Open Access Article
Open Access ArticlePatterned formation of enolate functional groups on the graphene basal plane†
Andrew
Cassidy
 *a,
Stine
Pedersen
a,
Hendrik
Bluhm
b,
Valentin
Calisti
c,
Thierry
Angot
c,
Eric
Salomon
c,
Régis
Bisson
*a,
Stine
Pedersen
a,
Hendrik
Bluhm
b,
Valentin
Calisti
c,
Thierry
Angot
c,
Eric
Salomon
c,
Régis
Bisson
 c and
Liv
Hornekær
a
c and
Liv
Hornekær
a
aDepartment of Physics and Astronomy, Aarhus University, Denmark. E-mail: amc@phys.au.dk
bChemical Sciences Division, Lawrence Berkeley National Lab, Berkeley, CA 94720, USA
cAix-Marseille Univ, CNRS, PIIM, Marseille, France
First published on 9th November 2018
Abstract
Chemical functionalization of graphene is one method pursued to engineer new properties into a graphene sheet. Graphene oxide is the most commonly used chemical derivative of graphene. Here we present experimental evidence for the formation of enolate moieties when oxygen atoms are added to the graphene basal plane. The exotic functional groups are stabilized by simultaneous bond formation between the graphene sheet and the underlying Ir(111) substrate. Scanning tunneling microscopy images demonstrate the patterned nature of C–O bond formation and X-ray photoelectron spectroscopy and high-resolution electron energy loss spectroscopy are used to characterize the enolate moiety. The results present a new mechanism for the formation of patterned graphene oxide and provide evidence of a functional group rarely considered for graphene oxide materials.
Graphene is a 2D semi-metal in which all carbon atoms are sp2 hybridized.1 Chemical functionalization of graphene is driven by the desire to engineer the physical and chemical properties of pristine graphene sheets, and to introduce new means of incorporating graphene interfaces with other materials.2–4 The addition of simple atomic species, such as hydrogen,5 oxygen6,7 or fluorine,8 has been shown to change the electronic band structure of graphene from that of a semi-metal to that of a semi-conductor or insulator.
Graphene oxide is a term used to describe graphene materials that have been subjected to oxidation reactions. Supported graphene oxide materials have been studied as sensor materials, for their potential magnetic properties and as clusters to form graphene oxide quantum dots.9–12 The growth of metallic nanoparticles on supported graphene sheets is also facilitated by the presence of C–O bonds at the graphene basal plane,4 with attachment or cleavage of particle growth initiators dependent on the nature of the C–O bond.13 This may be a viable method for atomic layer deposition on graphene films, allowing for integration of graphene sheets in more complex electronic devices.14 Consequently, the nature of the C–O bond formed during synthesis of graphene oxide is of general interest and importance.
It is widely assumed that an oxygen atom bonding to the basal plane of a graphene sheet is initially covalently bound through the formation of an epoxy group.15–18 The stability of different oxygen moieties on metal-supported graphene sheets was, however, recently explored for graphene islands on Ru(0001).19 Those authors, building on theoretical results from Jung et al.,20 provided the first experimental evidence for the existence of the enolate moiety when oxygen atoms bind to a graphene sheet supported by a metal substrate. Their calculations indicate that interaction with an underlying metallic substrate can guide the formation of enolate groups when an oxygen atom binds to one carbon atom, and the adjacent carbon atom is suitably positioned to bind down to an underlying metal atom. A 370 meV gain in energy was reported for the formation of an enolate group, versus an epoxy group, in certain areas of the moiré pattern formed between the graphene lattice and atoms from the Ru(0001) surface.19
Graphene on Ru(0001), however, is already a strongly coupled system with good evidence for the preexistence of a carbon–metal bond even before oxygen atoms are introduced.21 Hence, it is timely to consider the nature of the C–O bond for other, less strongly coupled systems. Graphene on Ir(111) (Gr/Ir(111)) demonstrates weak van der Waals interaction between the graphene sheet and the Ir(111) surface atoms.22 Moreover, single crystal graphene sheets with a low concentration of defects and highly oriented domains are routinely prepared on Ir(111) substrates following chemical vapour deposition.23
The attachment of oxygen atoms to Gr/Ir(111) has been reported previously but the possibility of enolate formation was not considered.15,16 Here, we revisit this system adding new high-resolution electron energy loss spectroscopy (HREELS) data to demonstrate that at a low flux of oxygen atoms, enolate formation dominates and occurs only at select sites on the Gr/Ir(111) surface, generating a long-range pattern of graphene oxide nano-dots.
