 (FR-Lys) by attaching morpholine to an ESIPT rhodol HBT fluorophore. The morpholine group serves as a targeting unit for the lysosome. Concurrently, the rhodol fragment can undergo a pH-modulated open/close reaction of its spirocyclic fragment; this enables the HBT moiety to provide a pH modulated ESIPT response (Scheme 4).30
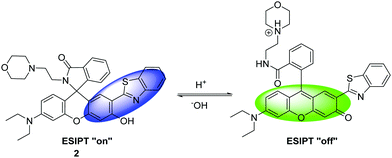 | ||
| Scheme 4 HBT-based rhodol fluorescent probe 2 for the monitoring of changes in pH in lysosomes. Measurement conditions: 100% PBS buffer (10 mM). | ||
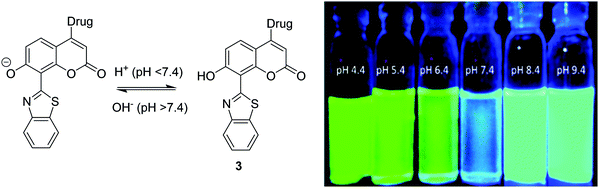
Interestingly, Singh et al. have reported a theranostic ESIPT pH probe 3 incorporating a HBT-7-hydroxy-coumarin fluorophore, which was then functionalised with a photocleavable unit containing the anticancer drug chlorambucil (Fig. 3). Probe 3 exhibited good ESIPT photophysical properties with a large Stokes shift of 151 nm. Moreover, this theranostic probe was shown to be pH sensitive. At physiological pH, the phenol is deprotonated, and no ESIPT takes place with only blue emission from the coumarin unit being observed. At the slightly acidic pH levels that are present in many cancer cells,31 the phenolate anion becomes protonated, thus turning on the ESIPT process that results in an increase in fluorescence emission at 516 nm. Photolysis of 3 using UV light at 365 nm was shown to result in efficient release of chlorambucil. Probe 3 was shown to internalize into cancer cells, while an MTT assay revealed low cytotoxicity towards the MDA-MB-231 cell line. Exposure to UV light, resulted in probe 3 demonstrating enhanced cytotoxicity due to the release of chlorambucil inside the cancer cell.32
Magnesium (Mg2+)
Magnesium (Mg2+) is an abundant element that plays a vital role in a number of cellular processes including cellular proliferation, a range of enzymatic reactions, cell death, DNA stabilisation and signal transduction.33 Changes in the concentrations of Mg2+ have been linked to a number of serious illnesses, including hypertension, diabetes, osteoporosis, renal failure and cardiac arrest. Consequently, it is of great importance to develop simple and efficient probes that allow for the monitoring of Mg2+ levels in real time.34Callan and co-workers designed a simple ESIPT probe 4 for the ratiometric detection of Mg2+. The Schiff base of 4 undergoes ESIPT to generate a keto tautomer, which enables efficient binding of Mg2+ (Scheme 5). Probe 4 was shown to display good selectivity and high sensitivity for the detection of low concentrations of Mg2+ (2 μM) in a THF/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system. It was shown to bind to Mg2+ with a 2
1) solvent system. It was shown to bind to Mg2+ with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and an association constant (K21) of (1.4 ± 0.1) × 104 M−2, meaning that it could be used potentially as a diagnostic probe for hypermagnesaemia (high level of Mg2+ in blood).35
1 stoichiometry and an association constant (K21) of (1.4 ± 0.1) × 104 M−2, meaning that it could be used potentially as a diagnostic probe for hypermagnesaemia (high level of Mg2+ in blood).35
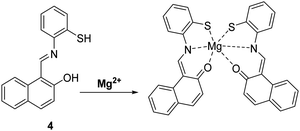 | ||
Scheme 5 A simple naphthalene Schiff base ESIPT fluorescent probe 4 for the detection of Mg2+. Measurement conditions: THF/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Zinc (Zn2+)
Zinc (Zn2+) is the second most abundant transition metal in nature, which acts as an essential element in a number of physiological processes.36,37 Changes in Zn2+ levels are closely associated with the onset of a number of diseases, including breast cancer, cerebral ischemia, Alzheimer's disease, Parkinson's disease, hypoxia-ischemia and epilepsy.38,39 Furthermore, a decrease in Zn2+ levels has been reported as a potential biomarker for the diagnosis of prostate cancer.40 Therefore, the ability to visualise Zn2+ in biological systems could potentially aid the understanding of its role in disease progression.Pang et al. developed a bis-HBO fluorescent probe 5 functionalised with the Zn2+-chelating di-(2-picolyl)amine (DPA) ligand. In the presence of Zn2+, probe 5 was shown to exhibit a large increase in enol fluorescence emission intensity (10-fold), which coincided with the appearance of a strong NIR keto emission peak at 710 nm (Scheme 6).41
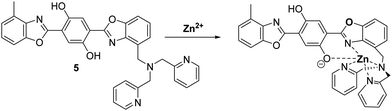 | ||
| Scheme 6 A bis-HBO fluorescent probe 5 for the detection of Zn2+ using the chelating ligand DPA. Measurement conditions: HEPES (50 mM, pH 7.2, 0.1 M KNO3 containing 5% DMSO). | ||
Tang et al. developed an ESIPT HBI-derived fluorescent probe 6 containing the Zn2+–ligand DPA, which had two fluorescence emission features due to a presumed ESIPT process. Addition of Zn2+ resulted in a significant ratiometric change in the fluorescence intensity due to inhibition of this ESIPT process (Scheme 7). Additionally, probe 6 overcame the limitations of other Zn2+-based probes by being able to clearly discriminate Zn2+ over Cd2+. Furthermore, the 6–Zn2+ complex exhibited a highly selective and ratiometric response towards S2−, caused by displacement of Zn2+ ions. This feature led to the suggestion that probe 6 could be used as a ratiometric probe to effect the sequential recognition of Zn2+ and S2− in aqueous media.42
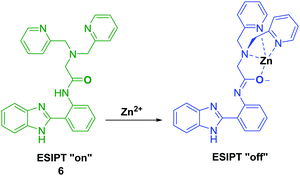 | ||
Scheme 7 Inhibition of the ESIPT process of 6 through the binding of Zn2+ using ligand DPA. Measurement conditions: HEPES/ACN (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (10 mM, pH 7.4). 2) (10 mM, pH 7.4). | ||
More recently, Yin and co-workers developed a thymolphthalein-HBI fluorophore 7, with hydroxyl and amino groups that provide a binding pocket for Zn2+ coordination – see Scheme 8. Probe 7 was shown to detect low concentrations of Zn2+ producing a rapid increase in fluorescence intensity within 5 seconds. In addition, probe 7 demonstrated excellent selectivity for Zn2+ over other metal ions and was shown to be able to image Zn2+ in living A549 cells.43
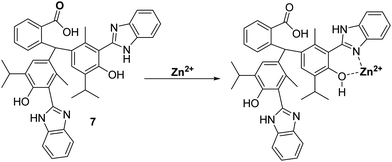 | ||
| Scheme 8 Thymolpthalein-based ESIPT fluorescent probe 7 for the detection of Zn2+ in A549 cells. Measurement conditions: EtOH. | ||
Kim et al. have developed a highly selective 2-(2′-tosylamidophenyl)thiazole fluorescent probe 8 for the detection of Zn2+ in ethanol (Scheme 9). It was shown to bind Zn2+ selectively in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry; however, it was also responsive to the presence of Cd2+. Bound Zn2+ could be displaced by the sequential addition of either hydrogen pyrophosphate or hydrogen phosphate, which led to an overall decrease in fluorescence intensity.44
1 stoichiometry; however, it was also responsive to the presence of Cd2+. Bound Zn2+ could be displaced by the sequential addition of either hydrogen pyrophosphate or hydrogen phosphate, which led to an overall decrease in fluorescence intensity.44
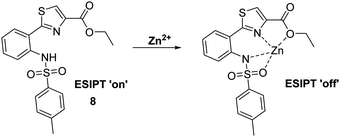 | ||
| Scheme 9 A thiazole-based ESIPT fluorescent probe 8 for the detection of Zn2+ through the inhibition of the ESIPT process. Measurement conditions: EtOH. | ||
Copper (Cu+ and Cu2+)
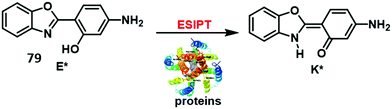
Copper is an essential element for all living species that acts as a redox active co-factor for a range of physiological processes, including respiration, cellular defence, signalling, metabolism and neurotransmitter biosynthesis.45 Dysregulation of copper has been associated with a number of diseases including neurodegeneration, which means the development of fluorescent probes for imaging copper in biological systems is highly desirable.46,47Govindaraju and co-workers developed a reaction-based HBT fluorescent probe 9 for the selective detection of Cu+ and Cu2+ (Scheme 10). Initially, the ESIPT process of probe 9 is blocked; however, the presence of Cu+/Cu2+ results in copper-mediated oxidative benzylic ether cleavage to release the HBT fluorophore. Probe 9 was shown to have a high sensitivity and an excellent selectivity for Cu+ (1 μM) in the presence of 2 mM glutathione (GSH), under conditions that mimic the reducing environment of the cell. Additionally, copper-mediated cleavage of probe 9 resulted in a large ratiometric change in the fluorescence intensity. This was taken as support for the notion that 9 could be used as a probe for copper in biological environments.48
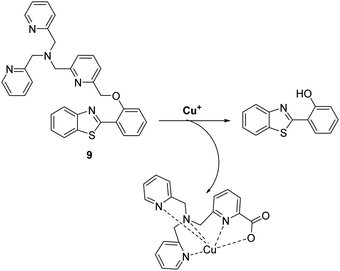 | ||
| Scheme 10 Reaction-based ESIPT fluorescent probe for the selective deprotection of probe 9 for the detection of Cu+. Measurement conditions: 50 mM HEPES, pH 7.2, 2 mM GSH. | ||
Similarly, Han et al. developed a reaction-based probe containing a HBT fluorophore for the selective detection of Cu2+. This system incorporates a picolinoyl ester unit that can undergo copper-mediated hydrolysis (Scheme 11). ESIPT of probe 10 is initially blocked; however, in the presence of Cu2+ a large increase in fluorescence intensity is observed. Probe 10 was shown to be selective and sensitive (LOD = 16.1 nM) for Cu2+ allowing it to be detected in living cells.49
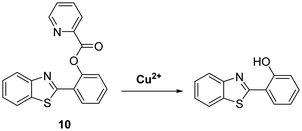 | ||
Scheme 11 Selective hydrolysis of picolinoyl ester of probe 10 and its use for the fluorescence detection of Cu2+. Measurement conditions: PBS/ACN (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) – pH 7.4. 2) – pH 7.4. | ||
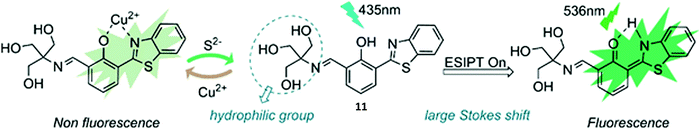
Guo et al. reported a reversible ESIPT fluorescent probe for the detection of Cu2+ and the sequential detection of S2− (Scheme 12). Probe 11 was shown to successfully bind to Cu2+ (10 μM), which resulted in quenching of the fluorescence emission intensity. Interestingly, the addition of S2− to Cu2+–11 restored the fluorescence emission due to displacement of Cu2+. Remarkably, a fluorescence “ON–OFF–ON” cycle could be repeated 5 times via the alternating addition of Cu2+ and S2−. Probe 11 displayed excellent selectivity for Cu2+ and a rapid response time. Excellent water solubility was conferred by the presence of its hydrophilic trihydroxyl group. A combination of these desirable features, enabled probe 11 to be used successfully to visualise Cu2+ and S2− in A549 cells.50
Similarly, Li et al. developed a HBT-derived fluorescent probe 12 for the reversible detection of Cu2+ and cysteine (Cys) (Scheme 13). Probe 12 was shown to coordinate Cu2+ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. This binding results in its fluorescence emission intensity being quenched due to the deactivation of its ESIPT process. Cys was shown to react with Cu2+–12 complex within 1 min to restore the initial fluorescence emission intensity, thus enabling probe 12 to be used to detect selectively exogenously administered Cu2+ and Cys in HeLa cells.51
1 stoichiometry. This binding results in its fluorescence emission intensity being quenched due to the deactivation of its ESIPT process. Cys was shown to react with Cu2+–12 complex within 1 min to restore the initial fluorescence emission intensity, thus enabling probe 12 to be used to detect selectively exogenously administered Cu2+ and Cys in HeLa cells.51
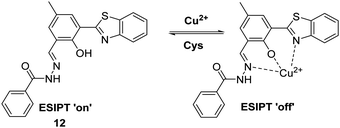 | ||
Scheme 13 An ESIPT-based fluorescent probe 12 for the reversible detection of Cu2+ and Cys. Measurement conditions: PBS/ACN (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), pH 7.4. 2), pH 7.4. | ||
Cobalt (Co2+)
Cobalt (Co2+) is an essential trace element that is present in cobalamins and metalloenzymes that catalyse a range of biological functions. Unfortunately, the high free redox activity of Co2+ makes it highly toxic to living cells. Therefore, the development of fluorescent probes for the detection of relatively low concentrations of free cobalt species is highly desirable.52Govindaraju et al. have developed a reaction-based HBT-based fluorescent probe 13 whose ESIPT process is initially blocked. In the presence of Co2+ oxidative cleavage at its benzylic position is facilitated (Scheme 14). This results in a remarkable ratiometric change in fluorescence intensity through the release of the HBT fluorophore. In fact, probe 13 demonstrates an excellent selectivity over other metal ions. Moreover, a minimum detection level for Co2+ of 20 μM was noted.48
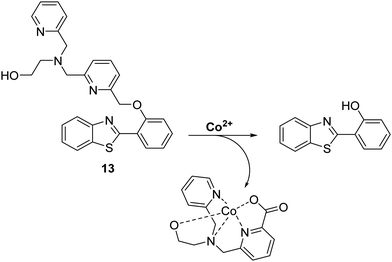 | ||
| Scheme 14 Reaction-based ESIPT fluorescent probe for Co2+ (13) and the selective deprotection events that underlie its efficacy. Measurement conditions: 50 mM HEPES, pH 7.2, 2 mM GSH. | ||
Iron (Fe2+ and Fe3+)
Iron is the most abundant transition metal found in the human body. It is essential to the functioning of numerous physiological processes. Iron homeostasis is normally tightly regulated, because excess levels of iron result in severe cell damage and organ damage through metal cation-mediated generation of excess reactive oxygen species (ROS).53 In fact, abnormal iron concentrations have been associated with several diseases including neurodegenerative diseases, anaemia, cancer and hepatitis.54,55Menges and co-workers developed a selective imidazole-based fluorescent ESIPT probe 14. This system was shown to bind to Fe3+ with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and “turn-off” the initial fluorescence (Scheme 15). These results suggest that this ESIPT fluorophore may be a highly promising sensing platform for the development of sensors for other analytes.56
1 stoichiometry and “turn-off” the initial fluorescence (Scheme 15). These results suggest that this ESIPT fluorophore may be a highly promising sensing platform for the development of sensors for other analytes.56
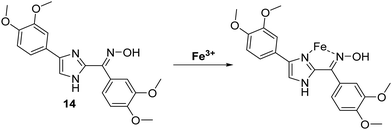 | ||
| Scheme 15 An imidazole-based ESIPT fluorescent probe 14 used for the fluorescence detection of Fe3+. Measurement conditions: DMSO. | ||
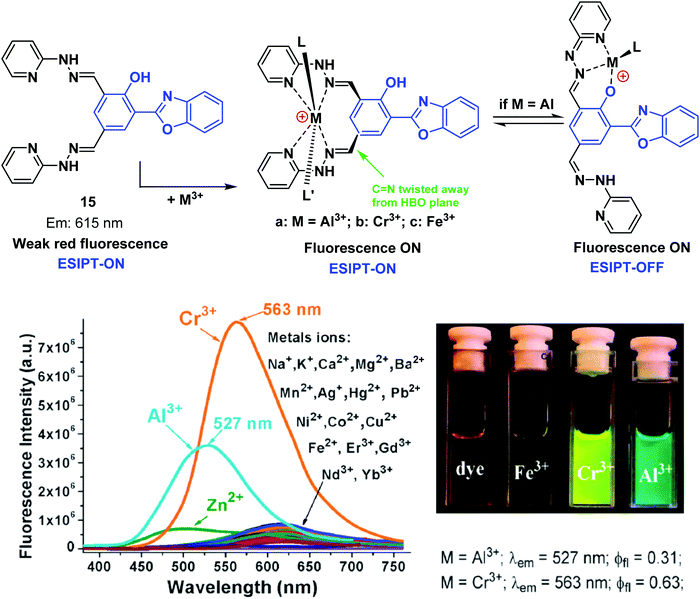
Pang and co-workers have developed a single ESIPT based fluorescent probe 15, that was used to selectively detect and discriminate between the trivalent metal ions Cr3+, Al3+, and Fe3+ in an H2O/EtOH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solvent system (Scheme 16). Probe 15 was functionalised with two hydrazine “Schiff bases”, which enabled selective binding of trivalent cations over divalent cations. A large increase in the fluorescence emission intensity was observed in the presence of an M3+ cation (Cr3+ and Al3+). Interestingly, an initial fluorescence increase was observed in the presence of Fe3+; however, the fluorescence emission was found to decrease over time, presumably as the result of tautomerisation. Probe 15 was shown to be effective at monitoring intracellular Cr3+ and Al3+ levels in human mesenchymal stem cells.57
2) solvent system (Scheme 16). Probe 15 was functionalised with two hydrazine “Schiff bases”, which enabled selective binding of trivalent cations over divalent cations. A large increase in the fluorescence emission intensity was observed in the presence of an M3+ cation (Cr3+ and Al3+). Interestingly, an initial fluorescence increase was observed in the presence of Fe3+; however, the fluorescence emission was found to decrease over time, presumably as the result of tautomerisation. Probe 15 was shown to be effective at monitoring intracellular Cr3+ and Al3+ levels in human mesenchymal stem cells.57
Mercury (Hg2+)
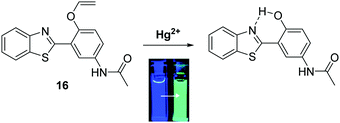
Mercury is a naturally occurring element found in the earth's crust that the World Health Organisation (WHO) considers to be one of the top ten chemicals with major public health concerns. Natural geographical events, such as erosion and volcanic eruptions, or industrial activities, such as smelting or chemical synthesis, are potential routes for the release of mercury into the environment.58 Mercury consumption can have devastating consequences to human health, as witnessed in Japan with the consumption of mercury species in contaminated fish that occurred in the Minamata incident.59 Therefore, it is extremely important to have access to methods for the qualitative and quantitative detection of mercury in drinking water.Ahn et al. developed a ratiometric fluorescent HBT ESIPT probe 16 for the detection of mercury species. The mode of action is based on mercury-promoted deprotection of a vinyl ether group to afford its corresponding phenol (Scheme 17). In the presence of mercury, the vinyl ether was found to be selectively hydrolysed. This turns on the ESIPT process, which resulted in a ratiometric change in fluorescence from blue to cyan emission. Probe 16 was shown to have high sensitivity (LOD = 20 ppb) and have an excellent selectively in detecting mercury species over various other metal ions. These attributes make probe 16 suitable for the analysis of drinking water. In fact, it was shown to react with mercury in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, thus indicating its potential for detecting highly toxic methylmercury.60
1 stoichiometry, thus indicating its potential for detecting highly toxic methylmercury.60
Yao et al. developed a novel ratiometric Hg2+ fluorescent probe 17 based on the ESIPT fluorophore, 2-(1-(p-tolyl)-1H-phenanthro[9,10-d]imidazol-2-yl)phenol, which incorporated a similar Hg2+ cleavable vinyl ether group (Scheme 18). The presence of Hg2+ resulted in a ratiometric change in the fluorescence intensity (I477/I380). A high sensitivity (LOD = 7.8 nM), in addition to an excellent selectively for Hg2+ over other metal ions, was seen. Additionally, it was shown that probe 17 has good cell permeability and could be used to visualise exogenously added Hg2+ in HeLa cells.61
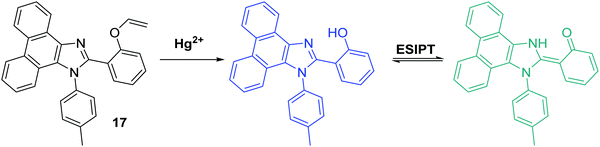 | ||
| Scheme 18 Vinyl protected probe 17 used for the ratiometric fluorescence detection of Hg2+ in aqueous solution and HeLa cells. Measurement conditions: PBS (pH 7.4) containing 1% ACN. | ||
Palladium (0, +2 and +4)
Palladium is used in a wide range of applications, including in dental appliances, as a catalyst for organic synthesis, and automotive emission control catalysts.62 Unfortunately, palladium at high concentrations is known to have toxic effects on biological systems, with low doses promoting allergic reactions.63 Therefore, given the potential occurrence of palladium in food, medicines, and water, there is significant interest in developing analytical methods to determine the concentrations of potential palladium contaminants.Bai et al. developed a ratiometric ESIPT flavone-based fluorescent probe 18 for the detection of Pd species that was shown to have good sensitivity (LOD for Pd(II) = 87 nM) and excellent selectivity over a range of other metal ions (Scheme 19). Probe 18 undergoes a Pd-catalysed depropargylation reaction to release the fluorophore whose phenolic group enables the ESIPT process to take place. Remarkably, probe 18 could be used to detect Pd(IV), Pd(II) and Pd(0) oxidation states, with Pd(II) and Pd(IV) producing the largest ratiometric changes in fluorescence intensity (I517/I412).64
