Dehydrocoupling of dimethylamine borane by titanocene: elucidation of ten years of inconsistency between theoretical and experimental descriptions†
Abstract
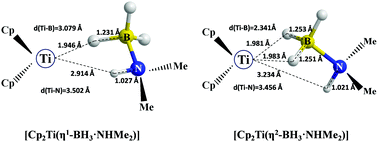
More than ten years ago, Manners and coworkers published the first experimental study on the efficiency of titanocene to catalyze the dehydrocoupling of dimethylamine borane (DMAB, T. Clark, C. Russell and I. Manners, J. Am. Chem. Soc., 2006, 128, 9582–9583). Several experimental investigations have shown that a two-step mechanism leads to the formation of a cyclic diborazane (Me2N–BH2)2via the linear diborazane (HNMe2–BH2–NMe2–BH3). This finding stood in contradiction to the following theoretical investigations of the reaction pathway. Herein, using dispersion-corrected density functional theory (DFT-D), we propose an energetically favored reaction mechanism in perfect agreement with the experimental findings. It is shown that van der Waals interactions play a prominent role in the reaction pathway. The formation of 3-center 2-electron interactions, classical dihydrogen bonds, and non-classical dihydrogen bonds was identified with the help of topological and localized orbital approaches.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...