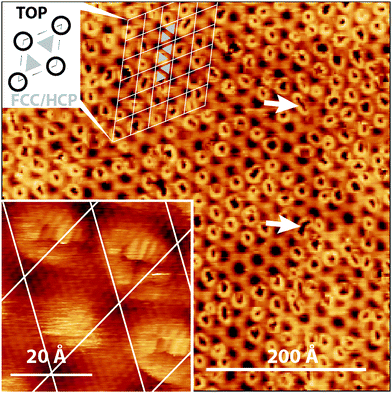
The slight lattice mis-match between the carbon atoms in the graphene sheet and the Ir(111) surface atoms leads to a long-range repeating moiré pattern.23 The moiré unit cell contains: TOP regions where Ir surface atoms are positioned directly beneath the center of a hexagonal ring in the graphene sheet; and HCP and FCC regions, where every second C atom is positioned directly above an Ir atom. HCP and FCC regions differ in the arrangement of Ir atoms in the second and third Ir layer. The remaining carbon atoms, in between TOP and HCP/FCC regions, show varying degrees of overlap with underlying surface Ir atoms. This repeating moiré pattern, with a 25.2 Å repeat-distance, dominates in STM imaging of the Gr/Ir(111) basal plane. Here we use an imaging mode such that TOP sites image as dark. Details on this assignment, and other experimental details, can be found in the ESI.†
When Gr/Ir(111) is exposed to a very low flux of O atoms to produce O–Gr/Ir(111) bond formation sites are imaged as doughnut-like, bright rings with a dark center, Fig. 1. In the image in Fig. 1, not all moiré sites have become oxidized with roughly one third of moiré unit cells remaining pristine. In the majority of moiré unit cells where oxidation has occurred, only the FCC site shows oxidation but in few areas, and examples are highlighted in Fig. 1, both the FCC and HCP sites show evidence for C–O bond formation. The inset shows an atomically resolved site, where O-atoms have landed on the graphene basal plane. The localized formation of C–O bonds, only at FCC and HCP sites, strongly suggests a role for the underlying Ir substrate.
In Fig. 2, X-ray photoelectron spectroscopy (XPS) data are presented for (a) Gr/Ir(111), (b) O–Gr/Ir(111), following exposure to a low flux of O atoms, and (c) the time evolution of the C1s peaks with increasing fluence of O atoms. Spectra in Fig. 2b were recorded after 50 min irradiation and represent a saturated coverage under these dosing conditions. The C1s data for Gr/Ir(111) show one peak, CC, fit using a Doniach-Šunjić function convoluted with a Gaussian, with a binding energy of 284.04 eV. After C–O bond formation, a new peak, CO, is introduced at 284.33 eV and the CC peak from pristine graphene is correspondingly reduced in intensity and has shifted to 284.09 eV. The CC binding energy shift following oxidation is likely induced by doping of the graphene sheet. There is also a contribution from a small third peak, CA at 285.84 eV. The data are consistent with reports on graphene oxide by others.15,16 Here, however, we show a strong preference for O addition as one unique type of C–O moiety, as represented by the CO peak.
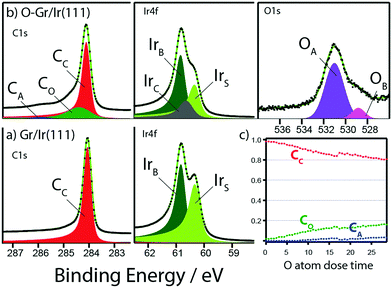 | ||
| Fig. 2 X-ray photoelectron spectroscopy data for (a) pristine graphene on Ir(111) and (b) oxidized graphene on Ir(111). Individual peak details are given in Table S1 (ESI†). (c) Time evolution of the relative area of the C1s peaks with increasing fluence of O atoms. | ||
Photoelectrons from the Ir4f core level, from Gr/Ir(111), show two peaks at 60.80 eV and 60.29 eV, for Ir bulk and surface atoms respectively.24 Thus the graphene sheet is not chemically interacting with the Ir surface.15 After oxidation, a new peak, at 60.60 eV, is introduced to represent Ir surface atoms that are involved in bond formation with the overlying graphene sheet. The pristine Ir surface peak is correspondingly reduced in intensity. There is no evidence in the Ir spectrum for Ir oxide deposits originating from the thermal cracker.