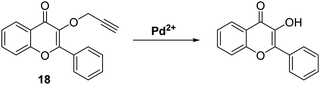 | ||
Scheme 19 Propargyl-protected probe 18 for the ratiometric fluorescence detection of Pd2+. Measurement conditions: HEPES/ACN (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Chen et al. developed two HBT-derived ESIPT-based fluorescent probes 19 and 20. Both rely on similar depropargylation reactions that are triggered by Pd2+. Again, this liberates a phenol, which allows the ESIPT process to occur (Fig. 4). Exposure to Pd2+, resulted in large increases in the fluorescence emission intensities of both probes. Interestingly, probe 20 showed greater sensitivity to Pd2+ than probe 19 (LOD = 14.6 nM vs. LOD = 285 nM, respectively). Both probes could be successfully incorporated into test strips that were able to detect low concentrations of Pd2+ (15 ppm) by eye.65
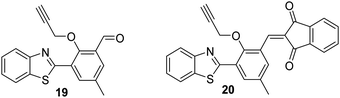 | ||
Fig. 4 Two ESIPT-based probes 19 and 20 that allow the detection of Pd2+. Measurement conditions: HEPES/THF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
Zeng et al. combined a HBT fluorophore with the electron acceptor 1,4-dimethylpyridin-1-ium iodide to develop a long-wavelength ESIPT fluorescent probe 21 that contains an extended π-electron framework. In the presence of Pd2+, probe 21 undergoes a palladium-mediated depropargylation reaction, resulting in a ratiometric change in fluorescence intensity (I635/I495) (Scheme 20). Probe 21 was shown to have the ability to detect multiple oxidation states of palladium (0, +2, +4). In the case of Pd2+, excellent selectivity and high sensitivity was seen with a LOD = 57 nM being recorded in a purely aqueous medium.66
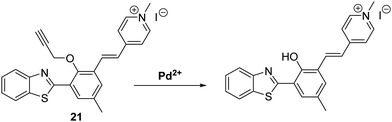 | ||
| Scheme 20 Propargyl-protected 21, a probe developed for the ratiometric fluorescence detection of Pd2+. Measurement conditions: PBS (10 mM, pH = 7.4). | ||
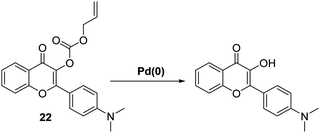
A different approach to palladium sensing was introduced by Liu et al. These researchers developed an allyl containing ESIPT dimethylamino-flavone probe 22 with the ability to detect a range of palladium (0, +2, and +4) species (Scheme 21). Probe 22 was found to react with Pd(0) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. This led to the suggestion that Pd(0) initially coordinates to the double bond to form a π-allyl complex that underwent decarboxylative elimination of the fluorophore, which allows the ESIPT process to take place. Probe 22 exhibited a large and rapid ratiometric change in fluorescence intensity (I522/I470) in response to Pd(0), with a LOD = 9.0 nM (1.5 min). This probe could be used to visualise Pd(0) in HeLa cells using two-photon fluorescence spectroscopy.67
1 stoichiometry. This led to the suggestion that Pd(0) initially coordinates to the double bond to form a π-allyl complex that underwent decarboxylative elimination of the fluorophore, which allows the ESIPT process to take place. Probe 22 exhibited a large and rapid ratiometric change in fluorescence intensity (I522/I470) in response to Pd(0), with a LOD = 9.0 nM (1.5 min). This probe could be used to visualise Pd(0) in HeLa cells using two-photon fluorescence spectroscopy.67
ESIPT fluorescence based sensors for anions
Pyrophosphate (P2O74−)
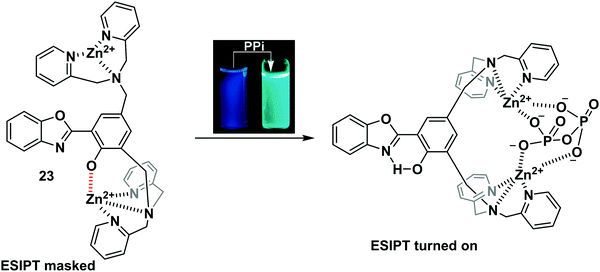
Pyrophosphate (PPi) is a biologically important anion, which is central to a number of biological processes, including ATP hydrolysis, DNA and RNA polymerisation, and a number of enzymatic reactions. Moreover, unregulated PPi production, degradation, and transport is associated with a range of diseases.68Pang and co-workers designed and synthesised an ESIPT-based fluorescent probe 23 containing a binuclear Zn2+ system. Coordination of PPi to Zn2+ triggers a large ratiometric fluorescence response, due to the ESIPT pathway being turned on (Scheme 22). Job plot analysis revealed that probe 23 is bound to PPi with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, a conclusion that was further supported by electrospray mass spectrometric studies. Probe 23 displayed excellent selectivity and was used to detect PPi released over the course of a PCR experiment, thus demonstrating its usefulness as a tool for bioanalytical applications.69
1 stoichiometry, a conclusion that was further supported by electrospray mass spectrometric studies. Probe 23 displayed excellent selectivity and was used to detect PPi released over the course of a PCR experiment, thus demonstrating its usefulness as a tool for bioanalytical applications.69
Superoxide (O2−)
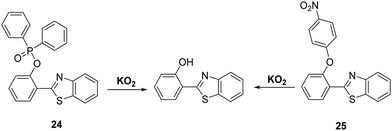
Superoxide (O2−) is known as the “primary” reactive oxygen species that is ultimately responsible for generating other ROS/RNS species, such as H2O2 and ONOO−. The production of superoxide mostly occurs in mitochondria, due to leakage of electrons from the electron transport chain to molecular oxygen. The high reactivity and short half-life of O2− makes it difficult to detect.70,71 O2− has been shown to be involved in a range of pathological conditions such as inflammation, aging, and ischemia-reperfusion injury.72,73 Therefore, the development of analytical tools for its real-time detection is highly desirable.Churchill and co-workers developed two HBT-derived fluorescent probes 24 and 25, that are functionalised with phosphinate and 4-nitrophenyl ether groups, respectively (Scheme 23). Initially, the ESIPT processes of both probes are blocked; however, reaction with O2− results in the phosphino group of 24 and the p-nitrophenyl group of 25 being cleaved to give the same free phenolic unit that turns on the ESIPT process. Exposure of probe 24 to O2− led to a 60-fold increase in fluorescence intensity, which enabled it to be used for cellular imaging studies. An MTT assay for probe 24 in SH-SY5Y cells provided initial evidence that this probe was not neurotoxic.74
Peroxynitrite (ONOO−)
Peroxynitrite (ONOO−), is a highly reactive nitrogen species (RNS) that is formed via the diffusion controlled reaction between superoxide (−O2) and nitric oxide (NO).75,76 Peroxynitrite is known for its destructive properties, causing irreversible damage to a range of biological molecules, such as lipids, proteins, and DNA.77 Peroxynitrite has been implicated as a key pathogenic factor for a number of diseases, including inflammation, cancer, ischemia-reperfusion injury, and neurodegenerative diseases.78–80 Therefore, the development of probes for peroxynitrite detection could allow for important advances in diagnostics and allow for improved staging of various medical conditions.Li et al. reported a simple and effective HBT-based ESIPT probe 26 that allows for the detection of ONOO− with a good selectivity profile and a fast response time (Scheme 24). Probe 26 was shown to be capable of crossing the blood–brain barrier (BBB) and could be used for two-photon fluorescence spectroscopy. This enabled probe 26 to be used for the visualisation of ONOO− in neurovascular ischemia progression in a live mouse brain.81 The excellent properties of probe 26 led to suggestion that it may have utility in visualising other pathophysiological processes that result in ONOO− formation.
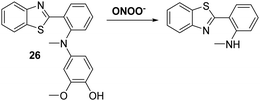 | ||
| Scheme 24 A reaction-based fluorescent probe 26 that allows for the rapid detection of ONOO− in cells and within a live mouse brain. Measurement conditions: PBS solution (10 mM, pH = 7.4). | ||
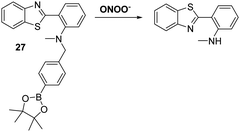
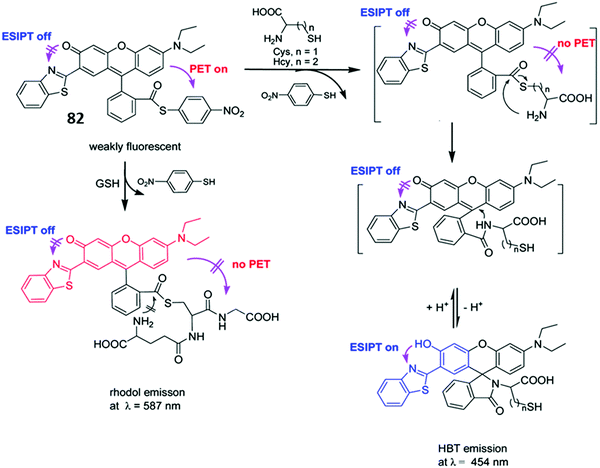
James and co-workers developed a boronate-based HBT probe for peroxynitrite 27. This system exploits the rapid ONOO−-mediated oxidation of a boronate ester fragment that is selective for ONOO− over hypochlorite and hydrogen peroxide (Scheme 25).82 A benzyl boronic ester ‘‘protecting’’ unit was used to block the ESIPT process initially. Exposure to ONOO− results in oxidative deprotection of the benzyl boronic ester fragment and leads to an increase in the fluorescence intensity. Probe 27 was shown to be cell permeable, which enabled it to be used to visualise ONOO− in live cell imaging experiments involving the HeLa and RAW 264.7 cell lines.83 More recently, James and co-workers developed a ratiometric boronate-based ESIPT probe for the visualisation of ONOO− within the endoplasmic reticulum.84
Sulfites (SO32−/HSO3−)
Sulfur dioxide (SO2) is a toxic pollutant that is produced by a variety of industrial processes,85–87 SO2 is also produced enzymatically in the cytosol and mitochondria of cells. It then equilibrates to form HSO3−, or SO32−. Endogenously generated SO2 acts as a signalling molecule in several physiological processes; however, elevated levels of SO2 have been associated with a number of diseases, including cardiovascular disease, cancer, and ischemia-reperfusion injury.88Song and co-workers have developed a levulinate ester-functionalised HBT fluorescent probe 28 for the ratiometric detection of SO32−. Exposure of probe 28 to this latter dianion triggers a cyclisation reaction that leads to cleavage of the levulinate ester fragment and release of the HBT fluorophore (Scheme 26). A large change in ratiometric fluorescence intensity (I468/I373) was observed when SO32− (0–1000 μM) was added. Moreover, probe 28 demonstrates excellent selectivity over other anions, illustrating its potential for the detection of SO32−.89
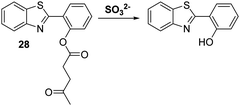 | ||
Scheme 26 Levulinate-functionalised HBT fluorescent probe 28, which allows for the triggered release of HBT and the detection of SO32−. Measurement conditions: PBS/CAN (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) pH = 7.4. 50) pH = 7.4. | ||
Hypochlorite (HOCl/ClO−)
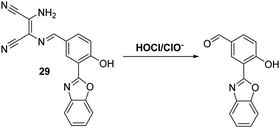
Myeloperoxidase (MPO) in leukocytes catalyses the reaction between chloride anions and H2O2 to generate hypochlorous acid (HOCl/ClO−), which is a biologically important reactive oxygen species generated by the immune system for its microbiocidal properties.90 Unfortunately, excess production of HOCl/ClO− can lead to uncontrolled damage of a range of biomolecules, such as amino acids, proteins, carbohydrates and lipids.91,92 As a consequence, HOCl/ClO− has been associated with cell and tissue damage, as well as a number of diseases.93 These deleterious properties provide an incentive to develop probes allowing for the effective detection of HOCl/ClO−.Yoon and co-workers have synthesised a diaminomaleonitrile (DMN)-based ESIPT fluorescent probe 29 for the detection of HOCl/ClO− (Scheme 27). Probe 29 undergoes HOCl/ClO− mediated imine hydrolysis to release the corresponding aldehyde (as confirmed by high resolution mass spectrometry). It is this release that responsible for the observed increase in fluorescence intensity. Probe 29 was shown to display excellent selectivity over other ROS/RNS, including ONOO− and H2O2. It also displayed high sensitivity, reacting fully with HOCl/ClO− in less than a minute. This enabled it to be used to monitor exogenous and endogenous HOCl/ClO− production in living cells and tissues using two-photon microscopic analyses.94
Goswami et al. designed a thiosemicarbazide HBT-based fluorescent probe 30 for the ratiometric detection of HOCl/ClO−. These latter species were proposed to disrupt the hydrogen bonding network of the probe, resulting in inhibition of the ESIPT process (Scheme 28). Probe 30 demonstrated high selectivity and sensitivity for HOCl/ClO− exposure which was accompanied by a colorimetric change from orange to blue. Probe 30 was shown to be able to detect different concentrations of HOCl/ClO− in tap water, illustrating its potential utility in monitoring the quality of drinking water.95
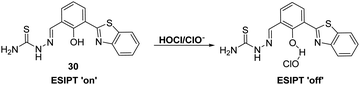 | ||
Scheme 28 A thiosemicarbazide HBT-based probe 30 for the selective detection of hypochlorite (HOCl/ClO−), including in tap water. Measurement conditions: ACN/H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). 50). | ||
Chen and co-workers developed a dithiolane functionalised HBT-based fluorescent probe 31 for the detection of HOCl/ClO−. In this case advantage was taken of the ability of hypochlorite to oxidise the dithiolane functionality to its corresponding fluorescent aldehyde (Scheme 29). This reactivity profile, which is reflected in a colour change from yellow to blue, enabled probe 31 to be used as a highly selective and sensitive probe for HOCl/ClO− with a LOD = 8.9 nM. Probe 31 was shown to be cell permeable and capable of detecting both exogenous and endogenous HOCl/ClO− in HeLa cells.96
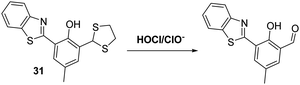 | ||
| Scheme 29 Fluorescent probe 31 that exploits the oxidation of a dithiolane functionalised HBT to permit the detection of hypochlorite. Measurement conditions: PBS (pH = 7.4, 10 mM). | ||
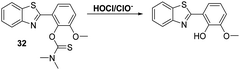
More recently, James and co-workers have developed a thiocarbamate functionalised HBT-OMe fluorescent probe 32 for the detection of HOCl/ClO− (Scheme 30). Addition of HOCl/ClO− resulted in rapid hydrolysis (<10 s) of the thiocarbamate linker resulting in a large increase in fluorescence intensity (>40-fold). This probe demonstrated high sensitivity (LOD = 0.16 nM) and excellent selectivity for hypochlorite over other ROS/RNS. It was capable of detecting exogenous and endogenous HOCl/ClO− in HeLa cells. Test strips impregnated with 32 were prepared and shown to be useful for the qualitative detection of different concentrations of HOCl/ClO− in water. Alternatively, 32 was shown to be capable of performing as a dual input logic gate for detecting Hg2+ and H2O2. Exposure of 32 to Hg2+ led to a significant increase in fluorescence intensity after 30 min; however, due to the large difference in response rates, no interference from Hg2+ was observed when probe 32 was used to test for HOCl/ClO− in drinking water.97
Cyanide (CN−)
Cyanide (CN−) is used extensively for industrial applications, including gold mining; however, it is one of the deadliest anionic poisons known. Its toxicity is ascribed to its ability to inhibit cytochrome-c oxidase, the final oxidase of the electron transport chain. Ultimately, this deactivates the electron transport chain and results in the inhibition of cellular respiration.98,99 Consequently, the ability to detect low levels of CN− in the environment and biological systems is extremely important.Malik et al. synthesised a salicylaldehyde functionalised fluorene ESIPT probe 33 for CN−. This system relies on reaction of CN− with the aldehyde group in 33. This affords a stable cyanohydrin product (Scheme 31) and results in a large increase in fluorescence intensity. With a calculated LOD of 0.06 ppm, it was found that probe 33 could be used to image CN− in living SH-SY5Y cells.100
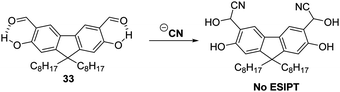 | ||
| Scheme 31 A salicylaldehyde functionalised fluorene fluorescent probe 33 for the detection of CN−. Measurement conditions: ACN only. | ||
Similarly, Nandi and co-workers developed a HBT-functionalised ratiometric fluorescent probe 34 for the detection of CN−. In this case the cyanide anion selectively reacts with the ortho-aldehyde functionalilty of 34 to afford a cyanohydrin that inhibits the ESIPT process (Scheme 32). The net result is a ratiometric change (I436/I521) in fluorescence intensity. The detection limit of probe 34 for the cyanide anion was 1.6 μM, a value that is below the WHO recommended level for CN− in drinking water (i.e., 1.9 μM).101
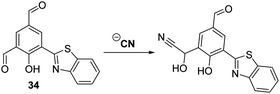 | ||
| Scheme 32 A selective HBT fluorescent probe 34 for the detection of CN−. Measurement conditions: ACN only. | ||
Fluoride (F−)
Fluoride (F−) occurs naturally at trace levels in all-natural water sources; however, an ability to detect F− has garnered considerable interest in recent years due to the significant health and environmental impact that can occur when this halide anion is present at higher concentrations.102Silyl ethers are widely used for the protection of alcohol and phenol functionalities, with silyl-ether protecting groups known to be selectively removed by the action of F− to afford their corresponding alcohols. Several research groups have exploited this reactivity profile to develop reaction-based fluorescent probes suitable for the detection of F−. For example, Yang and co-workers employed tert-butyldiphenylsilyl ether (TBDPS) to block the phenol group of probe 35 and inhibit the ESIPT process (Scheme 33). Exposure to a F− source results in cleavage of the O–Si bond which ‘turned on’ the ESIPT process leading to a ratiometric change in the fluorescence intensity (I560/I418). Probe 35 was shown to have excellent selectivity over other anions making it suitable for use in practical applications.103
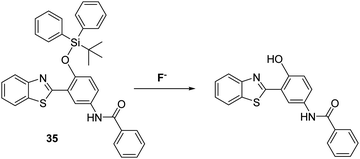 | ||
| Scheme 33 A silyl ether protected HBT-based fluorescent probe 35 for the detection of the fluoride anion. Measurement conditions: 100% H2O solution containing 2 mM CTAB. | ||
Saha et al. also blocked the phenol functionality of a HBT-derived naphthalene fluorophore with a tert-butyldimethylsilyl group to afford probe 36 (Scheme 34). In this case, the addition of F− led to a significant ratiometric change in the fluorescence intensity, an effect ascribed to deprotection of the O–Si bond, which allows an ESIPT process to occur efficiently. Test strips containing probe 36 were prepared and were shown to be effective for the qualitative detection of F−. Probe 36 was also found to be cell permeable and to be capable of imaging exogenously added F− in HeLa cells.104a,b
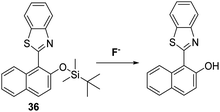 | ||
Scheme 34 A silyl ether protected naphthalene HBT fluorescent probe 36 for the fluoride anion. Measurement conditions: HEPES/CAN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) pH = 7.4. 8) pH = 7.4. | ||
Interestingly, Wu et al. developed two distinct HBT-based ESIPT fluorescent probes (37 and 38) for the detection of F−. Both systems displayed good selectivity and sensitivity105 to the fluoride anion and gave rise to a ratiometric response (Scheme 35). Probe 38 is believed to capable of detecting F−via formation of an N–H–F bonding interaction. This interaction stabilises the keto form involved in the ESIPT process, as shown in Scheme 35.
