A spectroscopic and ab initio study of the hydrogen peroxide–formic acid complex: hindering the internal motion of H2O2†
Abstract
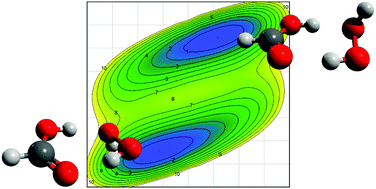
The microwave spectrum of the hydrogen-bonded hydrogen peroxide–formic acid complex was measured in the range from 4 to 17 GHz. Assignment of transitions and analyses of the spectrum were supported by ab initio wavefunction and density functional calculations. The detected conformer features a seven-membered hydrogen-bonded ring, in which the H-atom of one hydroxyl group of H2O2 and the O-atom of the other OH group are a hydrogen bond donor and acceptor, respectively, to the carboxyl group of formic acid. The rotational transitions show a tunnelling splitting, which is attributed to a wagging-like motion of the free H-atom of H2O2 from above to below the heavy atom plane of formic acid. Transitions between tunneling states are driven by the change in dipole moment accompanying this motion and were measured and analyzed. Ab initio analyses of the tunneling path reveal an asymmetric potential, which reflects the (transiently) chiral nature of the complex.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...