After oxidation the O1s signal is fit, predominantly, with 1 Gaussian peak, OA, with a binding energy of 531.07 eV and a second smaller peak, OB, at 528.83 eV. O1s data for O–Ir(111) show intensity at 530 eV and the absence of such a peak in the O–Gr/Ir(111) data illustrates that no Ir–O bond is formed.24Fig. 2c shows the evolution of the relative areas for the C1s peaks, with increasing fluence of O atoms. After 30 min dosing the coverage approximates saturation with an upper limit contribution from the CO peak at 17% and an upper limit 3% contribution from CA. We propose that the CO peak corresponds both to carbon atoms bound to O-atoms, via formation of an enolate, and to neighbouring carbon atoms simultaneously bound to the underlying Ir substrate.5 These percentages concur with analysis of STM images for surface coverage (S1). The size of such a cluster corresponds to the size of the FCC site in the moiré unit cell. This confirms that the most reactive sites for O atom addition are confined to the FCC region of the moiré. Experiments and DFT calculations illustrate that the position of an Ir atom directly beneath a carbon atom, and C–Ir bond formation, significantly increases the energy gain for C–H bond formation in FCC and HCP regions compared to similar bond formation in TOP regions, with a slight energetic preference for FCC regions.5 Jung et al. and Novotny et al., concluded that the same mechanism leads to the stabilization of enolate formation upon formation of C–O bonds for Gr/Cu, Gr/Ni and Gr/Ru.19,20 Novotny et al. demonstrated that enolate formation was favoured in FCC regions of the graphene Ru(0001) moiré.
We suggest that the CA peak is caused by either a small amount of epoxy group formation15,16 or carbonyl formation, which we justify using HREELS data.
HREELS data, are shown in Fig. 3. The spectrum for Gr/Ir(111) shows two distinct energy loss peaks; at 104 meV for losses incurred by scattering from the ZO phonons in graphene and a second peak at 199 meV, corresponding to scattering from the LO/TO phonons.28 After oxidation a new set of peaks is introduced, Fig. 3b. We interpret this spectrum in Table 1, in light of HREELS for H–Gr/Ir(111) reported by Kyhl et al.25 and the calculated vibrations of the enolate group reported by Jung et al.20
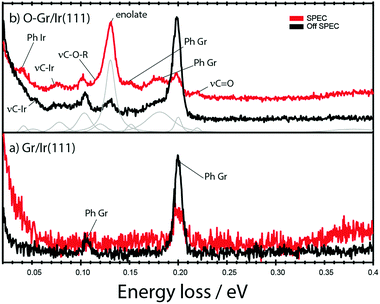 | ||
| Fig. 3 High-resolution electron energy loss spectroscopy data recorded (a) on pristine graphene on Ir(111) and (b) on graphene on Ir(111) exposed to a low flux of O atoms. In both cases curves are shown for data collected in the specular and out of specular directions following irradiation with a 20 eV electron beam. The curves have been normalized to the intensity of the elastic peak. See Table 1 for peak identifications. | ||
| Vibration mode | O–Gr/Ir(111) (meV) | O–Gr/Cu(111) (meV)20 | H–Gr/Ir(111) (meV)25 | Oxidized diamond films |
|---|---|---|---|---|
a Jung et al. calculated 6 vibrational modes for enolate groups at two distinct sites for free standing graphene, HOPG, Gr/Cu(111) and Gr/Ni(111) substrates. The values for O–Gr/Cu(111) are included here.
b The ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching vibration does not originate from phonons as in graphene but appears with similar energy losses.
c The region between 120–140 meV is complex for oxidized nano-diamond films and peaks assigned to ν(C–O–O–C), δ(CH3), δ(HC C) stretching vibration does not originate from phonons as in graphene but appears with similar energy losses.
c The region between 120–140 meV is complex for oxidized nano-diamond films and peaks assigned to ν(C–O–O–C), δ(CH3), δ(HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), aromatic δ(ring-H) and ν(C–O–H) in the literature overlap with the clear peak at 130 meV identified in Fig. 3. CH), aromatic δ(ring-H) and ν(C–O–H) in the literature overlap with the clear peak at 130 meV identified in Fig. 3.