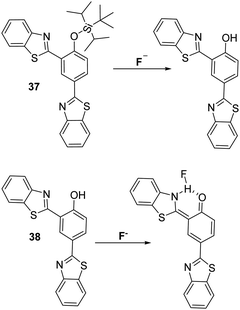 | ||
| Scheme 35 Two HBT fluorescent probes 37 and 38, which employ two different strategies for the detection of the fluoride anion. Measurement conditions: THF. | ||
Wang and co-workers synthesised a benzoselenadiazole-based diarylamine-based ESIPT probe 39 for F− (Scheme 36). This highly selective probe contains a strong electron withdrawing 4-nitrophenyl group, whose presence increases the acidity of the N–H bond. Addition of excess F− to probe 39 leads to deprotonation of the N–H proton (observed by 1H NMR spectroscopy). This results in a ratiometric change in the fluorescence intensity (I478/I671) of probe 39, as well as an easily-visualized colour change.106
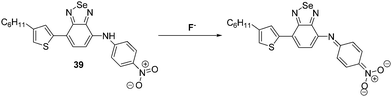 | ||
| Scheme 36 A benzoselenadiazole-based diarylamine ESIPT fluorescent probe 39. This system permits detection of the fluoride as the result of a deprotonation event. Measurement conditions: DMSO. | ||
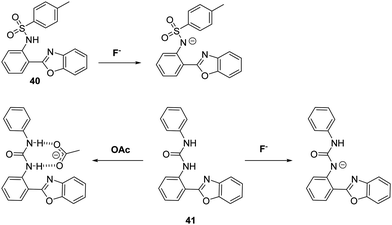
Peng and co-workers developed two HBO-based anion fluorescent probes for the detection of F− (Scheme 37). The first HBO derivative was functionalised with a p-toluenesulfonyl unit to afford probe 40. This system could be used to detect F−, CH3COO−, and H2PO4− salts via deprotonation of the sulfonamide group and a corresponding inhibition of the ESIPT process. In the case of probe 41 the inherent ESIPT process could be inhibited either by F−-promoted deprotonation of the urea unit, or via formation of a strong CH3COO−–urea intermolecular hydrogen bond complex. Interestingly, exposure of probe 41 to a F− or CH3COO− source resulted in different ratiometric responses, thus allowing these two anions to be distinguished.107
ESIPT fluorescence based sensors for small neutral molecules
Hydrogen sulphide (H2S)
Hydrogen sulphide (H2S) has recently been reported as an endogenous gaseous transmitter that regulates several physiological and pathological processes, including those involved in neurotransmission, vasodilation, inflammation, atherosclerosis, oxidative stress, and inhibition of insulin signaling.108–111 Therefore, the development of a fluorescent probe for the intracellular detection of H2S could help advance our fundamental understanding of its role in pathophysiological processes.Qian and co-workers developed and designed a fluorescent probe 42 for the detection of H2S through the functionalisation of the ESIPT fluorophore HMBT with a H2S reactive unit, namely 2-(pyridin-2-yl-disulfanyl)benzoic acid (Scheme 38). Exposure of probe 42 to H2S results in cleavage of the S–S bond. This produces a free –SH intermediate, which subsequently cyclises to release HMBT. Probe 42 was shown to exhibit high sensitivity (LOD = 0.12 μM) towards H2S, producing a 30-fold fluorescence enhancement within 2 minutes. Probe 42 was also shown to display excellent selectivity over other biologically relevant species, including other thiol-containing analytes (GSH, HCys, and Cys), presumably because these latter species are unable to form the requisite –SH intermediate. The potential utility of probe 42 for biological applications was demonstrated through fluorescence imaging of H2S in HeLa cells containing CTAB.112
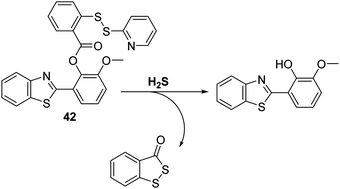 | ||
| Scheme 38 HMBT-based fluorescent probe 42, a system that permits the selective detection of H2S in HeLa cells. Measurement conditions: Tris–HCl buffer (20 mM, pH = 7.4) containing 1 mM CTAB. | ||
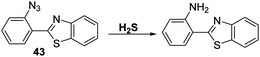
An alternative strategy for the detection of H2S involves exploitation of the well-known H2S-mediated reduction reactions of aryl azides to the corresponding amines to generate a fluorescence response.113,114 This approach is embodied in the azide-functionalised HBT derivative 43 prepared by Guo et al. (Scheme 39). Initially, probe 43 was found to be non-fluorescent; however, addition of H2S resulted in a remarkably large fluorescence increase (∼1150-fold). Probe 43 was shown to be extremely sensitive (LOD = 0.78 nM) for H2S with relatively fast response times (3–10 min). It also exhibited excellent selectivity against other biologically relevant analytes. Probe 43 could be used to image exogenous and endogenously H2S in B16 cells in the presence of CTAB.115
Zeng et al. developed a HBT-derived long-wavelength fluorescent probe 44 functionalised with a H2S-responsive 2,4-dinitrophenyl ether unit. In this case exposure to H2S results in nucleophilic aromatic substitution at the 2,4-dinitrophenyl fragment. This, in turn, releases the free phenol of the fluorophore and enables the ESIPT process to take place (Scheme 40). The net result is a large increase in the fluorescence intensity, ascribed to emission from the keto form (λem = 620 nm). A large Stokes shift (240 nm) is also observed. Probe 44 was shown to be highly selective for H2S over a range of biologically relevant analytes; however, it did respond to high concentrations of GSH and Cys (ca. 10 mM and 1 mM, respectively).116a Mahapatra and co-workers developed a similar fluorescent probe that was shown to reversibly detect S2− and Zn2+ ions once the 2,4-dinitrophenol ether unit was removed.116b The same group have developed an ESIPT probe that can be used to detect picric acid an explosive and environmental pollutant.116c
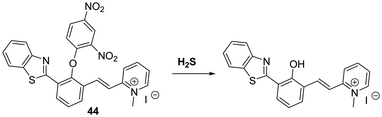 | ||
Scheme 40 A H2S-mediated SNAr cleavage endows probe 44 with an ability to detect H2S with good selectivity. Measurement conditions: PBS/ACN (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) pH = 7.4. 50) pH = 7.4. | ||
Homocysteine (HCys)
Homocysteine (HCys) is a naturally occurring amino thiol that is exclusively derived from the demethylation of methionine (Met). Free HCys is either re-methylated to Met facilitated by folate and vitamin B12 as cofactors or transformed into cysteine (Cys) via a condensation reaction with serine catalysed by cystathionine β-synthase with vitamin B6 acting as a co-factor. Due to the structural similarity with Met, HCys can enter the first step of protein synthesis and is recognised by ‘methionyl t-RNA synthetase’ (MetRS). However, the error-editing activity of MetRS prevents the incorporation of HCys into proteins. As a by-product of this error-editing process, HCys thiolactone (HTL) is formed. HTL can non-enzymatically modify proteins through the irreversible covalent attachment to lysine residues known as ‘protein N-homocysteinylation’.117,118 This results in structural alterations, loss of enzymatic function, and induces protein aggregation. Elevated levels of HCys (hyperhomocysteinemia) have been associated with pregnancy disorders, cardiovascular, and neurodegenerative diseases.119–121 This provides a particular incentive to generate probes for this neutral species.Tang et al. developed a simple HBI-based fluorescent probe 45 for the selective detection of HCys over other amino acids, including Cys and GSH (Scheme 41). HCys and Cys have previously been shown to form thiazolidine and thiazine adducts in the presence of aldehydes. By exploiting this reactivity, probe 45 was capable of distinguishing between HCys and Cys. This selectivity was ascribed to the formation of 5- and 6-membered heterocycles in an analyte-specific sense, chemistry that effected the ESIPT emission to differing extents. As a result of 45 demonstrating excellent selectivity, probe 45 was used in cellular imaging experiments. Probe 45 was shown to detect intracellular HCys in MCF-7 cells, leading to the suggestion that it might find utility in biological applications.122
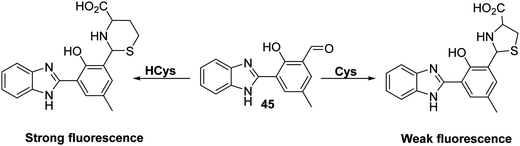 | ||
Scheme 41 A HBI-based fluorescent probe 45 that was found to display differing responses to HCys and Cys. Measurement conditions: HEPES/EtOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) pH = 7.4. 1) pH = 7.4. | ||
Cysteine (Cys)
Cysteine (Cys) is a biologically significant thiol-containing amino acid that plays a vital role in a number of biological processes including protein synthesis, cellular detoxification, and metabolism. However, abnormal levels of Cys are associated with a number of health problems, including cancer, neurological disorders, and motor neuron diseases.123–127 Therefore, the development of an analytical tool for the detection of Cys over other thiol-containing biologically important species is highly desirable.The ability of the thiol group of Cys to undergo conjugate addition reactions to acrylate esters, with concomitant intramolecular amide formation has been exploited to create turn on ESIPT-based fluorescent probes whose phenolic group are protected by Cys-reactive acrylate fragments. Strongin et al. reported an acrylate-functionalised HMBT fluorescent probe 46, which was used to detect cysteine (Cys) and homocysteine (HCys) in EtOH/PBS (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Scheme 42). The presence of very low concentrations of Cys/HCys (LOD = 0.11 and 0.18 μM, respectively) resulted in thiol-based conjugate addition to the acrylate group of probe 46 resulting a subsequent amino cyclisation reaction; this afforded the HMBT fluorophore and the respective lactam and gave rise to a large change in the ratiometric emission intensity (I487/I377). Probe 46 was found to possess excellent selectivity for Cys/HCys over other biologically relevant amino acids. Moreover, probe 46 was shown to be capable of discriminating Cys over HCys due to disparities in the rate of 7- vs. 8-membered ring-formation, a difference that was reflected in different spectroscopic and kinetic profiles for each analyte.128
2) (Scheme 42). The presence of very low concentrations of Cys/HCys (LOD = 0.11 and 0.18 μM, respectively) resulted in thiol-based conjugate addition to the acrylate group of probe 46 resulting a subsequent amino cyclisation reaction; this afforded the HMBT fluorophore and the respective lactam and gave rise to a large change in the ratiometric emission intensity (I487/I377). Probe 46 was found to possess excellent selectivity for Cys/HCys over other biologically relevant amino acids. Moreover, probe 46 was shown to be capable of discriminating Cys over HCys due to disparities in the rate of 7- vs. 8-membered ring-formation, a difference that was reflected in different spectroscopic and kinetic profiles for each analyte.128
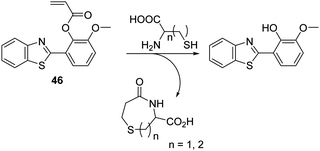 | ||
Scheme 42 An acrylate functionalised HMBT fluorescent probe 46 that allows for the selective detection of Cys and HCys. Measurement conditions: HEPES/EtOH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) – pH = 7.4. 2) – pH = 7.4. | ||
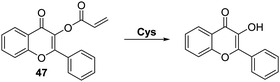
Pang and co-workers prepared a related acrylate-functionalised flavone-based fluorescence probe 47 that allowed for the selective detection of Cys over other biological thiols. Exposure of 47 produced a large ratiometric change in the fluorescence intensity, with a lower detection limit of <1 μM being observed (Scheme 43). Probe 47 was shown to exhibit excellent selectivity over other α-amino acids. This permitted its use in cellular imaging experiments, where it was shown to have low cytotoxicity and good cell permeability.129
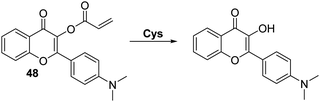
Similarly, Feng and co-workers reported an acrylate modified dimethylamino-flavone-based fluorescent probe 48 for Cys. This particular system demonstrated Cys-mediated acrylate cleavage within 5 min at low concentrations of Cys (LOD = 0.2 μM) (Scheme 44). A large ratiometric change in fluorescence intensity (I550/I471) and a large Stokes shift (>130 nm) were observed. Probe 48 was found to display excellent selectivity over other amino acids and could be used to detect intracellular levels of Cys in HeLa cells.130
Glutathione (GSH)
GSH is a natural tripeptide (γ-L-glutamyl-L-cysteinyl-glycine), which mainly exists in its thiol reduced form (GSH) over its corresponding disulphide-oxidised (GSSG) form.131 GSH is present in millimolar concentrations in most cells, where it functions as an antioxidant. Elevated levels of GSH are often seen under conditions of oxidative stress and, in fact, the susceptibility of cells towards ROS/RNS species is strongly correlated with intracellular GSH levels.132,133 Moreover, dysregulation of GSH homeostasis is associated with a number of diseases, including AIDS, liver damage, cancer, and neurodegenerative diseases.134–136Chen et al. reported a 2,4-dinitrophenyl ether HBT-based fluorescent probe 49 for the detection of GSH. In the presence of CTAB exposure of probe 49 to GSH results in cleavage of the 2,4-dinitrophenyl group to release the ESIPT fluorophore and an increase in the fluorescence intensity (15-fold) at 485 nm (Scheme 45). The presence of CTAB and the resulting formation of micelles was believed helpful in catalysing the nucleophilic aromatic substitution reaction between GSH and probe 49 by maximising the hydrophobic and electrostatic interactions between the positive charge of CTAB and the negative charge of the carboxylate groups of GSH. Probe 49 proved effective in detecting low concentrations of GSH (LOD = 0.81 μM). It also displayed excellent selectivity over other amines, including HCys and Cys. This selectivity was rationalized in terms of GSH having a negatively charged carboxylate group. Probe 49 was use to image exogenously added GSH in HeLa cells, thus demonstrating its potential utility in biological applications.137
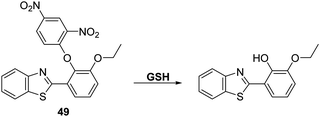 | ||
| Scheme 45 A HBT-based fluorescent probe 49 for the detection of GSH catalysed by CTAB. Measurement conditions: HEPES buffer, 20 mM, pH = 7.4 containing 1 mM CTAB. | ||
Hydrogen peroxide (H2O2)
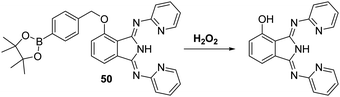
Hydrogen peroxide (H2O2) is a weak oxidant and a weak nucleophile that is able to diffuse freely across cell membranes and into cells. H2O2 is known to be critical to a number of physiological processes, including cell proliferation, differentiation, apoptosis, and cell signalling.138 However, the accumulation of H2O2 has been implicated in a number of pathological conditions, including cancer, aging, and neurodegenerative diseases.139–142 There is thus a demand for fluorescent probes that might permit the selective detection and quantification of H2O2 in biological milieus.A commonly employed strategy for the detection of H2O2 involves the H2O2-mediated oxidation of functionalized aryl boronates to their corresponding phenols thereby generating a fluorescence response.143 Song et al. developed a fluorescent probe 50 for the detection of H2O2via functionalisation of the phenol group of the ESIPT dye, 1,3-bis(bispyridin-2-ylimino)isoindolin-4-ol, with an aromatic boronate ester unit. This functionalisation blocks the ESIPT process (Scheme 46). Addition of H2O2 to probe 50 in PBS (pH = 7.4) containing CTAB produced a large fluorescence increase (54-fold) and a remarkably large Stokes shift (217 nm). Probe 50 was shown to display sensitivity towards H2O2 (LOD = 9.4 nM) and to react fully within 60 min in the presence of 80 equiv. of H2O2. Probe 50 was found to have excellent selectivity for H2O2 over other ROS/RNS species as well as other biologically relevant analytes. Probe 50 was shown to be membrane permeable in 4T1 cells, which were imaged successfully in the presence of exogenous H2O2.144
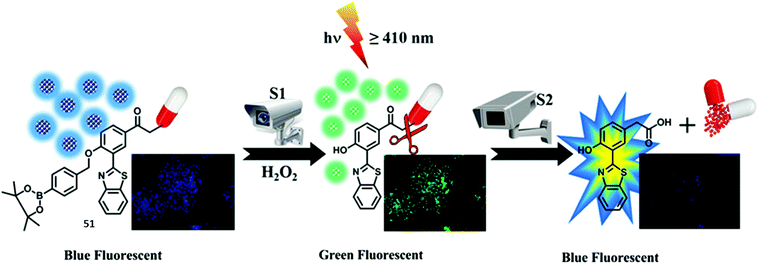
Singh et al. developed a ‘two-step surveillance’ ESIPT HBT-based probe 51 for the detection of H2O2 where photolysis (≥410 nm) allowed for the release of the anticancer drug chlorambucil (Scheme 47). Nanoformulation was carried out to produce nanoparticles containing probe 51 that provided for relatively good penetration and retention in cancer cells. Initially, the ESIPT process is blocked by the presence of a benzyl boronic ester; however, exposure to H2O2 results in oxidative cleavage and gives rise to a large ratiometric change in the fluorescence intensity (I518/I448). Subsequent exposure of probe 51 to visible light (≥410 nm) leads to release of chlorambucil, as monitored by reverse-phase high-performance liquid chromatography (RP-HPLC). Remarkably, exposure of probe 51 to visible light (≥410 nm) led to a decrease in the fluorescence intensity of the keto emission (518 nm) with only a fluorescence emission at 450 nm being observed. Probe 51 was shown to be membrane permeable which allowed it to be used to image ratiometrically H2O2 in HeLa cells (blue to green). Subjecting HeLa cells containing probe 51 to irradiation for 15 min was found to produce change in the colour of the emitted light from green to blue. This colour change was taken as evidence of chlorambucil release. Further support for this conclusion came from the enhanced cytotoxicity of probe 51 upon irradiation as inferred from an MTT assay.145
Yan et al. developed a mitochondria-targetable NIR fluorescent probe 52 for the detection of H2O2. In this case, exposure of the probe to the analyte results in a decrease in the emission intensity at 539 nm and an increase in the emission intensity at 669 nm. A large Stokes shift of 357 nm is also seen (Scheme 48). Probe 52 was shown to have high sensitivity for H2O2 (LOD = 0.59 μM) and good selectivity over other ROS/RNS species, as well as other biologically relevant analytes. Probe 52 was found to localise in the mitochondria of A549 cells where it could be used to image both exogenous and endogenous H2O2.146
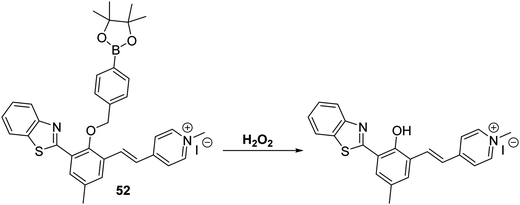 | ||
Scheme 48 A NIR-mitochondria targeting ESIPT fluorescent probe 52 that allows for the ratiometric detection of hydrogen peroxide in A549 cells. Measurement conditions: HEPES/ACN (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) pH = 7.4. 50) pH = 7.4. | ||
Li and co-workers have developed a clever dual functional theranostic probe 53 that generates a fluorescence response and releases a therapeutic agent in the presence of H2O2 (Scheme 49). Probe 53 is comprised of HBT whose phenolic group is esterified with an aspirin moiety that blocks the ESIPT process. Probe 53 was shown to detect H2O2 at low concentrations (LOD = 2.5 μM) and with good selectivity over other reactive species, including ONOO−. Probe 53 was used to image H2O2 in EA.hy926 endothelial cells. It was also shown to alleviate endothelial injury, with the endothelial-protective effect of probe 53 being demonstrated in terms of reduced thrombosis formation in zebrafish models.147
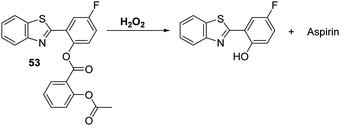 | ||
Scheme 49 A hydrogen peroxide responsive theranostic probe 53 that allows for endothelial injury imaging and protection. Measurement conditions: PBS/EtOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) pH = 7.4. 50) pH = 7.4. | ||
Nitric oxide (NO)
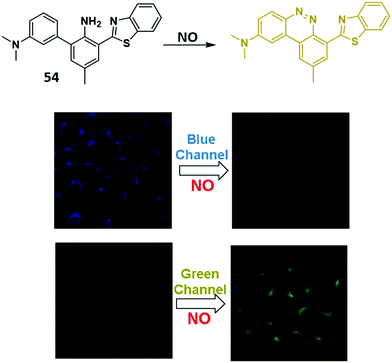
Nitric oxide (NO) is a small signalling molecule that is generated by three different nitric oxide synthases (NOS) that catalyse the oxidative conversion of L-arginine to L-citrulline. NO is known to mediate many physiological processes, such as neurotransmission, blood pressure regulation, smooth muscle relaxation, and immune regulation. However, uncontrolled NO production can lead to nitrosative stress, which is implicated in neurodegenerative diseases and ischemic-reperfusion injury.75,76,148,149 Therefore, the development of fluorescence-based probes for NO could provide useful tools allowing for its direct detection and real-time monitoring in biological environments.Yoon and co-workers developed a simple HBT-based fluorescent probe 54 for the selective and ratiometric detection of NO (Scheme 50). Initially, probe 54 exhibited a fluorescence emission peak at 470 nm due to the ESIPT process. However, the presence of NO resulted in the formation of a longer wavelength benzo[c]cinnoline derivative leading to a ratiometric change in fluorescence intensity (I560/I470). The formation of a benzo[c]cinnoline derivative was confirmed by mass spectrometry. Probe 54 displayed good selectivity and sensitivity (LOD = 17 nM) for NO over other relevant analytes. It was also shown to be cell permeable and could be used to image exogenously added 1,1-diethyl-2-hydroxy-2-nitroso-hydrazine (DEA NONOate – an NO donor) in HeLa cells.150
Nitroxyl (HNO)
Nitroxyl (HNO) is the one-electron reduced form of NO that demonstrates a unique chemical and biological profile compared to NO. HNO exists in two electronic forms, a singlet and triplet state. Biologically, 1HNO is a small neutral molecule (pKa ≈ 11.4), which allows it to freely cross cell membranes and engage in several redox reactions with biological reductants and oxidants. HNO has been found to have a number of beneficial physiological properties therefore HNO is emerging in the literature as a potential pharmacological agent. Some of these properties include increasing cardiac output, protective effects against myocardial ischemia injury, and anticancer properties.151–155 Therefore, the development of a fluorescent probe for its detection could provide a suitable tool for understanding the role in biological systems.In 2009, King et al. reported that HNO can react with ortho-triarylphosphines methyl benzoates to give the corresponding phosphine oxides and an aza-ylide (Scheme 51).156 The resulting intermediate can rearrange to afford an amide and a phosphine oxide (Staudinger ligation).
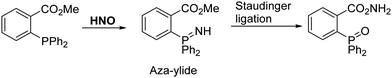 | ||
Scheme 51 HNO induced Staudinger ligation developed by King et al.156 Reaction conditions: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD3CN/Tris buffer. 1 CD3CN/Tris buffer. | ||
Yin and co-workers employed this reactivity to develop the first ESIPT fluorescent probe 55 functionalised with a HNO-reactive diphenylphosphinobenzoyl group (Scheme 52). As expected, addition of sodium trioxodinitrate – Na2N2O3 (Angeli's salt) as a HNO donor (0–30 μM) gave a ∼9.5 fold ratiometric change in fluorescence (I460/I380). Probe 55 exhibited high sensitivity (LOD = 0.98 μM) and selectivity for HNO and was used to image HNO in HeLa cells (Fig. 5).157
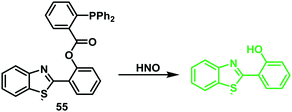 | ||
Scheme 52 A HNO-reactive diphenylphosphine-based ESIPT fluorescent probe 55. This system permitted the ratiometric detection of HNO in HeLa cells. Measurement conditions: PBS/ACN (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) pH = 7.4. 3) pH = 7.4. | ||
Zhu et al. developed a flavone-based fluorescent probe 56 for HNO. This was achieved by functionalising the hydroxyl group of flavone with an HNO-cleavable diphenylphosphinobenzoyl recognition unit (Scheme 53). Probe 56 was shown to exhibit a selective response to HNO over other biological oxidants and reductants and to permit the rapid detection of HNO (LOD = 0.13 μM). Importantly, probe 56 could be used to detect HNO in complex biological samples (serum) demonstrating its potential utility in clinical applications.158
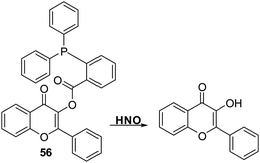 | ||
Scheme 53 A diphenylphosphine-based 3-hydroxyflavone fluorescent probe 56 that could be used for the detection of HNO in aqueous media and in serum. Measurement conditions: PBS/EtOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), pH = 7.3. 4), pH = 7.3. | ||
Ozone (O3)
Ozone (O3) is located in the stratosphere, where its presence acts to protect the Earth from harmful UV light. However, in the lower troposphere, O3 is known to be harmful and has been associated with the onset of respiratory diseases.159 In addition, it is known that antibodies can catalyse the production of the previously unknown oxidant, dihydrogen trioxide (H2O3) and O3 from 1O2, for the purpose of immune defense.160 For these reasons, the development of fluorescent probes for the detection of O3 is highly desirable from both a health and safety perspective and to gain a greater understanding of its biological function.Wang et al. have reported a simple HBT-based fluorescent probe 57 for the detection of O3. The ESIPT process of probe 57 is initially blocked through alkylation of the phenol group of HBT with 4-bromo-1-butene. O3 reacts with the terminal alkene to afford an aldehyde that undergoes β-elimination to release the HBT fluorophore and acrolein (Scheme 54). This cleavage process liberates the phenol group turning on the ESIPT process. This in turn leads to a large increase in the fluorescence intensity. Probe 57 was shown to exhibit high sensitivity for O3 (LOD = 34 nM) and an excellent selectivity for O3 over other ROS/RNS species. In addition, filter paper impregnated with probe 57 was found capable of detecting qualitatively various concentrations of O3, thus demonstrating its potential utility as a tool for monitoring environmental levels of O3.161
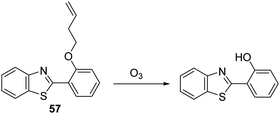 | ||
| Scheme 54 1-Butene-functionalised HBT fluorescent probe 57 that permits the rapid detection of ozone. Measurement conditions: PBS buffer, 10 mM, pH = 7.1. | ||
Hydrazine (NH2NH2)
Hydrazine (NH2NH2) is a chemical reagent that is widely used in the pharmaceutical, chemical, aerospace, and agricultural industries.162–164 However, it is highly toxic, displaying mutagenic and carcinogenic effects that can cause serious damage to major organs and the central nervous system.165–167 Therefore, it is vital to have analytical methods that allow its detection since these could allow for its monitoring in the event of accidental release into the environment.Zhu and co-workers functionalised a flavone fluorophore with 4-bromobutyric acid to produce probe 58. As prepared the ESIPT process in probe 58 is blocked and only the enol-derived emission is observed (Scheme 55). However, addition of NH2NH2 to a DMSO/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution of probe 58 results in nucleophilic substitution of bromide and subsequent amino-carbonyl cyclisation onto the ester to release the flavone fluorophore. This results in a large increase in the fluorescence intensity (∼55 fold increase) due to the formation of the keto form. Probe 58 was shown to have high sensitivity for NH2NH2 (LOD = 0.15 μM) and excellent selectivity over other amine-containing analytes. Probe 58 could be used to image exogenously added NH2NH2 in living HeLa cells.168 It could also be formulated into test strips that permitted the qualitative detection of different concentrations of NH2NH2 under conditions of UV light irradiation.
1, v/v) solution of probe 58 results in nucleophilic substitution of bromide and subsequent amino-carbonyl cyclisation onto the ester to release the flavone fluorophore. This results in a large increase in the fluorescence intensity (∼55 fold increase) due to the formation of the keto form. Probe 58 was shown to have high sensitivity for NH2NH2 (LOD = 0.15 μM) and excellent selectivity over other amine-containing analytes. Probe 58 could be used to image exogenously added NH2NH2 in living HeLa cells.168 It could also be formulated into test strips that permitted the qualitative detection of different concentrations of NH2NH2 under conditions of UV light irradiation.
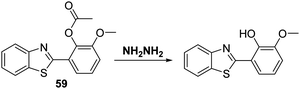
Recently, Huang and co-workers functionalised a HMBT fluorophore with acetic anhydride to afford probe 59, wherein the inherent ESIPT process is blocked (Scheme 56). Addition of NH2NH2 to a PBS buffer solution containing 1 mM CTAB and probe 59 resulted in the nucleophilic cleavage of the acetate group and release of the HMBT fluorophore. This engendered a large ratiometric fluorescence response (I480/I371). Probe 59 was found to possess high sensitivity for hydrazine and excellent selectivity over other nucleophilic anionic species. Moreover, renewable test strips impregnated with probe 59 were developed; these proved capable of qualitatively and quantitatively detecting NH2NH2 in aqueous media or in the gaseous state. These strips were thought to exploit the solid state emitting nature of this specific ESIPT fluorophore, a feature seen in several other systems.9 The protocol embodied in the test strips containing 59 is relatively unexplored and it was suggested that it could be used for the real time monitoring of NH2NH2 gas released from various sources, including those ostensibly contributing to air pollution (Scheme 56).169
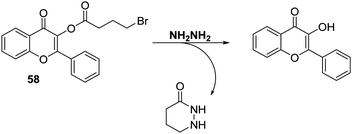 | ||
Scheme 55 3-Hydroxyflavone probe 58, a system that permits the rapid detection of hydrazine in HeLa cells. Measurement conditions: PBS/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) pH = 7.0. 9) pH = 7.0. | ||
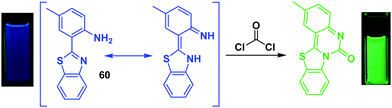
Phosgene (COCl2)
Phosgene (COCl2) is a colourless and highly toxic gas that was used as a chemical weapon during World War I. Exposure to COCl2 results in serious damage to the lungs and respiratory tract leading to asphyxia, pulmonary emphysema, and death.170,171 However, COCl2 is still widely used in industry for the synthesis of pesticides and pharmaceuticals;172 therefore, COCl2 is a potential threat to human health and safety and so the availability of fluorescent probes for its detection is highly desirable.Yoon and co-workers developed a colorimetric and ratiometric fluorescent probe 60 for the selective detection of COCl2 (Scheme 57). Initially, probe 60 has a fluorescence emission at 445 nm due to an ESIPT effect. Exposure to COCl2 results in the carbamoylation of probe 60 to form a tetracyclic urea (structure confirmed by 1H NMR spectroscopy and mass spectrometry) characterized by a fluorescence emission feature at 496 nm. Reaction of COCl2 with probe 60 also results in a colorimetric change (colourless to yellow) and a ratiometric change in the fluorescence intensity (I495/I445). High sensitivity and selectivity was observed for phosgene in chloroform and in the gas phase. Probe 60 could be incorporated into filter papers giving test strips that could be used for the qualitative detection of different concentrations of COCl2.173
Boronic acids (R-B(OH)2)
Boronic acids (R-B(OH)2) are one of the most commonly used compounds in synthetic organic and medicinal chemistry. They also represent a functional group that can be used as a recognition unit for the sensing of biological analytes.143,174,175 This provides a cogent rationale to develop probes for boronic acids. Their availability might allow the progress of an organic transformation that employs a boronic acid to be monitored readily or permit its presence as a contaminant in a pharmaceutical process to be detected more easily.Raines and co-workers developed a quinolone-based fluorescent probe 61 that was able to bind and detect boronic-acid functionalised compounds selectively via disruption of an ESIPT process (Scheme 58). Probe 61 was shown to be able to detect phenylboronic acid over a range of 0–20 μM. A 1 mM solution of probe 61 was developed by this group as a dip for the visualisation of boronic acids spotted onto TLC plates. In addition, probe 61 was used to detect the presence of m-aminophenylboronic acid on agarose functionalised beads, thus demonstrating its potential utility in materials chemistry applications.176a Similarly, Mahapatra and co-workers developed an ESIPT probe that can be used to detect perborate.176b
ESIPT fluorescence based sensors for biomacromolecules
Esterases
Esterases represent a broad class of enzymes that catalyse the hydrolysis of esters that are all-important in biology and which play a central role in biotechnology, including food processing, production of alcohol, cellular signalling, material transport, and metabolism.177,178 Therefore, the development of a fluorescent probe for the detection of esterase activity is a particularly attractive goal since it could provide new tools for use in manufacturing and biological analysis.Li et al. reported a flavone-based fluorescent probe 62 that could be used to evaluate esterase activity via fluorescence emission monitoring and 19F NMR spectroscopy (Scheme 59). Initially, the ESIPT process of probe 62 was blocked by an acetate group; however addition of an esterase results in hydrolysis of the acetate group and release of the flavone fluorophore. This release results in an enhancement in the fluorescence intensity at 510 nm (∼27 fold) which is ascribed to the presence of the more emissive keto tautomer. 19F NMR spectral studies provided further evidence that hydrolysis of the acetate group had occurred. Specifically, the fluorine signal of the probe was seen to shift from δF −111.57 to −111.69 ppm upon treatment with an esterase. The combination of these two detection methods, enabled probe 62 to be used for the real-time detection of esterase. In fact, probe 62 allowed for the confocal fluorescence imaging of esterase activity in HeLa cells.179
Li and co-workers developed a flavone-based probe 63 that is capable of discriminating between live and dead cells. Its efficacy depends on the fact that cellular esterases are normally inactive inside dead cells (Scheme 60). Probe 63 contains an esterase cleavable acetate group that only exhibits an enol tautomer emission at 440 nm. Esterase-mediated acetate hydrolysis in live HeLa cells, results in a keto tautomer-derived emission at 570 nm being turned on. Two-photon spectroscopic analysis of probe 63 revealed that it is capable of detecting dead tumour cells that had been killed via pre-treatment with H2O2 and UV. Probe 63 could also be used to discriminate between live and dead zebrafish, thus demonstrating its use as a potentially powerful visualising agent for use in molecular biological applications.180
Human carboxylesterase (CE)
Mammalian carboxylesterases are a multigene family of α/β-hydrolase enzymes that are widely distributed throughout the body. They catalyse the hydrolysis of a range of structurally diverse exogenous and endogenous substances (e.g., fatty acid esters, toxins, and drugs). In humans, most carboxylesterases have been identified as carboxylesterase 1 (CE1) and carboxylesterase 2 (CE2), which differ in terms of their distribution throughout the body. CE2 is the major carboxylesterase isoform found in the intestine where it mediates the hydrolysis of most oral ester prodrugs. This means it is an important biomarker for determining first-pass metabolism of a drug of interest. In addition, CE2 is believed to be overexpressed in human colon cancer tissue, thus making CE2 an important biomarker. Being able to detect it could aid in the development of new prodrugs and advance our understanding its role in disease.181–183Yang et al. have developed a flavone-based fluorescent probe 64 for the selective and sensitive detection of human carboxylesterase 2 (CE2) activity (Scheme 61). The inherent ESIPT process for probe 64 is blocked through functionalisation of the hydroxyl unit of the flavone fluorophore with a 4-ethylbenzoyl chloride unit. Addition of CE2 to probe 64 results in a colorimetric change (clear to light yellow) and a ratiometric change in the fluorescence intensity (I528/I392). Probe 64 was shown to display high sensitivity with a limit of detection for CE2 activity that was lower than 0.5 μg mL−1. Excellent selectivity over other hydrolase enzymes, including CE1, was also seen. Probe 64 could be used to evaluate the activity of a series of different CE2 inhibitors and to image CE2 activity in HeLa cells.184
Phosphatases
Alkaline phosphatase (ALP) is an enzyme that catalyses the dephosphorylation of a range of biological substrates, including phosphorylated proteins, nucleic acids, and other small molecules. More importantly, ALP is an important biomarker in medical diagnostics as changes in expression levels of ALP are associated with a range of pathological processes.185–187Wu et al. reported a phosphorylated flavone-based fluorescent probe 65 that allows for the detection of ALP based on the modulation of its inherent ESIPT effect (Scheme 62). The phosphate group of probe 65 functions as an ESIPT blocking agent and as a recognition unit for ALP, with only enol emission being observed for the parent probe. ALP can hydrolyse the phosphate group to generate a free hydroxyl group resulting in an ESIPT process being turned on as reflected in a strong keto form-based emission signal. Probe 65 exhibited excellent selectivity for ALP activity over other phosphatase enzymes and proved capable of detecting very low levels of activity (0.032 U L−1). Probe 65 could be used to detect endogenous ALP levels in human serum samples and to be internalised into HeLa cells where it could be used to image endogenous ALP activity.188
Han et al. have developed two HBT-based fluorescent probes 66-AMP functionalised with the nucleotides adenosine monophosphate (AMP) and 66-GMP guanosine monophosphate (GMP), with the aim of achieving greater selectivity for phosphatase activity (Scheme 63). Initially, both probes displayed enol emission due to the ESIPT process being blocked. However, in the presence of ALP a large increase in fluorescence emission intensity at 512 nm was observed corresponding to emission from the keto form. Both probes displayed an excellent selectivity for ALP over other types of phosphatase. The biological potential of these probes was demonstrated by imaging endogenous ALP activity in real time in both HeLa cells and a zebrafish model.189
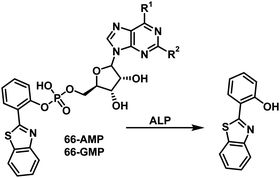 | ||
| Scheme 63 Nucleotide monophosphate-functionalised fluorescent probes 66-AMP and 66-GMP developed for the selection detection of ALP. Measurement conditions: PBS buffer (100 mM, pH = 7.4). | ||
More specifically, Kim and co-workers developed an effective fluorescent probe 67 for the detection of protein tyrosine phosphatase-MKP-6 (PTP MKP-6) activity (Scheme 64). PTP MKP-6 is thought to inhibit the apoptosis of gastric cancer cells; therefore, the ability to detect low levels of MKP-6 activity is potentially important for early cancer diagnosis.190,191 Probe 67 was prepared by phosphorylating the phenol group of HBT resulting in the inhibition of the ESIPT process. Remarkably, probe 67 was shown to be a highly selective substrate for PTP MKP-6 with phosphate hydrolysis resulting in a significant increase in the keto form-derived emission. The selectivity of this phosphatase activity was confirmed through addition of sodium vanadate which is a known inhibitor of PTP MKP-6; under these latter conditions no fluorescence emission was observed.