|
||||
| Phonon Ir | 41 | 40 | ||
| νC–Ir | 61 | 52 | ||
| νC–Ir | 78 | 72 | ||
| ZO graphene | 104 | 100 | 100(ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C))b C))b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
|
| ν(C–O–C) epoxy | 120 | 57,26 125,27 135,27 140c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
||
| Enolate | 130 | 28, 29, 54, 125, 145a | ||
| LO/TO phonons in graphane-like clusters | 152 | 158 | ||
| ZO phonons in graphane-like clusters | 181 | 178 | ||
| TO/LO graphene | 199 | 195 | 200–205(ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C))b C))b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
|
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) carbonyl O) carbonyl |
219 | 220,26 22027 | ||
The LO/TO and ZO phonons modes for graphene persist and in addition signals arise from scattering loses associated with: the vibration of the newly formed C–Ir bonds, reported in relation to Fig. 2, at 61 meV and 78 meV; LO/TO and ZO phonons associated with the newly formed graphane-like clusters reported in relation to Fig. 1 at 152 meV and 181 meV respectively. Three peaks, at 120 meV, 130 meV and 219 meV, can be associated with vibration of the newly formed C–O bonds and the intensity of these peaks varies with the angle at which scattered electrons are detected. In particular the peak at 130 meV is prominent in the specular direction but reduced in out-of-specular directions, indicating formation of a very polar bond.
Where available, experimental reports on the characterization of oxidized carbon substrates by HREELS are listed in Table 1.26,27,29 There is consensus that the energy loss peak at 219 meV can be associated with vibration of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond indicating that we have formation of carbonyl species. The corresponding small HREELS intensity explains the origin of the smaller peak OB, at 528.83 eV and CA at 285.84 eV in XPS data discussed in Fig. 2. Energy losses at 120–135 meV have consistently been associated with vibrations from ether and epoxy groups but the strong polar nature of the peak at 130 meV observed here, and the simultaneous formation of the C–Ir bond, points to the identification of an enolate group. The vibrational energies for the 6 vibrational modes of the enolate moiety calculated by Jung et al. are consistent with this assignment.20 Jung et al. report a substrate dependent vibration between 25–29 meV that is likely hidden by the elastic peak in Fig. 3b. The vibration at 49–58 meV for collective motions of the entire CO group normal to the graphene plane overlaps with νC–Ir, reported by Kyhl et al.25
O bond indicating that we have formation of carbonyl species. The corresponding small HREELS intensity explains the origin of the smaller peak OB, at 528.83 eV and CA at 285.84 eV in XPS data discussed in Fig. 2. Energy losses at 120–135 meV have consistently been associated with vibrations from ether and epoxy groups but the strong polar nature of the peak at 130 meV observed here, and the simultaneous formation of the C–Ir bond, points to the identification of an enolate group. The vibrational energies for the 6 vibrational modes of the enolate moiety calculated by Jung et al. are consistent with this assignment.20 Jung et al. report a substrate dependent vibration between 25–29 meV that is likely hidden by the elastic peak in Fig. 3b. The vibration at 49–58 meV for collective motions of the entire CO group normal to the graphene plane overlaps with νC–Ir, reported by Kyhl et al.25
We have demonstrated that O atoms covalently bind to the graphene basal plane selectively at sites dictated by the underlying Ir(111) substrate. The spectroscopic evidence supports the view that these new bonds take the form of enolates and microscopy demonstrates the patterned nature of bond formation. Future work will focus on exploiting the potential of using this new functional group to perform further chemistry at the graphene basal plane, and to engineer interfaces with other materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from The Danish Council for Independent Research (grant no. 5137-00049B), Innovation Fund Denmark (NIAGRA) (grant no. 5184-00018B), The European Research Council (CoG GRANN, grant no. 648551), and VILLUM FONDEN via the Centre of Excellence for Dirac Materials (grant no. 11744). Affiliation with the Center for Integrated Materials Research (iMAT) at Aarhus University is gratefully acknowledged. We thank The Advanced Light Source, which is supported by the Director, Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy at LBNL under contract no. DE-AC02-05CH11231.References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Electric field effect in atomically thin carbon films, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- N. M. Bardhan, P. V. Kumar, Z. Li, H. L. Ploegh, J. C. Grossman, A. M. Belcher and G. Y. Chen, Enhanced Cell Capture on Functionalized Graphene Oxide Nanosheets through Oxygen Clustering, ACS Nano, 2017, 11, 1548–1558 CrossRef CAS PubMed.