Nitroreductases (NTR)
Hypoxia is an important condition found in many solid tumours which is caused by the limited availability of molecular oxygen in bodily tissues and is a state that has long been associated with tumour development.192,193 Nitroreductase enzymes (NTR) are flavin-based oxidoreductases that catalyse the reduction of nitroaromatics to their corresponding amino groups. NTRs can be divided into two functional classes: Type I NTRs – oxygen insensitive and Type II NTRs that only function under extreme hypoxic conditions.194,195 Therefore, owing to the enhanced activity of Type II NTRs under hypoxic conditions the development of fluorescent probes for NTR detection is of particular importance in cancer research and diagnosis.Wang and co-workers reported a NTR fluorescent probe 68 for the selective detection of nitroreductase (NTR) activity. In this probe, ESIPT activity is blocked via attachment to the known hypoxic trigger 4-nitroimidazole (Scheme 65). Addition of NTR and nicotinamide adenine dinucleotide (NADH) leads to catalytic reduction of the nitro group, cleavage of the benzylic ether bond, and release of an activated ESIPT fluorophore. Probe 68 was shown to be sensitive towards NTR activity (LOD = 63 ng mL−1) as reflected in a significant enhancement in its fluorescence intensity at 560 nm. Probe 68 was shown to have an excellent selectivity for NTR over other reductants, such as dithiothreitol and other biologically relevant analytes. Probe 68 was found to be cell permeable and capable of detecting changes in the levels of oxygen in HeLa cells, presumably via differences in NTR activity.196
Proteases
Proteases are important enzymes responsible for protein catabolism that generate amino acids through the hydrolysis of amide bonds. Proteases regulate and control the activity of many proteins, modulate protein–protein interactions, and contribute to cellular signalling. They are known to influence cell proliferation, DNA replication and transformation, wound repair, inflammation, necrosis, and apoptosis. Moreover, changes in proteolytic activity have been associated with a number of pathological conditions, such as cancer, neurodegenerative disorders, inflammation, and cardiovascular disease.197 As a result, proteases are important biomarkers for drug delivery and diagnostics. Thus, the development of fluorescence based probes to evaluate protease activity is viewed as a highly desirable research objective.198Hasserodt et al. developed a rapid response system for the detection of leucine aminopeptidase (LAP) using the hydroxyphenylquinazoline (HPQ) fluorescent probes 69 and 70. These two probes undergo self-immolative 1,2-diamine-based cyclisation in the presence of LAP to release insoluble HPQ and the corresponding cyclic urea (Scheme 66). Probe 70 exploits the Thorpe–Ingold effect, which facilitates rapid cyclisation to release HPQ. Additionally, the charge present in the leucine recognition unit ensures good aqueous solubility for both probes. In fact, a high OFF–ON detection sensitivity for LAP was seen with no false positive signals being observed over the course of 24 hours.199
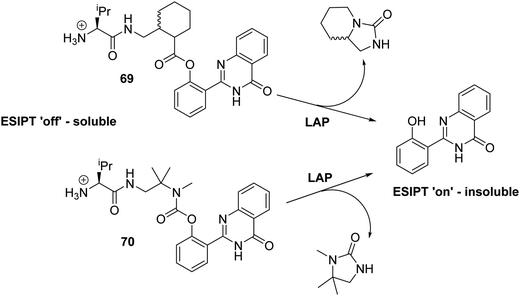 | ||
| Scheme 66 Leucine-functionalised HPQ fluorescent probes 69 and 70 that were developed to allow for the rapid and selective detection of LAP. Measurement conditions: pH = 7.5. | ||
ESIPT probes for interrogating biomolecular interactions
Biological macromolecular recognition depends on the formation of multiple protein–protein and protein–nucleic acid interactions. Therefore, the development of fluorescence-based probes that can selectively interrogate these processes could prove useful.200Mely and co-workers have exploited the environmentally sensitive nature of flavone ESIPT dyes to evaluate the microenvironments of single and double-stranded DNA fragments. In their work, they designed and developed flavone-based fluorescent probes, such as 71 and 72, that were functionalised with a polycationic spermine unit (Fig. 6). Upon binding to a double-stranded DNA (dsDNA), a ca. 16-fold ratiometric change in fluorescence intensity was seen for probes 71 and 72. This increase in fluorescence intensity is believed to result from intercalation of the probes into dsDNA, a binding event that serves to shield them effectively from water molecules. Both probes exhibited a smaller change in ratiometric fluorescence intensity in the presence of single-stranded DNA (ssDNA). This finding was considered consistent with the higher degree of exposure to water molecules expected in the case of ssDNA relative to dsDNA. Importantly, probes 71 and 72 can thus be used discriminate between ssDNA and dsDNA, a finding that was viewed as underscoring the potential utility of these probes in exploring DNA–protein interactions.201
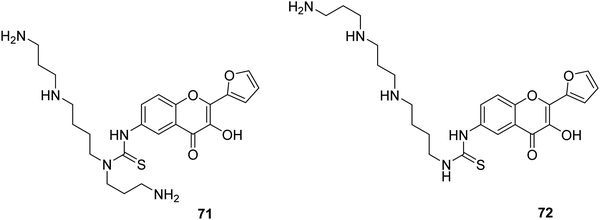 | ||
| Fig. 6 3-Hydroxyflavone probes 71 and 72 used for the interrogation of DNA binding. Measurement conditions: PBS buffer pH 7.0. | ||
Mely and co-workers further targeted peptide–oligonucleotide interactions by means of probe 73 that consists of the flavone ESIPT fluorophore covalently attached to the N-terminus of a peptide corresponding to the Zn-finger recognition domain of HIV-1 nucleocapsid protein (NC) – Fig. 7. Addition of the target oligonucleotides (ODN) to the probe-labelled peptide 73 gives rise to a large change in the ratiometric fluorescence intensity. The ratiometric response was found to be dependent upon the ODN sequence, a finding that enabled the peptide–ODN binding parameters to be determined for multiple ODN binding sites. This work provides a platform technology that illustrates how ESIPT dye-labelled peptides could be used to investigate peptide–ODN interactions.202
More recently, Mely and co-workers reported new probe systems 74–77 that display higher ratiometric sensitivity to solvent polarity (Fig. 8). These systems were covalently attached to the N-terminus of a Tat (44–61) peptide that corresponded to the binding domain of the HIV-1 Tat protein. These ESIPT labelled-peptides produced dramatic ratiometric changes in the fluorescence intensity and could be used to investigate hydration levels in the vicinity of the peptide/oligonucleotide complex, revealing a solvation trend that decreases in the following order short ssDNAs ≫ long ssDNAs > DNA hairpins > dsDNAs.203
Exploiting the environmentally sensitive nature of ESIPT fluorophores, Mely and co-workers developed a dodecyl functionalised 3-hydroxyflavone probe 78. This system is selectively incorporated into the outer leaflet of cell plasma membranes and can monitor changes in the outer membrane lipid content occurring during the initial steps of cell apoptosis (Scheme 67). It was found that probe 78 underwent a real time ratiometric change in its fluorescence during the course of apoptosis. Probe 78 could be used to monitor cellular events involving changes in the membrane content, such as those that occur during uncontrolled cell division. Therefore, it was suggested that probe 78 could be used to monitor cancer metathesis at the molecular level and hence evaluate the efficacy of anticancer drugs.205
Triplex DNA is formed via the sequence-selective recognition of the major groove of a dsDNA by an additional third strand of DNA. DNA triplexes have been shown to have a number of biological activities, including regulation of gene expression, DNA damage and repair. Additionally, triplex DNA has received considerable attention due to its importance in gene regulation, as a molecular switch, and for sensor development. Shao and co-workers screened a number of flavone-based probes for binding to triplex DNA. The natural flavonoid Fisetin (FIS) was found to undergo a large ratiometric change in the fluorescence intensity (I538/I460) in the presence of triplex DNA (Scheme 68). FIS was shown to have excellent selectivity for triplex DNA relative to ssDNA, dsDNA, i-motifs, and DNA/RNA G-quadruplexes. In fact, FIS was shown to stabilise the triplex DNA structure via a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 interaction with both sequence termini.204
1 interaction with both sequence termini.204
Membrane proteins normally contain a large number of transmembrane α-helices, whose expression levels often differ between normal and diseased cells.206 Liu et al. designed an HBT-based fluorescent probe 79 whose keto emission levels were shown to correlate positively with the α-helix content of proteins (Scheme 69). Probe 79 was found to accumulate in cell membranes, where its fluorescence output could be used to detect the presence of the α-helices of membrane proteins. The changes in fluorescence output could be used to distinguish between healthy and prostate cancerous cells since the latter are typically characterized by higher levels of membrane proteins. Therefore, this system could be used as a possible diagnostic tool for the early detection of prostate cancer.207
Pang and co-workers prepared a flavone-based fluorescent probe 80 that was functionalised with a lysosome-directing morpholine unit (Fig. 9). The environmental sensitivity of probe 80, meant that its fluorescence emission intensity at 560 nm increased ∼75 fold on moving from polar to non-polar medium. Co-localisation studies confirmed that the probe could be used for the selective visualisation of lysosomes affording ‘wash-free’ stains that could be used for the real time imaging of lysosomes (Fig. 9).208
Fluorescence based sensors with a combined fluorescence mechanism
Combination of fluorescence mechanisms such as ESIPT with photoinduced electron transfer (PET), or ESIPT with aggregation induced emission (AIE), enables the favourable attributes of both processes to be combined to overcome the limitations of many ESIPT dyes.PET-based fluorescent probes result in a significant change in fluorescence intensity (‘off to on’, or ‘on to off’) in the presence of a target analyte with the emission wavelength remaining unchanged.209 Therefore, the combination of PET and ESIPT can afford a fluorescent probe that exhibits a dual emission spectrum with amplified sensitivity and selectivity.210a,210b Wang and co-workers reported a flavone-based fluorescent probe 81 functionalised with the PET fluorescence quenching unit DNBS (dinitrobenzenesulfonyl) (Scheme 70). As prepared, probe 81 is non-fluorescent, due to PET. However, addition of biological thiols (Cys, HCys, and GSH) results in SNAr cleavage of the dinitroaryl group. This produces a free phenol and gives rise to a large fluorescence response. Probe 81 demonstrated excellent selectivity for biological thiols and could be used to image these analytes in HeLa cells.
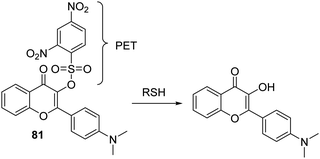 | ||
Scheme 70 A combined PET + ESIPT fluorescent probe 81 that proved effective for the detection of biological thiols. Measurement conditions: PBS/EtOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) – pH = 7.4. 4) – pH = 7.4. | ||
Strongin and co-workers reported a HBT rhodol thioester fluorescent probe 82. This system exploits the fluorescence emission of a combined PET + ESIPT construct to distinguish between GSH and Cys/Hcy (Scheme 71). Addition of Cys/Hcy to probe 82 results in a tandem native chemical ligation (NCL) reaction that affords a deconjugated spirolactam. This NCL reaction results in the quinone of the rhodol unit being transformed into a phenolic group and triggers an ESIPT process that affords a large increase in the fluorescence emission intensity at 454 nm (ESIPT on, no PET). Conversely, addition of GSH, only results in a transthioesterification reaction; this prevents the intramolecular PET process and leads to a large fluorescence response at 587 nm (ESIPT off, no PET). Probe 82 was shown to image biological thiols in HepG2 cells successfully, thus demonstrating its potential for use in biological applications.211
AIE is a unique fluorescence phenomenon that was discovered by Tang et al., wherein a weakly fluorescent probe in the solution state becomes highly fluorescent in the aggregate state. It thus stands in contrast to the traditional self-quenching that occurs for fluorophores at high concentrations.212 AIE normally results in fluorescent probes with a high signal-to-noise ratio and excellent photostability. However, AIE probes generally require good solubility in water in order to ensure a low background signal. ESIPT + AIE probes rely on an intramolecular proton transfer event to induce a change in the fluorescence intensity associated with the ESIPT process but can be designed to favour the formation of aggregates, thus enhancing an AIE-based signal.
Tang et al. reported a phosphorylated chalcone derived fluorescent probe 83 that combines the AIE + ESIPT mechanism and allows for the visualization of ALP activity in living cells (Scheme 72). As prepared, probe 83 is water soluble and emits at 539 nm in aqueous buffer. However, the addition of ALP results in a ratiometric change in the fluorescence emission (I641/I539) due to phosphate hydrolysis and aggregation of the resultant phenol product (which is also responsible for the ESIPT effect). Probe 83 was shown to be sensitive towards ALP activity (LOD = 0.15 mU mL−1) over a range of 0–150 mU mL−1. These attributes allowed it to be used to map ALP activity in HeLa cells.213
Zhang and co-workers designed and developed a novel AIE + ESIPT probe 84. This system combines in one molecular construct tetraphenylethene (TPE), a classic AIE luminogen, and the ESIPT fluorophore HBT (Scheme 73). This combination was expected to the synergistic benefit of both fluorescence mechanisms, namely a high solid-state fluorescence quantum yield (ΦF = 0.97) and a large Stokes shift (>200 nm). In the case of 84, functionalisation with DNBS afforded a probe that was suitable for the imaging biothiols in cells. In fact, probe 84 displayed high sensitivity (LOD = 72 nM) and excellent selectivity over other biologically relevant analytes.214
Lin and co-workers reported an aggregate based AIE + ESIPT fluorescent probe 85 for SO2/SO32−-that contains a leuvinate ester as a SO32−-reactive unit (Scheme 74). As prepared probe 85 is weakly emissive at 422 nm. However, exposure to an aqueous solution of SO32− results in the leuvinate ester being cleaved and gives rise to a large ratiometric change in the fluorescence emission intensity (I567/I470). The formation of aggregates was confirmed using dynamic light scattering (DLS), experiments that revealed the formation of aggregates with an average size of 60 nm. Probe 85 exhibited high sensitivity (LOD = 0.9 μM), and was able to detect SO2 ratiometrically in live cells and zebrafish models.215
Singh and co-workers designed an AIE + ESIPT imaging agent/prodrug probe 86 for the real-time monitoring of drug release (Scheme 75). This AIE + ESIPT drug delivery system (DDS) displayed a number of ideal properties, including: (i) excitation: >410 nm; (ii) drug release only occurring from an aggregated state, and (iii) a ratiometric response to photoactivation. Probe 86 contains two drug molecules and was formulated into nanoparticles to enhance selectivity, permeability, and retention in tumour cells. Irradiation for 5 minutes resulted in significant cytotoxicity, a finding ascribed to the photoinduced release of the drug molecules. This putative release was accompanied by a ratiometric change in the observed fluorescence in HeLa cells.216
Zhang and co-workers developed a novel solid-state AIE + ESIPT fluorophore (HTPQ and TPE), probe 87, whose phenolic unit was functionalised with a phosphate unit to inhibit the ESIPT processes (Scheme 76). Exposure to ALP resulted in phosphate hydrolysis, a conversion that produced an insoluble fluorophore that aggregated after a short period of time to produce a 100-fold fluorescence increase in the emission intensities at 550 nm. The insoluble diffusion-resistant properties of the cleavage product of probe 87, enabled ALP to be successfully visualised at the cell membranes of HeLa cells. Probe 87 was also used to visualise different ALP activity levels in Saos-2 and U-2OS osteosarcoma cells, thus enabling the role of ALP in the development of physiological and pathological processes to be determined.217
Hu et al. developed an AIE + ESIPT HPQ-based fluorescent probe 88 that was designed to allow for the enzymatic detection of β-galactosidase (β-Gal). This is an enzyme that catalyses the hydrolysis of oligosaccharides containing β-galactoside linkages. Overexpression of β-Gal is widely recognised as a biomarker for cell senescence and primary ovarian cancer.218,219 The inherent ESIPT process of probe 88 was blocked through attachment of galactose to the phenolic unit of HPQ. In the presence of β-Gal, the galactose unit of probe 88 is cleaved turning on the ESIPT process, whilst allowing the formation of aggregates resulting in a large increase in fluorescence emission intensity at 495 nm (Scheme 77). Probe 88 was used successfully for imaging β-Gal activity in OVCAR-3 cells with the insoluble nature of HPQ resulting in the fluorophore being retained in living cells. These are features that make this probe particularly suitable for intracellular imaging applications.220
Liu and co-workers developed a salicylaldehyde-derived azine fluorescent probe 89 that was designed to display both an AIE and an ESIPT response to β-Gal (Scheme 78). Upon addition of β-Gal, the galactose recognition units were cleaved. This activated an ESIPT process whilst also producing aggregates, as evidenced by the strong enhancement in fluorescence emission (820 fold) and a large Stokes shift (190 nm). Probe 89 was shown to display high sensitivity (LOD = 0.014 U mL−1) and excellent selectivity for β-Gal activity. Its cellular retention and use in imaging of C6/LacZ cells was aided by administrating it as a colloidal solution.221
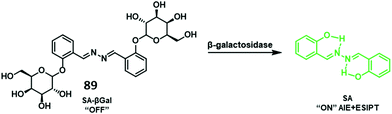 | ||
| Scheme 78 A salicylaldehyde azine AIE + ESIPT probe 89 developed to allow for the monitoring of β-galactosidase activity in C6/LacZ cells. Measurement conditions: PBS buffer (10 mM, pH = 7.4). | ||
Liu and co-workers reported a morpholine-functionalised salicylaldehyde azine fluorescent probe 90 for the in situ visualisation of lysosomal esterase activity. As prepared, the acetate groups present in probe 90 serve to block the ESIPT process but also act as recognition units for esterases (Scheme 79). Treatment with esterase results in generation of free phenol groups and a ‘turning on’ of the ESIPT process, as well as aggregate formation. The net result is a large increase in the fluorescence emission intensity at 532 nm (Stokes shift = 176 nm). Probe 90 was shown to target lysosomes and to produce an enzyme-dependent fluorescence response in MCF-7 cells within 8 min. Additionally, probe 90 was found capable of tracking lysosome movements over time in the presence of chloroquine, an agent that is known to induce the movement of lysosomes.222
Tong reported a salicylaldehyde azine-derived fluorescent probe 91 that contains a hydroxyl ESIPT donor masked by a Cys-reactive acryloyl group. Addition of Cys results in hydrolysis of the acrylate group and release the AIE + ESIPT fluorophore (Scheme 80). Probe 91 was shown to permit the selective detection of Cys over the 0–30 μM concentration with a limit of detection as low as 0.46 μM. This probe also proved capable of measuring concentrations of Cys in fetal bovine serum.223
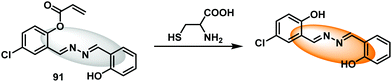 | ||
Scheme 80 An AIE + ESIPT-based fluorescent probe 91 for the detection of cysteine. Measurement conditions: PBS/DMSO (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) – pH = 7.4. 3) – pH = 7.4. | ||
Yoon and co-workers reported a similar p-methoxy-salicylaldehyde azine-derived fluorescent probe 92 that has both hydroxyl units of the ESIPT unit protected with Cys-reactive acryloyl groups. Addition of Cys caused the cleavage of both acryloyl groups and formation of aggregates. This resulted in a large increase in the fluorescence intensity at 505 nm. A large Stokes shift of 165 nm is also observed (Scheme 81). Probe 92 displayed excellent selectivity towards Cys over other biothiols (HCys and GSH) and could be used to detect Cys selectively in HeLa cells.224
Jiang and co-workers developed a Schiff base fluorescent probe 93 containing two phenolic groups. In the presence of CN−, this system undergoes an oxidative cyclization to form HBO (Scheme 82). Probe 93 displayed a strong fluorescence emission intensity in the presence of CN− in a micellar environment due to a combined ESIPT process and AIE effect. Probe 93 was shown to display excellent selectivity and high sensitivity for CN− with a LOD = 0.59 μM. Notably, this latter value is lower than the WHO guideline for CN− in drinking water (1.9 μM). Finally, test papers were prepared based on 93 that enabled the practical detection of CN−.225
 | ||
| Scheme 82 Schiff-base fluorescent probe 93. This system undergoes a cyanide anion-induced oxidative cyclisation to form an active ESIPT fluorophore, HBO. Measurement conditions: H2O containing CTAB. | ||
Attachment of an electron acceptor to an ESIPT fluorophore can afford near infrared (NIR) probes that can be used to carry out ratiometric sensing at lower emission energies. Probes with longer excitation/emission wavelengths minimise background auto-fluorescence from proteins and photodamage to biological samples. They also permit far greater tissue penetration, a feature that is useful in the in vivo imaging of tissues.
Zhao and co-workers reported an ESIPT + FRET based fluorescent probe 94 for the endogenous detection of bisulfite (HSO3−) in liver cancer cells. This system incorporates an ESIPT flavone fluorophore conjugated to a hemicyanine moiety and displays a high FRET efficiency (Fig. 10). Addition of HSO3−/SO32− results in a decrease in fluorescence emission at 590 nm and a concomitant increase in the emission intensity at 530 nm. This is ascribed to the nucleophilic attack of SO32− at the alkene bond of the hemicyanine moiety, resulting in emission from the keto form of the flavone being turned on. Probe 94 was shown to have good sensitivity (LOD = 16 nM) and to produce a large ratiometric change in the fluorescence intensity (I530/I590) upon exposure to HSO3−/SO32− over a 1.5–22.5 μM concentration range. This allowed it to be used to image endogenous levels in HepG2 cells.226
 | ||
Fig. 10 A FRET + ESIPT-based fluorescent probe 94 for the detection of bisulfite in HepG2 cells. Measurement conditions: PBS/DMF (7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) – pH = 7.4. 2.5) – pH = 7.4. | ||
Feng et al. reported a long-wavelength HBT–hemicyanine hybrid, probe 95. This system combines intramolecular charge transfer (ICT) and ESIPT effects and was found to give rise to a rapid colorimetric and ratiometric fluorescence response upon exposure to the bisulfite anion (Scheme 83). Conjugate addition of HSO3− to the alkene functionality in 95 eliminates the ICT pathway, resulting in only the ESIPT emission of the HBT being observed. Probe 95 was shown to detect HSO3− with good sensitivity in aqueous solution. A linear response was seen over a 0–25 μM concentration range, with a detection limit of 56 nM being determined. This probe could be used to detect exogenous HSO3− in food samples and HeLa cells.227
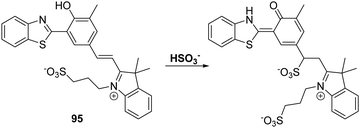 | ||
Scheme 83 A combined ICT + ESIPT fluorescent probe 95 for the detection of HSO3−. Measurement conditions: PBS/EtOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) – pH = 7.4. 4) – pH = 7.4. | ||
Pang et al. developed a near-infrared (NIR) lysosomal fluorescent probe 96 that could be used to monitor pH levels (Scheme 84).228 This ESIPT probe exhibits a large Stokes shift 234 nm with a NIR-emission (tautomer = 700 nm). The phenolic proton is proposed to act as a switch and to provide a strong fluorescence emission at pH 5 that could be used to selectively visualise lysosome organelles.
 | ||
| Scheme 84 A NIR-lysosomal targeting ESIPT + FRET probe 96 for the imaging of lysosomes in normal human lung fibroblast cells. Measurement conditions: H2O at various pH. | ||
Conclusion
Excited State Intramolecular Proton Transfer (ESIPT) is a unique four-level photochemical process that relies on extremely fast enol to keto phototautomerization (kESIPT > 1012 s−1) pathways to enable fluorescence based detection of reactive analytes. As a result of this rapid photochemical process, ESIPT fluorophores exhibit a number of particularly attractive attributes that have been used in the design of fluorescent probes. ESIPT-based probes have been designed to enable ratiometric detection of a specific analyte. This is a key attribute that provides direct information on the concentration of the analyte in question without the need for calibration. Moreover, the ESIPT emission is readily influenced by the environment. As a consequence, it is often found that the presence of polar and hydrogen bond donating solvents results in an inhibition of the ESIPT process with no keto (K*) emission being observed. This property could be viewed as a disadvantage. However, this property has been used to help image a range of biomacromolecules. A specific example includes the development of ESIPT-fluorophores functionalised with the lysosome-directing morpholine group by Pang and co-workers as discussed above. In an aqueous biological environment, the probes were non-fluorescent. Once they entered the hydrophobic pocket of lysosomes a large fluorescence increase was observed. Co-localisation studies confirmed that the probes were selective for the visualisation of lysosomes and effective as ‘wash-free’ stains thus making them suitable imaging agents for the real time monitoring.A particularly interesting and exciting development has been the combination of ESIPT with other fluorescence mechanisms. The systems produced as the result of this intellectual and chemical linking generally combine the favourable attributes of both mechanisms and override many of the limitations of ESIPT. Aggregate induced emission (AIE), where systems are weakly fluorescent in solution but highly fluorescent when aggregated, has emerged as a promising complement to ESIPT. Specifically, the combination of AIE and ESIPT can be used to overcome the environmentally sensitive nature of the ESIPT process, since the formation of aggregates favours the sensitive nature of the ESIPT emission. Similarly, the attachment of an additional fluorophore to an ESIPT fluorophore (ESIPT and FRET) enables the development of longer wavelength/near infrared (NIR) probes that show promise for the detection of biologically relevant analytes in vivo. Lastly, the use of photoinduced electron transfer (PET)-based fluorescent probes can produce systems that display significant changes in the fluorescence intensity (‘off to on’ or ‘on to off’) in the presence of a target analyte with the emission wavelength remaining unchanged. This can give rise to amplified sensitivity and selectivity.
Overall, ESIPT is emerging an important sensing mechanism for the detection of biologically and/or environmentally important species. In this review, we have selected representative examples up to and including work published by August 2018. While not fully comprehensive in its coverage, an effort has been made to be as thorough as possible while focusing on examples that we hope will allow the reader to appreciate the unique aspects of ESIPT and how it can be used for the development of fluorescence-based probes. We believe that ESIPT fluorophores will continue to provide an important foundation on which to build. We further predict that its use in combination with other fluorescence mechanisms will allow new probes to be constructed that overcome the limitations of many current detection methods while allowing for the development of an ever-increasing number of practical fluorescence based sensors and imaging agents.229 It is our hope that this review will inspire the readers to take up the challenge of these new, not-yet-conceived probes.
“There's Nature and she's going to come out the way She is. So therefore when we go to investigate we shouldn’t predecide what it is we’re looking for only to find out more about it. Now you ask: “Why do you try to find out more about it?” If you began your investigation to get an answer to some deep philosophical question, you may be wrong. It may be that you can’t get an answer to that particular question just by finding out more about the character of Nature. But that's not my interest in science; my interest in science is to simply find out about the world and the more I find out the better it is…” Richard P. Feynman (1918–1988)
Abbreviations
| 3-HF | 3-Hydroxyflavone |
| α | Alpha |
| β | Beta |
| β-Gal | Beta-galactosidase |
| δ | Delta, chemical shift |
| μM | Micromolar |
| ACN | Acetonitrile |
| Ag+ | Silver ion |
| AIE | Aggregation induced emission |
| Al3+ | Aluminium ion |
| ALP | Alkaline phosphatase |
| AMP | Adenosine monophosphate |
| Angeli's salt (Na2N2O3) | Sodium trioxodinitrate |
| Ar | Aryl |
| Ba2+ | Barium ion |
| BBB | Blood brain barrier |
| Br | Bromine |
| Ca2+ | Calcium ion |
| Cd2+ | Cadmium ion |
| CD3CN | Deuterated acetonitrile |
| CE | Human carboxylesterase |
| CE1 | Human carboxylesterase 1 |
| CE2 | Human carboxylesterase 2 |
| CH3COO− | Acetate ion |
| Cl− | Chloride anion |
| ClO− | Hypochlorite anion |
| CN− | Cyanide anion |
| Co2+ | Cobalt ion |
| COCl2 | Phosgene |
| Cr | Chromium |
| Cr3+ | Chromium ion |
| CTAB | Cetyl trimethylammonium bromide |
| Cu+/2+ | Copper ion |
| Cys | Cysteine |
| DEA NONOate | 1,1-Diethyl-2-hydroxy-2-nitroso-hydrazine |
| DLS | Dynamic light scattering |
| DMF | Dimethylformamide |
| DMN | Diaminomaleonitrile |
| DMSO | Dimethylsulfoxide |
| DNA | Deoxyribose nucleic acid |
| DNBS | Dinitrobenzenesulfonyl |
| DPA | Dipicolylamine |
| DPBS | Dulbecco's phosphate buffer saline |
| dsDNA | Double stranded DNA |
| E | Enol |
| Er3+ | Erbium ion |
| ESIPT | Excited state intramolecular proton transfer |
| Et | Ethyl |
| EtOH | Ethanol |
| F− | Fluoride ion |
| Fe2+/3+ | Iron ion |
| FIS | Fisetin |
| FRET | Förster resonance energy transfer |
| Gd2+ | Gadolinium ion |
| GMP | Guanosine monophosphate |
| GSH | Glutathione |
| h | Hours |
| H+ | Proton |
| HBI | 2-(2′-Hydroxyphenyl)benzimidazole |
| HBO | 2-(2-Hydroxyphenyl)-benzoxazole |
| HBT | 2-(2-Hydroxyphenyl)-benzothiazole |
| HCys | Homocysteine |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| Hg2+ | Mercury ion |
| HIV | Human immunodefiency virus |
| HMBT | 2-(2′-Hydroxy-3′-methoxyphenyl)benzothiazole |
| hMSCs | Human mesenchymal stem cells |
| HNO | Nitroxyl ion |
| HO− | Hydroxyl anion |
| H2O | Water |
| H2O2 | Hydrogen peroxide |
| H2O3 | Dihydrogen trioxide |
| HOCl | Hypochlorite |
| H2PO4− | Dihydrogen phosphate |
| HPQ | Hydroxyphenylquinazoline |
| H2S | Hydrogen Sulphide |
| HSO3− | Bisulfite ion |
| HTL | Homocysteine thiolactone |
| I | Intensity |
| ICT | Intramolecular charge transfer |
| K | Keto |
| K+ | Potassium ion |
| KNO3 | Potassium nitrate |
| KO2 | Potassium superoxide |
| LAP | Leucine aminopeptidase |
| LOD | Limit of detection |
| Me | Methyl |
| MeOH | Methanol |
| Met | Methionine |
| MetRS | Methionyl t-RNA synthetase |
| Mg2+ | Magnesium ion |
| Min | Minutes |
| mL | Millilitres |
| mM | Millimolar |
| Mn2+ | Manganese ion |
| MPO | Myeloperoxidase |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| mU | MilliUnits |
| Na+ | Sodium ion |
| NaCl | Sodium chloride |
| NADH | Nicotinamide adenine dinucleotide |
| Na2N2O3 | Angeli's salt |
| NC | Nucleocapsid protein |
| NCL | Native chemical ligation |
| Nd3+ | Neodymium ion |
| NEt3 | Triethylamine |
| N2H4 | Hydrazine |
| Ni2+ | Nickel ion |
| NIR | Near-infrared |
| nm | Nanometres |
| nM | Nanomolar |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NTR | Nitroreductase |
| 1O2 | Singlet Oxygen |
| O2 | Oxygen |
| O2− | Superoxide |
| O3 | Ozone |
| OAc | Acetate |
| ODN | Oligonucleotides |
| OH | Hydroxyl radical |
| OMe | Methoxy |
| ONOO− | Peroxynitrite |
| Pb2+ | Lead ion |
| PBS | Phosphate buffer saline |
| PCR | Polymerase chain reaction |
| Pd | Palladium |
| Pd2+ | Palladium ion |
| PET | Photoinduced electron transfer |
| Ph | Phenyl |
| ppm | Parts per million |
| ppb | Parts per billion |
| PTP | Protein tyrosine phosphatase |
| PPi | Pyrophosphate |
| R | Unspecified generic group |
| R-B(OH)2 | Boronic acids |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
| RPT | Reverse proton transfer |
| S | Sulfur |
| S2− | Sulfide |
| SNAr | Nucleophilic aromatic substitution |
| SO2 | Sulfur dioxide |
| SO32− | Sulfite ion |
| ssDNA | Single stranded DNA |
| Tat | Trans-activator of transcription |
| TBDMS | tert-Butyldimethylsilyl |
| TBDPS | tert-Butyldiphenyllsilyl ether |
| TBS | Tris-buffered saline |
| THF | Tetrahydrofuran |
| TLC | Thin layer chromatography |
| TPE | Tetraphenylethene |
| UV | Ultraviolet |
| WHO | World Health Organisation |
| Yb3+ | Ytterbium ion |
| Zn2+ | Zinc ion |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
ACS thanks the EPSRC for a studentship. LW wishes to thank China Scholarship Council and the University of Bath for supporting his PhD work in the UK. ACS, LW, SDB and TDJ would like to thank the EPSRC and the University of Bath for funding. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award and Sophia University for a visiting professorship. HHH, XPH, HT thank the Natural Science Foundation of China (No. 21788102, 21722801 and 21572058), the Program of Introducing Talents of Discipline to Universities (B16017) and the Shanghai Rising-Star Program (16QA1401400) (XPH) for generous financial support. BZT thanks the National Natural Science Foundation of China (21788102) and the Innovation and Technology Commission of Hong Kong (ITC-CNERC14SC01) for financial support. JY acknowledges a grant from the National Creative Research Initiative programs of the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2012R1A3A2048814). JLS thanks the National Institutes of Health (RGM103790A) and the Robert A. Welch Foundation (F-0018) for support. We would like to thank Lauren Gwynne and Bethany L. Patenall for proof reading the manuscript.References
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- (a) Y. N. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC; (b) W. Sun, S. Guo, C. Hu, J. Fan and X. Peng, Chem. Rev., 2016, 116(14), 7768–7817 CrossRef CAS PubMed.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Sci., 2018, 9, 3672–3676 RSC.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2017, 53, 12822–12825 RSC.
- A. C. Sedgwick, A. Hayden, B. Hill, S. D. Bull, R. B. P. Elmes and T. D. James, Front. Chem. Sci. Eng., 2018, 12, 311–314 CrossRef CAS.
- A. C. Sedgwick, J. E. Gardiner, G. Kim, M. Yevglevskis, M. D. Lloyd, A. T. A. Jenkins, S. D. Bull, J. Yoon and T. D. James, Chem. Commun., 2018, 54, 4786–4789 RSC.
- A. Weller, Naturwissenschaften, 1955, 42, 175–176 CrossRef CAS.
- J. Z. Zhao, S. M. Ji, Y. H. Chen, H. M. Guo and P. Yang, Phys. Chem. Chem. Phys., 2012, 14, 8803–8817 RSC.
- V. S. Padalkar and S. Seki, Chem. Soc. Rev., 2016, 45, 169–202 RSC.
- J. E. Kwon and S. Y. Park, Adv. Mater., 2011, 23, 3615–3642 CrossRef CAS PubMed.