- J. E. Johns and M. C. Hersam, Atomic covalent functionalization of graphene, Acc. Chem. Res., 2013, 46, 77–86 CrossRef CAS PubMed.
- J. E. Johns, J. M. P. Alaboson, S. Patwardhan, C. R. Ryder, G. C. Schatz and M. C. Hersam, Metal oxide nanoparticle growth on graphene via chemical activation with atomic oxygen, J. Am. Chem. Soc., 2013, 135, 18121–18125 CrossRef CAS PubMed.
- J. H. Jørgensen, A. G. Čabo, R. Balog, L. Kyhl, M. N. Groves, A. M. Cassidy, A. Bruix, M. Bianchi, M. Dendzik, M. A. Arman, L. Lammich, J. I. Pascual, J. Knudsen, B. Hammer, P. Hofmann and L. Hornekaer, Symmetry-Driven Band Gap Engineering in Hydrogen Functionalized Graphene, ACS Nano, 2016, 10, 10798–10807 CrossRef PubMed.
- B. Huang, H. Xiang, Q. Xu and S. H. Wei, Overcoming the phase inhomogeneity in chemically functionalized graphene: the case of graphene oxides, Phys. Rev. Lett., 2013, 110, 1–5 Search PubMed.
- K. Schulte, N. a. Vinogradov, M. L. Ng, N. Mårtensson and A. B. Preobrajenski, Bandgap formation in graphene on Ir(111) through oxidation, Appl. Surf. Sci., 2012, 267, 74–76 CrossRef.
- M. Chen, H. Zhou, C. Qiu, H. Yang, F. Yu and L. Sun, Layer-dependent fluorination and doping of graphene via plasma treatment, Nanotechnology, 2012, 23, 115706 CrossRef PubMed.
- Y. Wen, C. L. Yen, L. Yan, H. Kono, S. H. Lin and Y. C. Ling, Magnetism-tuning strategies for graphene oxide based on magnetic oligoacene oxide patches model, Phys. Chem. Chem. Phys., 2018, 20, 3678–3686 RSC.
- W. Xie, L.-T. Weng, C.-K. Chan, K. L. Yeung and C.-M. Chan, Reactions of SO2 and NH3 with epoxy groups on the surface of graphite oxide powder, Phys. Chem. Chem. Phys., 2018, 20, 6431–6439 RSC.
- Y. Zhang, C. Yang, D. Yang, Z. Shao, Y. Hu, J. Chen, L. Yuwen, L. Weng, Z. Luo and L. Wang, Reduction of graphene oxide quantum dots to enhance the yield of reactive oxygen species for photodynamic therapy, Phys. Chem. Chem. Phys., 2018, 20, 17262–17267 RSC.
- N. M. Bardhan, P. V. Kumar, Z. Li, H. L. Ploegh, J. C. Grossman, A. M. Belcher and G. Y. Chen, Enhanced Cell Capture on Functionalized Graphene Oxide Nanosheets through Oxygen Clustering, ACS Nano, 2017, 11, 1548–1558 CrossRef CAS PubMed.
- C. J. Tainter and G. C. Schatz, Reactive Force Field Modeling of Zinc Oxide Nanoparticle Formation, J. Phys. Chem. C, 2016, 120, 2950–2961 CrossRef CAS.
- R. H. J. Vervuurt, W. M. M. E. Kessels and A. A. Bol, Atomic Layer Deposition for Graphene Device Integration, Adv. Mater. Interfaces, 2017, 1700232, 1700232 CrossRef.
- R. Larciprete, S. Fabris, T. Sun, P. Lacovig, A. Baraldi and S. Lizzit, Dual path mechanism in the thermal reduction of graphene oxide, J. Am. Chem. Soc., 2011, 133, 17315–17321 CrossRef CAS PubMed.
- N. A. Vinogradov, K. Schulte, M. L. Ng, A. Mikkelsen, E. Lundgren, N. Mårtensson and A. B. Preobrajenski, Impact of atomic oxygen on the structure of graphene formed on Ir(111) and Pt(111), J. Phys. Chem. C, 2011, 115, 9568–9577 CrossRef CAS.