- J. S. Wu, W. M. Liu, J. C. Ge, H. Y. Zhang and P. F. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
- P. T. Chou, Y. C. Chen, W. S. Yu, Y. H. Chou, C. Y. Wei and Y. M. Cheng, J. Phys. Chem. A, 2001, 105, 1731–1740 CrossRef CAS.
- M. T. Ignasiak, C. Houee-Levin, G. Kciuk, B. Marciniak and T. Pedzinski, ChemPhysChem, 2015, 16, 628–633 CrossRef CAS PubMed.
- D. Ghosh, S. Batuta, S. Das, N. A. Begum and D. Mandal, J. Phys. Chem. B, 2015, 119, 5650–5661 CrossRef CAS PubMed.
- S. J. Schmidtke, D. F. Underwood and D. A. Blank, J. Am. Chem. Soc., 2004, 126, 8620–8621 CrossRef CAS PubMed.
- M. J. Paterson, M. A. Robb, L. Blancafort and A. D. DeBellis, J. Am. Chem. Soc., 2004, 126, 2912–2922 CrossRef CAS PubMed.
- E. Hadjoudis and I. M. Mavridis, Chem. Soc. Rev., 2004, 33, 579–588 CAS.
- Y. Nakane, T. Takeda, N. Hoshino, K. Sakai and T. Akutagawa, J. Phys. Chem. A, 2015, 119, 6223–6231 CrossRef CAS PubMed.
- N. Sarkar, K. Das, S. Das, A. Datta, D. Nath and K. Bhattacharyya, J. Phys. Chem., 1995, 99, 17711–17714 CrossRef.
- K. Das, N. Sarkar, A. K. Ghosh, D. Majumdar, D. N. Nath and K. Bhattacharyya, J. Phys. Chem., 1994, 98, 9126–9132 CrossRef CAS.
- M. H. Lee, J. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 4185–4191 RSC.
- W. Aoi and Y. Marunaka, BioMed Res. Int., 2014, 598986, DOI:10.1155/2014/598986.
- P. Swietach, R. D. Vaughan-Jones, A. L. Harris and A. Hulikova, Philos. Trans. R. Soc., B, 2014, 369 DOI:10.1098/rstb.2013.0099.
- V. S. Patil, V. S. Padalkar, K. R. Phatangare, V. D. Gupta, P. G. Umape and N. Sekar, J. Phys. Chem. A, 2012, 116, 536–545 CrossRef CAS PubMed.
- V. S. Patil, V. S. Padalkar, A. B. Tathe and N. Sekar, Dyes Pigm., 2013, 98, 507–517 CrossRef CAS.
- Y. P. Chan, L. Fan, Q. H. You, W. H. Chan, A. W. M. Lee and S. M. Shuang, Tetrahedron, 2013, 69, 5874–5879 CrossRef CAS.
- D. J. Colacurcio and R. A. Nixon, Ageing Res. Rev., 2016, 32, 75–88 CrossRef CAS PubMed.
- A. Kogot-Levin, M. Zeigler, A. Ornoy and G. Bach, Pediatr. Res., 2009, 65, 686–690 CrossRef CAS PubMed.
- B. Winchester, A. Vellodi and E. Young, Biochem. Soc. Trans., 2000, 28, 150–154 CrossRef CAS PubMed.
- Q. Q. Wang, L. Y. Zhou, L. P. Qiu, D. Q. Lu, Y. X. Wu and X. B. Zhang, Analyst, 2015, 140, 5563–5569 RSC.
- Y. Kato, S. Ozawa, C. Miyamoto, Y. Maehata, A. Suzuki, T. Maeda and Y. Baba, Cancer Cell Int., 2013, 13 DOI:10.1186/1475-2867-13-89.
- S. Barman, S. K. Mukhopadhyay, M. Gangopadhyay, S. Biswas, S. Dey and N. D. P. Singh, J. Mater. Chem. B, 2015, 3, 3490–3497 RSC.
- H. Rubin, BioEssays, 2005, 27, 311–320 CrossRef CAS PubMed.
- M. Liu, X. Yu, M. Li, N. X. Liao, A. Y. Bi, Y. P. Jiang, S. Liu, Z. C. Gong and W. B. Zeng, RSC Adv., 2018, 8, 12573–12587 RSC.
- N. Singh, N. Kaur, R. C. Mulrooney and J. F. Callan, Tetrahedron Lett., 2008, 49, 6690–6692 CrossRef CAS.
- J. M. Berg and Y. G. Shi, Science, 1996, 271, 1081–1085 CrossRef CAS PubMed.
- S. L. Sensi, P. Paoletti, A. I. Bush and I. Sekler, Nat. Rev. Neurosci., 2009, 10, 780–791 CrossRef CAS PubMed.
- S. Alam and S. L. Kelleher, Nutrients, 2012, 4, 875–903 CrossRef CAS PubMed.
- C. T. Chasapis, A. C. Loutsidou, C. A. Spiliopoulou and M. E. Stefanidou, Arch. Toxicol., 2012, 86, 521–534 CrossRef CAS PubMed.
- S. K. Ghosh, P. Kim, X. A. Zhang, S. H. Yun, A. Moore, S. J. Lippard and Z. Medarova, Cancer Res., 2010, 70, 6119–6127 CrossRef CAS PubMed.
- Y. Q. Xu and Y. Pang, Chem. Commun., 2010, 46, 4070–4072 RSC.
- L. J. Tang, M. J. Cai, P. Zhou, J. Zhao, K. L. Zhong, S. H. Hou and Y. J. Bian, RSC Adv., 2013, 3, 16802–16809 RSC.
- F. J. Huo, Q. Wu, J. Kang, Y. B. Zhang and C. X. Yin, Sens. Actuators, B, 2018, 262, 263–269 CrossRef CAS.
- M. An, B. Y. Kim, H. Seo, A. Helal and H. S. Kim, Spectrochim. Acta, Part A, 2016, 169, 87–94 CrossRef CAS PubMed.
- R. A. Festa and D. J. Thiele, Curr. Biol., 2011, 21, R877–R883 CrossRef CAS PubMed.
- C. Cheignon, M. Tomas, D. Bonnefont-Rousselot, P. Faller, C. Hureau and F. Collin, Redox Biol., 2018, 14, 450–464 CrossRef CAS PubMed.
- D. J. Waggoner, T. B. Bartnikas and J. D. Gitlin, Neurobiol. Dis., 1999, 6, 221–230 CrossRef CAS PubMed.
- D. Maity, V. Kumar and T. Govindaraju, Org. Lett., 2012, 14, 6008–6011 CrossRef CAS PubMed.
- C. Y. Yang, Y. Chen, K. Wu, T. Wei, J. L. Wang, S. S. Zhang and Y. F. Han, Anal. Methods, 2015, 7, 3327–3330 RSC.
- H. Wang, D. L. Shi, J. Li, H. Y. Tang and Y. Guo, Sens. Actuators, B, 2018, 256, 600–608 CrossRef CAS.
- Y. M. Shen, X. Y. Zhang, C. X. Zhang, Y. Y. Zhang, J. L. Jin and H. T. Li, Spectrochim. Acta, Part A, 2018, 191, 427–434 CrossRef CAS PubMed.
- S. Okamoto and L. D. Eltis, Metallomics, 2011, 3, 963–970 RSC.
- G. Papanikolaou and K. Pantopoulos, Toxicol. Appl. Pharmacol., 2005, 202, 199–211 CrossRef CAS PubMed.
- T. A. Rouault, Nat. Chem. Biol., 2006, 2, 406–414 CrossRef CAS PubMed.
- I. De Domenico, D. M. Ward and J. Kaplan, Nat. Rev. Mol. Cell Biol., 2008, 9, 72–81 CrossRef CAS PubMed.
- B. Kuzu, M. Tan, Z. Ekmekci and N. Menges, J. Lumin., 2017, 192, 1096–1103 CrossRef CAS.
- J. F. Wang, Y. B. Li, N. G. Patel, G. Zhang, D. M. Zhou and Y. Pang, Chem. Commun., 2014, 50, 12258–12261 RSC.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- M. Harada, Crit. Rev. Toxicol., 1995, 25, 1–24 CrossRef CAS PubMed.
- M. Santra, B. Roy and K. H. Ahn, Org. Lett., 2011, 13, 3422–3425 CrossRef CAS PubMed.
- B. Gu, L. Y. Huang, N. X. Mi, P. Yin, Y. Y. Zhang, X. M. Tu, X. B. Luo, S. L. Luo and S. Z. Yao, Analyst, 2015, 140, 2778–2784 RSC.
- J. Kielhorn, C. Melber, D. Keller and I. Mangelsdorf, Int. J. Hyg. Environ. Health, 2002, 205, 417–432 CrossRef CAS PubMed.
- J. C. Wataha and C. T. Hanks, J. Oral Rehabil., 1996, 23, 309–320 CrossRef CAS PubMed.
- B. Liu, H. Wang, T. S. Wang, Y. Y. Bao, F. F. Du, J. Tian, Q. B. A. Li and R. K. Bai, Chem. Commun., 2012, 48, 2867–2869 RSC.
- T. T. Chen, T. W. Wei, Z. J. Zhang, Y. H. Chen, J. Qiang, F. Wang and X. Q. Chen, Dyes Pigm., 2017, 140, 392–398 CrossRef CAS.
- T. Gao, P. F. Xu, M. H. Liu, A. Y. Bi, P. Z. Hu, B. Ye, W. Wang and W. B. Zeng, Chem. – Asian J., 2015, 10, 1142–1145 CrossRef CAS PubMed.
- W. F. Luo, J. Li and W. S. Liu, Org. Biomol. Chem., 2017, 15, 5846–5850 RSC.
- R. A. Terkeltaub, Am. J. Physiol.: Cell Physiol., 2001, 281, C1–C11 CrossRef CAS PubMed.
- W. H. Chen, Y. Xing and Y. Pang, Org. Lett., 2011, 13, 1362–1365 CrossRef CAS PubMed.
- M. D. Brand, C. Affourtit, T. C. Esteves, K. Green, A. J. Lambert, S. Miwa, J. L. Pakay and N. Parker, Free Radical Biol. Med., 2004, 37, 755–767 CrossRef CAS PubMed.
- M. Hayyan, M. A. Hashim and I. M. AlNashef, Chem. Rev., 2016, 116, 3029–3085 CrossRef CAS PubMed.
- K. Niizuma, H. Yoshioka, H. Chen, G. S. Kim, J. E. Jung, M. Katsu, N. Okami and P. H. Chan, Biochim. Biophys. Acta, Mol. Basis Dis., 2010, 1802, 92–99 CrossRef CAS PubMed.
- G. W. Kim, T. Kondo, N. Noshita and P. H. Chan, Stroke, 2002, 33, 809–815 CrossRef CAS PubMed.
- D. P. Murale, H. Kim, W. S. Choi and D. G. Churchill, Org. Lett., 2013, 15, 3946–3949 CrossRef CAS PubMed.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS PubMed.
- J. S. Beckman and W. H. Koppenol, Am. J. Physiol., 1996, 271, C1424–C1437 CrossRef CAS PubMed.
- P. Ascenzi, A. di Masi, C. Sciorati and E. Clementi, BioFactors, 2010, 36, 264–273 CrossRef CAS PubMed.
- H. Ischiropoulos and J. S. Beckman, J. Clin. Invest., 2003, 111, 163–169 CrossRef CAS PubMed.
- P. Sarchielli, F. Galli, A. Floridi and V. Gallai, Amino Acids, 2003, 25, 427–436 CrossRef CAS PubMed.
- D. A. Wink, Y. Vodovotz, J. Laval, F. Laval, M. W. Dewhirst and J. B. Mitchell, Carcinogenesis, 1998, 19, 711–721 CrossRef CAS PubMed.
- X. Li, R.-R. Tao, L.-J. Hong, J. Cheng, Q. Jiang, Y.-M. Lu, M.-H. Liao, W.-F. Ye, N.-N. Lu, F. Han, Y.-Z. Hu and Y.-H. Hu, J. Am. Chem. Soc., 2015, 137, 12296–12303 CrossRef CAS PubMed.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radical Biol. Med., 2009, 47, 1401–1407 CrossRef CAS PubMed.
- A. C. Sedgwick, X. L. Sun, G. Kim, J. Yoon, S. D. Bull and T. D. James, Chem. Commun., 2016, 52, 12350–12352 RSC.
- L. Wu, Y. Wang, M. Weber, L. Liu, A. C. Sedgwick, S. D. Bull, C. Huang and T. D. James, Chem. Commun., 2018, 54, 9953–9956 RSC.
- J. A. Bernstein, N. Alexis, C. Barnes, I. L. Bernstein, A. Nel, D. Peden, D. Diaz-Sanchez, S. M. Tarlo and P. B. Williams, J. Allergy Clin. Immunol., 2004, 114, 1116–1123 CrossRef PubMed.
- H. Traboulsi, N. Guerrina, M. Iu, D. Maysinger, P. Ariya and C. J. Baglole, Int. J. Mol. Sci., 2017, 18, 243, DOI:10.3390/ijms18020243.
- T. M. Chen, J. Gokhale, S. Shofer and W. G. Kuschner, Am. J. Med. Sci., 2007, 333, 249–256 CrossRef PubMed.
- X. B. Wang, J. B. Du and H. Cui, Life Sci., 2014, 98, 63–67 CrossRef CAS PubMed.
- S. Chen, P. Hou, J. X. Wang and X. Z. Song, RSC Adv., 2012, 2, 10869–10873 RSC.
- A. Strzepa, K. A. Pritchard and B. N. Dittel, Cell. Immunol., 2017, 317, 1–8 CrossRef CAS PubMed.
- M. J. Davies, J. Clin. Biochem. Nutr., 2011, 48, 8–19 CrossRef CAS PubMed.
- S. J. Klebanoff, J. Leukocyte Biol., 2005, 77, 598–625 CrossRef CAS PubMed.
- B. S. van der Veen, M. P. J. de Winther and P. Heeringa, Antioxid. Redox Signaling, 2009, 11, 2899–2937 CrossRef CAS PubMed.
- L. Chen, S. J. Park, D. Wu, H. M. Kim and J. Yoon, Dyes Pigm., 2018, 158, 526–532 CrossRef CAS.
- A. Manna and S. Goswami, New J. Chem., 2015, 39, 4424–4429 RSC.
- C. C. Chang, F. Wang, J. Qiang, Z. J. Zhang, Y. H. Chen, W. Zhang, Y. Wang and X. Q. Chen, Sens. Actuators, B, 2017, 243, 22–28 CrossRef CAS.
- L. Wu, Q. Yang, L. Liu, A. C. Sedgwick, A. J. Cresswell, S. D. Bull, C. Huang and T. D. James, Chem. Commun., 2018, 54, 8522–8525 RSC.
- J. L. Way, Annu. Rev. Pharmacol. Toxicol., 1984, 24, 451–481 CrossRef CAS PubMed.
- R. Koenig, Science, 2000, 287, 1737–1738 CrossRef CAS PubMed.
- M. K. Bera, C. Chakraborty, P. K. Singh, C. Sahu, K. Sen, S. Maji, A. K. Das and S. Malik, J. Mater. Chem. B, 2014, 2, 4733–4739 RSC.
- S. Goswami, A. Manna, S. Paul, A. K. Das, K. Aich and P. K. Nandi, Chem. Commun., 2013, 49, 2912–2914 RSC.
- A. Ghosh, K. Mukherjee, S. K. Ghosh and B. Saha, Res. Chem. Intermed., 2013, 39, 2881–2915 CrossRef CAS.
- R. Hu, J. A. Feng, D. H. Hu, S. Q. Wang, S. Y. Li, Y. Li and G. Q. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915–4918 CrossRef CAS PubMed.
- (a) S. Goswami, A. K. Das, A. Manna, A. K. Maity, H. K. Fun, C. K. Quah and P. Saha, Tetrahedron Lett., 2014, 55, 2633–2638 CrossRef CAS; (b) A. K. Mahapatra, S. Mondal, S. K. Manna, K. Maiti, R. Maji, S. Samim, D. Mandal, M. R. Uddin and S. Mandal, Supramol. Chem., 2016, 28, 693–706 CrossRef CAS.
- S. D. Liu, L. W. Zhang, P. P. Zhou, Y. Yang and W. S. Wu, Sens. Actuators, B, 2018, 255, 401–407 CrossRef CAS.
- C. Saravanan, S. Easwaramoorthi, C. Y. Hsiow, K. Wang, M. Hayashi and L. Wang, Org. Lett., 2014, 16, 354–357 CrossRef CAS PubMed.
- Y. K. Wu, X. J. Peng, J. L. Fan, S. Gao, M. Z. Tian, J. Z. Zhao and S. Sun, J. Org. Chem., 2007, 72, 62–70 CrossRef CAS PubMed.
- H. Kimura, Antioxid. Redox Signaling, 2010, 12, 1111–1123 CrossRef CAS PubMed.
- R. Wang, Antioxid. Redox Signaling, 2010, 12, 1061–1064 CrossRef CAS PubMed.
- M. Wang, J. Zhu, Y. Pan, J. D. Dong, L. L. Zhang, X. R. Zhang and L. Zhang, J. Neurosci. Res., 2015, 93, 487–494 CrossRef CAS PubMed.
- R. Wang, Physiol. Rev., 2012, 92, 791–896 CrossRef CAS PubMed.
- Z. Xu, L. Xu, J. Zhou, Y. F. Xu, W. P. Zhu and X. H. Qian, Chem. Commun., 2012, 48, 10871–10873 RSC.
- H. A. Henthorn and M. D. Pluth, J. Am. Chem. Soc., 2015, 137, 15330–15336 CrossRef CAS PubMed.
- V. S. Lin, A. R. Lippert and C. J. Chang, Redox Biol., 2015, 554, 63–80 CAS.
- J. Y. Zhang and W. Guo, Chem. Commun., 2014, 50, 4214–4217 RSC.
- (a) P. F. Xu, M. H. Liu, T. Gao, H. L. Zhang, Z. W. Li, X. Y. Huang and W. B. Zeng, Tetrahedron Lett., 2015, 56, 4007–4010 CrossRef CAS; (b) P. Karmakar, S. Manna, S. S. Ali, U. N. Guria, R. Sarkar, P. Datta, D. Mandal and A. K. Mahapatra, New J. Chem., 2018, 42, 76–84 RSC; (c) K. Maiti, A. K. Mahapatra, A. Gangopadhyay, R. Maji, S. Mondal, S. S. Ali, S. Das, R. Sarkar, P. Data and D. Mandal, ACS Omega, 2017, 2, 1583–1593 CrossRef CAS.
- G. S. Sharma, T. Kumar, T. A. Dar and L. R. Singh, Biochim. Biophys. Acta, 2015, 1850, 2239–2245 CrossRef CAS PubMed.
- K. Karolczak and B. Olas, Physiol. Res., 2009, 58, 623–633 CAS.
- P. Ganguly and S. F. Alam, Nutr. J., 2015, 14 DOI:10.1186/1475-2891-14-6.
- U. Hubner, I. Koch, U. Retzke and W. Herrmann, Geburtshilfe Frauenheilkd., 2003, 63, 990–998 CrossRef.
- M. P. Mattson and T. B. Shea, Trends Neurosci., 2003, 26, 137–146 CrossRef CAS PubMed.
- L. J. Tang, J. Z. Shi, Z. L. Huang, X. M. Yan, Q. Zhang, K. L. Zhong, S. H. Hou and Y. J. Bian, Tetrahedron Lett., 2016, 57, 5227–5231 CrossRef CAS.
- N. Mohorko, A. Petelin, M. Jurdana, G. Biolo and Z. Jenko-Praznikar, BioMed Res. Int., 2015, 2015, 418681 Search PubMed.
- P. B. E. Woolsey, J. Altern. Complement. Med., 2008, 14, 1159–1164 CrossRef PubMed.
- J. Lin, I. M. Lee, Y. Q. Song, N. R. Cook, J. Selhub, J. E. Manson, J. E. Buring and S. M. Zhang, Cancer Res., 2010, 70, 2397–2405 CrossRef CAS PubMed.
- L. J. Wang, P. Y. Lin, L. Yu, Y. C. Huang, C. C. Wu, S. T. Hsu, C. C. Chen, C. Mian-Yoon, C. H. Lin and C. F. Hung, Schizophr. Res., 2018, 192, 391–397 CrossRef PubMed.
- X. F. Wang and M. S. Cynader, J. Neurosci., 2001, 21, 3322–3331 CrossRef CAS PubMed.
- X. F. Yang, Y. X. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690–10693 CrossRef CAS PubMed.
- B. Liu, J. F. Wang, G. Zhang, R. K. Bai and Y. Pang, ACS Appl. Mater. Interfaces, 2014, 6, 4402–4407 CrossRef CAS PubMed.
- Y. Liu, D. H. Yu, S. S. Ding, Q. Xiao, J. Guo and G. Q. Feng, ACS Appl. Mater. Interfaces, 2014, 6, 17543–17550 CrossRef CAS PubMed.
- S. C. Lu, Mol. Aspects Med., 2009, 30, 42–59 CrossRef CAS PubMed.
- J. S. Armstrong, K. K. Steinauer, B. Hornung, J. M. Irish, P. Lecane, G. W. Birrell, D. M. Peehl and S. J. Knox, Cell Death Differ., 2002, 9, 252–263 CrossRef CAS PubMed.
- M. Nakamura, V. H. Thourani, R. S. Ronson, D. A. Velez, X. L. Ma, S. Katzmark, J. Robinson, L. S. Schmarkey, Z. Q. Zhao, N. P. Wang, R. A. Guyton and J. Vinten-Johansen, Circulation, 2000, 102, 332–338 CrossRef.
- N. Ballatori, S. M. Krance, S. Notenboom, S. J. Shi, K. Tieu and C. L. Hammond, Biol. Chem., 2009, 390, 191–214 CAS.
- G. Y. Wu, Y. Z. Fang, S. Yang, J. R. Lupton and N. D. Turner, J. Nutr., 2004, 134, 489–492 CrossRef CAS PubMed.
- C. B. Pocernich and D. A. Butterfield, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822, 625–630 CrossRef CAS PubMed.
- X. T. Ren, F. Wang, J. Lv, T. W. Wei, W. Zhang, Y. Wang and X. Q. Chen, Dyes Pigm., 2016, 129, 156–162 CrossRef CAS.
- J. L. Martindale and N. J. Holbrook, J. Cell. Physiol., 2002, 192, 1–15 CrossRef CAS PubMed.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205–214 CrossRef CAS PubMed.
- T. Finkel and N. J. Holbrook, Nature, 2000, 408, 239–247 CrossRef CAS PubMed.
- P. D. Ray, B. W. Huang and Y. Tsuji, Cell. Signalling, 2012, 24, 981–990 CrossRef CAS PubMed.
- E. H. Sarsour, M. G. Kumar, L. Chaudhuri, A. L. Kalen and P. C. Goswami, Antioxid. Redox Signaling, 2009, 11, 2985–3011 CrossRef CAS PubMed.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef CAS PubMed.
- X. J. Liu, H. H. Tian, L. Yang, Y. A. Su, M. Guo and X. Z. Song, Sens. Actuators, B, 2018, 255, 1160–1165 CrossRef CAS.
- S. Biswas, J. Das, S. Barman, B. R. Pinninti, T. K. Maiti and N. D. P. Singh, ACS Appl. Mater. Interfaces, 2017, 9, 28180–28184 CrossRef CAS PubMed.
- L. J. Tang, M. Y. Tian, H. B. Chen, X. M. Yan, K. L. Zhong and Y. J. Bian, Dyes Pigm., 2018, 158, 482–489 CrossRef CAS.
- C. K. Wang, J. Cheng, X. G. Liang, C. Tan, Q. Jiang, Y. Z. Hu, Y. M. Lu, K. Fukunaga, F. Han and X. Li, Theranostics, 2017, 7, 3803–3813 CrossRef PubMed.
- T. Malinski, J. Alzheimer's Dis., 2007, 11, 207–218 CAS.
- U. Forstermann and W. C. Sessa, Eur. Heart J., 2012, 33, 829–837 CrossRef PubMed.
- L. Chen, D. Wu and J. Yoon, Sens. Actuators, B, 2018, 259, 347–353 CrossRef CAS.
- C. H. Switzer, W. Flores-Santana, D. Mancardi, S. Donzelli, D. Basudhar, L. A. Ridnour, K. M. Miranda, J. M. Fukuto, N. Paolocci and D. A. Wink, Biochim. Biophys. Acta, 2009, 1787, 835–840 CrossRef CAS PubMed.
- T. Y. Dai, Y. Tian, C. G. Tocchetti, T. Katori, A. M. Murphy, D. A. Kass, N. Paolocci and W. D. Gao, J. Physiol., 2007, 580, 951–960 CrossRef CAS PubMed.
- M. Feelisch, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 4978–4980 CrossRef CAS PubMed.
- M. E. Shoman and O. M. Aly, Curr. Top. Med. Chem., 2016, 16, 2464–2470 CrossRef CAS PubMed.
- V. Shafirovich and S. V. Lymar, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 7340–7345 CrossRef CAS PubMed.
- J. A. Reisz, E. B. Klorig, M. W. Wright and S. B. King, Org. Lett., 2009, 11, 2719–2721 CrossRef CAS PubMed.
- H. M. Lv, Y. Chen, J. Lei, C. T. Au and S. F. Yin, Anal. Methods, 2015, 7, 3883–3887 RSC.
- X. D. Jin, X. L. Sun, X. Y. Di, X. Q. Zhang, H. Huang, J. N. Liu, P. W. Ji and H. J. Zhu, Sens. Actuators, B, 2016, 224, 209–216 CrossRef CAS.
- B. Brunekreef and S. T. Holgate, Lancet, 2002, 360, 1233–1242 CrossRef CAS.
- R. A. Lerner and A. Eschenmoser, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3013–3015 CrossRef CAS PubMed.
- L. Yu, Y. Y. Li, H. Yu, K. Zhang, X. W. Wang, X. F. Chen, J. Yue, T. X. Huo, H. W. Ge, K. A. Alamry, H. M. Marwani and S. H. Wang, Sens. Actuators, B, 2018, 266, 717–723 CrossRef CAS.
- U. Ragnarsson, Chem. Soc. Rev., 2001, 30, 205–213 RSC.
- B. Toth, In Vivo, 2000, 14, 299–319 CAS.
- B. A. Aigner, U. Darsow, M. Grosber, J. Ring and S. G. Plotz, Dermatology, 2010, 221, 300–302 CrossRef CAS PubMed.
- B. Ritz, Y. X. Zhao, A. Krishnadasan, N. Kennedy and H. Morgenstern, Epidemiol., 2006, 17, 154–161 CrossRef PubMed.
- S. Garrod, M. E. Bollard, A. W. Nichollst, S. C. Connor, J. Connelly, J. K. Nicholson and E. Holmes, Chem. Res. Toxicol., 2005, 18, 115–122 Search PubMed.
- S. D. Zelnick, D. R. Mattie and P. C. Stepaniak, Aviat., Space Environ. Med., 2003, 74, 1285–1291 Search PubMed.
- X. D. Jin, C. Z. Liu, X. M. Wang, H. Huang, X. Q. Zhang and H. J. Zhu, Sens. Actuators, B, 2015, 216, 141–149 CrossRef CAS.
- Y. Wang, H. Xiang, R. Zhao and C. Huang, Analyst, 2018, 143, 3900–3906 RSC.
- D. Evison, D. Hinsley and P. Rice, Br. Med. J., 2002, 324, 332–335 CrossRef CAS PubMed.
- J. Borak and W. F. Diller, J. Occup. Environ. Med., 2001, 43, 110–119 CrossRef CAS.
- H. Babad and A. G. Zeiler, Chem. Rev., 1973, 73, 75–91 CrossRef CAS.
- L. Chen, D. Wu, J. M. Kim and J. Yoon, Anal. Chem., 2017, 89, 12596–12601 CrossRef CAS PubMed.
- W. L. Zhai, X. L. Sun, T. D. James and J. S. Fossey, Chem. – Asian J., 2015, 10, 1836–1848 CrossRef CAS PubMed.
- A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412–443 RSC.
- (a) M. R. Aronoff, B. VanVeller and R. T. Raines, Org. Lett., 2013, 15, 5382–5385 CrossRef CAS PubMed; (b) A. K. Mahapatra, S. Mondal, S. K. Manna, K. Maiti, R. Maji, S. S. Ali, S. Mandal, M. R. Uddin and D. K. Maiti, ChemistrySelect, 2016, 1, 375–383 CrossRef CAS.
- T. Panda and B. S. Gowrishankar, Appl. Microbiol. Biotechnol., 2005, 67, 160–169 CrossRef CAS PubMed.
- P. Glynn, Biochem. J., 1999, 344, 625–631 CrossRef CAS PubMed.
- J. Hu, K. Cheng, Q. Wu, D. S. Ding, C. G. Li and Z. Li, Mater. Chem. Front., 2018, 2, 1201–1206 RSC.
- M. G. Tian, J. Sun, Y. H. Tang, B. L. Dong and W. Y. Lin, Anal. Chem., 2018, 90, 998–1005 CrossRef CAS PubMed.
- S. P. Sanghani, S. K. Quinney, T. B. Fredenburg, Z. J. Sun, W. I. Davis, D. J. Murry, O. W. Cummings, D. E. Seitz and W. F. Bosron, Clin. Cancer Res., 2003, 9, 4983–4991 CAS.
- D. Liu, J. Gao, C. L. Zhang, X. H. Ren, Y. Liu and Y. J. Xu, Pharmazie, 2011, 66, 888–893 CAS.
- M. Hosokawa, Molecules, 2008, 13, 412–431 CrossRef CAS PubMed.
- L. Feng, Z. M. Liu, J. Hou, X. Lv, J. Ning, G. B. Ge, J. N. Cui and L. Yang, Biosens. Bioelectron., 2015, 65, 9–15 CrossRef CAS PubMed.
- G. Abboud and N. Kaplowitz, Drug Saf., 2007, 30, 277–294 CrossRef CAS PubMed.
- E. Mornet, Orphanet J. Rare Dis., 2007, 2 DOI:10.1186/1750-1172-2-40.
- J. L. Millan and W. H. Fishman, Crit. Rev. Clin. Lab. Sci., 1995, 32, 1–39 CrossRef CAS PubMed.
- Q. H. Hu, F. Zeng, C. M. Yu and S. Z. Wu, Sens. Actuators, B, 2015, 220, 720–726 CrossRef CAS.
- Y. Jia, P. Li and K. L. Han, Chem. – Asian J., 2015, 10, 2444–2451 CrossRef CAS PubMed.
- L. Bai, S. O. Yoon, P. D. King and J. L. Merchant, Cell Death Differ., 2004, 11, 663–673 CAS.
- T. I. Kim, H. J. Kang, G. Han, S. J. Chung and Y. Kim, Chem. Commun., 2009, 5895–5897 RSC.
- J. M. Brown and W. R. William, Nat. Rev. Cancer, 2004, 4, 437–447 CrossRef CAS PubMed.
- R. B. P. Elmes, Chem. Commun., 2016, 52, 8935–8956 RSC.
- E. M. Williams, R. F. Little, A. M. Mowday, M. H. Rich, J. V. E. Chan-Hyams, J. N. Copp, J. B. Smaill, A. V. Patterson and D. F. Ackerley, Biochem. J., 2015, 471, 131–153 CrossRef CAS PubMed.
- G. N. Parkinson, J. V. Skelly and S. Neidle, J. Med. Chem., 2000, 43, 3624–3631 CrossRef CAS PubMed.
- W. P. Feng, Y. C. Wang, S. Z. Chen, C. Wang, S. X. Wang, S. H. Li, H. Y. Li, G. Q. Zhou and J. C. Zhang, Dyes Pigm., 2016, 131, 145–153 CrossRef CAS.
- C. Lopez-Otin and J. S. Bond, J. Biol. Chem., 2008, 283, 30433–30437 CrossRef CAS PubMed.
- B. Law and C. H. Tung, Bioconjugate Chem., 2009, 20, 1683–1695 CrossRef CAS PubMed.
- O. Thorn-Seshold, M. Vargas-Sanchez, S. McKeon and J. Hasserodt, Chem. Commun., 2012, 48, 6253–6255 RSC.
- C. I. Cheng, Y. P. Chang and Y. H. Chu, Chem. Soc. Rev., 2012, 41, 1947–1971 RSC.
- A. S. Klymchenko, V. V. Shvadchak, D. A. Yushchenko, N. Jain and Y. Mely, J. Phys. Chem. B, 2008, 112, 12050–12055 CrossRef CAS PubMed.
- V. V. Shvadchak, A. S. Klymchenko, H. de Rocquigny and Y. Mely, Nucleic Acids Res., 2009, 37, e25, DOI:10.1093/nar/gkn1083.
- O. M. Zamotaiev, V. Y. Postupalenko, V. V. Shvadchak, V. G. Pivovarenko, A. S. Klymchenko and Y. Mely, Bioconjugate Chem., 2011, 22, 101–107 CrossRef CAS PubMed.
- Y. Wang, Y. H. Hu, T. Wu, X. S. Zhou and Y. Shao, Anal. Chem., 2015, 87, 11620–11624 CrossRef CAS PubMed.
- V. V. Shynkar, A. S. Klymchenko, C. Kunzelmann, G. Duportail, C. D. Muller, A. P. Demchenko, J. M. Freyssinet and Y. Mely, J. Am. Chem. Soc., 2007, 129, 2187–2193 CrossRef CAS PubMed.
- D. P. Ng, B. E. Poulsen and C. M. Deber, Biochim. Biophys. Acta, 2012, 1818, 1115–1122 CrossRef CAS PubMed.
- N. Jiang, C. L. Yang, X. W. Dong, X. L. Sun, D. Zhang and C. L. Liu, Org. Biomol. Chem., 2014, 12, 5250–5259 RSC.
- K. A. Bertman, C. S. Abeywickrama, H. J. Baumann, N. Alexander, L. Mcdonald, L. P. Shriver, M. Konopka and Y. Pang, J. Mater. Chem. B, 2018, 6, 5050–5058 RSC.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- (a) M. H. Lan, J. S. Wu, W. M. Liu, H. Y. Zhang, W. J. Zhang, X. Q. Zhuang and P. F. Wang, Sens. Actuators, B, 2011, 156, 332–337 CrossRef CAS; (b) A. Gangopadhyay, S. S. Ali, U. N. Guria, S. K. Samanta, R. Sarkar, P. Datta and A. K. Mahapatra, New J. Chem., 2018 10.1039/c8nj03369b.
- X. F. Yang, Q. Huang, Y. G. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177–2183 RSC.
- J. D. Luo, Z. L. Xie, J. W. Y. Lam, L. Cheng, H. Y. Chen, C. F. Qiu, H. S. Kwok, X. W. Zhan, Y. Q. Liu, D. B. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- Z. G. Song, R. T. K. Kwok, E. G. Zhao, Z. K. He, Y. N. Hong, J. W. Y. Lam, B. Liu and B. Z. Tang, ACS Appl. Mater. Interfaces, 2014, 6, 17245–17254 CrossRef CAS PubMed.
- Q. Chen, C. M. Jia, Y. F. Zhang, W. Du, Y. L. Wang, Y. Huang, Q. Y. Yang and Q. Zhang, J. Mater. Chem. B, 2017, 5, 7736–7742 RSC.
- Y. Liu, J. Nie, J. Niu, W. S. Wang and W. Y. Lin, J. Mater. Chem. B, 2018, 6, 1973–1983 RSC.
- S. Biswas, R. Mengji, S. Barman, V. Venugopal, A. Jana and N. D. P. Singh, Chem. Commun., 2018, 54, 168–171 RSC.
- H. W. Liu, K. Li, X. X. Hu, L. M. Zhu, Q. M. Rong, Y. C. Liu, X. B. Zhang, J. Hasserodt, F. L. Qu and W. H. Tan, Angew. Chem., 2017, 56, 11788–11792 CrossRef CAS PubMed.
- S. K. Chatterjee, M. Bhattacharya and J. J. Barlow, Cancer Res., 1979, 39, 1943–1951 CAS.
- G. P. Dimri, X. H. Lee, G. Basile, M. Acosta, C. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereirasmith, M. Peacocke and J. Campisi, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 9363–9367 CrossRef CAS.
- W. G. Yang, X. X. Zhao, J. Zhang, Y. Zhou, S. M. Fan, H. Sheng, Y. Cao and Y. H. Hu, Dyes Pigm., 2018, 156, 100–107 CrossRef CAS.
- L. Peng, M. Gao, X. L. Cai, R. Y. Zhang, K. Li, G. X. Feng, A. J. Tong and B. Liu, J. Mater. Chem. B, 2015, 3, 9168–9172 RSC.
- M. Gao, Q. L. Hu, G. X. Feng, B. Z. Tang and B. Liu, J. Mater. Chem. B, 2014, 2, 3438–3442 RSC.
- H. L. Liu, X. Y. Wang, Y. Xiang and A. J. Tong, Anal. Methods, 2015, 7, 5028–5033 RSC.
- L. Cui, Y. Baek, S. Lee, N. Kwon and J. Yoon, J. Mater. Chem. C, 2016, 4, 2909–2914 RSC.
- C. S. Liang and S. M. Jiang, Analyst, 2017, 142, 4825–4833 RSC.
- D. P. Li, Z. Y. Wang, H. Su, J. Y. Miao and B. X. Zhao, Chem. Commun., 2017, 53, 577–580 RSC.
- H. Y. Zhang, Z. J. Huang and G. Q. Feng, Anal. Chim. Acta, 2016, 920, 72–79 CrossRef CAS PubMed.
- D. Dahal, L. McDonald, X. M. Bi, C. Abeywickrama, F. Gombedza, M. Konopka, S. Paruchuri and Y. Pang, Chem. Commun., 2017, 53, 3697–3700 RSC.
- A. Sedgwick, W.-T. Dou, J.-B. Jiao, L. Wu, G. T. Williams, A. T. A. Jenkins, S. D. Bull, J. L. Sessler, X.-P. He and T. D. James, J. Am. Chem. Soc., 2018 DOI:10.1021/jacs.8b08457.
| This journal is © The Royal Society of Chemistry 2018 |