- M. Z. Hossain, J. E. Johns, K. H. Bevan, H. J. Karmel, Y. T. Liang, S. Yoshimoto, K. Mukai, T. Koitaya, J. Yoshinobu, M. Kawai, A. M. Lear, L. L. Kesmodel, S. L. Tait and M. C. Hersam, Chemically homogeneous and thermally reversible oxidation of epitaxial graphene, Nat. Chem., 2012, 4, 305–309 CrossRef CAS PubMed.
- S. Katano, T. Wei, T. Sasajima, R. Kasama and Y. Uehara, Localized electronic structures of graphene oxide studied using scanning tunneling microscopy and spectroscopy, Phys. Chem. Chem. Phys., 2018, 20, 17977–17982 RSC.
- Z. Novotny, M.-T. Nguyen, F. P. Netzer, V.-A. Glezakou, R. Rousseau and Z. Dohnálek, Formation of Supported Graphene Oxide: Evidence for Enolate Species, J. Am. Chem. Soc., 2018, 140, 5102–5109 CrossRef CAS PubMed.
- J. Jung, H. Lim, J. Oh and Y. Kim, Functionalization of Graphene Grown on Metal Substrate with Atomic Oxygen: Enolate vs Epoxide, J. Am. Chem. Soc., 2014, 136, 8528–8531 CrossRef CAS PubMed.
- D. Martoccia, P. R. Willmott, T. Brugger, M. Björck, S. Günther, C. M. Schlepütz, A. Cervellino, S. A. Pauli, B. D. Patterson, S. Marchini, J. Wintterlin, W. Moritz and T. Greber, Graphene on Ru(0001): A 25 × 25 supercell, Phys. Rev. Lett., 2008, 101, 1–4 CrossRef PubMed.
- R. Brako, D. Šokčević, P. Lazić and N. Atodiresei, Graphene on the Ir(111) surface: From van der Waals to strong bonding, New J. Phys., 2010, 12, 113016 CrossRef.
- A. T. N’Diaye, J. Coraux, T. N. Plasa, C. Busse and T. Michely, Structure of epitaxial graphene on Ir(111), New J. Phys., 2008, 10, 043033 CrossRef.
- M. Bianchi, D. Cassese, A. Cavallin, R. Comin, F. Orlando, L. Postregna, E. Golfetto, S. Lizzit and A. Baraldi, Surface core level shifts of clean and oxygen covered Ir(111), New J. Phys., 2009, 11, 063002 CrossRef.
- L. Kyhl, R. Balog, T. Angot, L. Hornekær and R. Bisson, Hydrogenated graphene on Ir(111): a high-resolution electron energy loss spectroscopy study of the vibrational spectrum, Phys. Rev. B, 2016, 93, 115403 CrossRef.
- S. Ghodbane, T. Haensel, Y. Coffinier, S. Szunerits, R. Boukherroub, S. I. U. Ahmed, J. A. Schaefer, D. Steinm, F. D. C. Gmbh, I. D. R. Interdisciplinaire, D. N. Iemn, D. Steinmüller-Nethl, R. Boukherroub, S. I. U. Ahmed and J. A. Schaefer, HREELS investigation of the surfaces of nanocrystalline diamond films oxidized by different processes, Langmuir, 2010, 26, 18798–18805 CrossRef CAS PubMed.
- Z. Shpilman, I. Gouzman, E. Grossman, R. Akhvlediani and A. Hoffman, Oxidation of diamond films by atomic oxygen: High resolution electron energy loss spectroscopy studies, J. Appl. Phys., 2007, 102, 114914 CrossRef.
- M. Endlich, A. Molina-Sánchez, L. Wirtz and J. Kröger, Screening of electron-phonon coupling in graphene on Ir(111), Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 1–4 CrossRef.
- M. Acik, G. Lee, C. Mattevi, A. Pirkle, R. M. Wallace, M. Chhowalla, K. Cho and Y. Chabal, The Role of Oxygen during Thermal Reduction of Graphene Oxide Studied by Infrared Absorption Spectroscopy, J. Phys. Chem. C, 2011, 115, 19761–19781 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail, XPS fitting parameters and extra STM images. See DOI: 10.1039/c8cp05730c |
| This journal is © the Owner Societies 2018 |