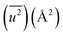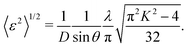Open Access Article
Open Access ArticleSize effects on rhodium nanoparticles related to hydrogen-storage capability†
Chulho
Song
 a,
Anli
Yang
a,
Osami
Sakata
a,
Anli
Yang
a,
Osami
Sakata
 *abc,
L. S. R.
Kumara
*abc,
L. S. R.
Kumara
 a,
Satoshi
Hiroi
a,
Satoshi
Hiroi
 b,
Yi-Tao
Cui
b,
Yi-Tao
Cui
 d,
Kohei
Kusada
e,
Hirokazu
Kobayashi
e and
Hiroshi
Kitagawa
d,
Kohei
Kusada
e,
Hirokazu
Kobayashi
e and
Hiroshi
Kitagawa
 ef
ef
aSynchrotron X-ray Station at SPring-8, Research Network and Facility Services Division, National Institute for Materials Science, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan. E-mail: SAKATA.Osami@nims.go.jp; Tel: +81 (0)791 58 1970
bSynchrotron X-ray Group, Research Center for Advanced Measurement and Characterization, National Institute for Materials Science, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan
cDepartment of Innovative and Engineered Materials, Tokyo Institute of Technology, 4259-J3-16, Nagatsuta, Midori, Yokohama 226-8502, Japan
dSynchrotron Radiation Laboratory, The Institute for Solid State Physics, The University of Tokyo, 1-490-2 Kouto, Shingu-cho Tatsuno, Hyogo 679-5165, Japan
eDivision of Chemistry, Graduate School of Science, Kyoto University, Kitashirakawa Oiwake-cho, Sakyo-ku, Kyoto 606-8502, Japan
fINAMORI Frontier Research Center, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
First published on 23rd May 2018
Abstract
To unveil the origin of the hydrogen-storage properties of rhodium nanoparticles (Rh NPs), we investigated the electronic and crystal structures of the Rh NPs using various synchrotron based X-ray techniques. Electronic structure studies revealed that the hydrogen-storage capability of Rh NPs could be attributed to their more unoccupied d-DOSs than that of the bulk near the Fermi level. Crystal structure studies indicated that lattice distortion and mean-square displacement increase while coordination number decreases with decreasing particle size and the hydrogen-absorption capability of Rh NPs improves to a greater extent with increased structural disorder in the local structure than with that in the mean structure. The smallest Rh NPs, having the largest structural disorder/increased vacancy spaces and the smallest coordination number, exhibited excellent hydrogen-storage capacity. Finally, from the bond-orientational order analysis, we confirmed that the localized disordering is distributed more over the surface part than the core part and hydrogen can be trapped on the surface part of Rh NPs which increases with a decrease in NP diameter.
1 Introduction
The study of materials enabling the efficient storage and safe transport of fuel gases, particularly hydrogen gas, has mainly focused on areas such as metal–organic frameworks,1–3 carbon materials,4,5 and hydrogen-storage alloys.6–8 Hydrogen-storage alloys are metallic materials that have a unique ability to store hydrogen compactly and safely as a consequence of the stability of their hydrides.9,10 Recently, nanoparticles of such metals have attracted much attention as advanced materials of new hydrogen-storage alloys.11–13 Rhodium (Rh) is an effective catalyst for various chemical reactions in the 4d transition metal series.14–16 The synthesis of metal nanoparticles (NPs) to improve their catalytic activities and obtain unexpected properties is a very hot topic owing to the quantum size effects and high surface-area-to-volume ratios exhibited by the NPs.17–19 For instance, while bulk Rh metal does not absorb hydrogen, Rh NPs do.14 Kusada et al. observed that the hydrogen-storage capacity of Rh NPs increases with decreasing particle size.20 They also reported that the enthalpy of hydrogen storage in the Rh NPs changes from endothermic to exothermic with decreasing particle sizes, with critical sizes between 7.1 and 10.5 nm. These phenomena might be caused by a change in the electronic state of Rh with decreasing particle size.10,14 However, the origin of the hydrogen-storage properties and performance of Rh NPs can hardly be described as one parameter and has not yet been revealed. Therefore, understanding the electronic and crystal structures of such NPs is very important for understanding their catalyst properties. In particular, the absorption of hydrogen by metal NPs is expected to occur in their core part as well as on their surface part.X-ray photoelectron spectroscopy (XPS) is a useful technique for probing the electronic properties of solids.21,22 However, as described before,23 it is very difficult to detect a bulk electronic structure of NPs with a wrapping layer by using conventional XPS. Synchrotron-based hard X-ray photoelectron spectroscopy (HAXPES), which has high brilliance and exhibits large probing depths, is widely used to investigate the bulk electronic structure of materials24,25 and this kind of NPs.23 High-energy X-ray diffraction (HEXRD)26,27 and extended X-ray absorption fine structure (EXAFS)28–31 analysis using synchrotron X-rays are also useful tools for observing structural information in long- and short-range orders. Furthermore, a reverse Monte Carlo (RMC) modeling method allows us to generate 3-dimensional structure models from HEXRD data.32
In this study, we investigated the electronic and crystal structures of Rh NPs of various particle sizes (2.4, 4.0, 7.1, and 10.5 nm), using synchrotron-based X-ray techniques including the HAXPES, X-ray absorption fine structure (XAFS), and HEXRD coupled with the RMC modeling method. To help unveil the origin of their hydrogen-absorption ability, we analysed the particle size dependence of the d-band center, lattice distortion, coordination number, long- and short-range order, mean-square displacement, and volume fraction of cavities of the Rh NPs.
2 Experimental
2.1 Sample preparation
We investigated Rh NPs of four different sizes (2.4–10.5 nm), as shown in Table 1. The Rh NPs were fabricated by the chemical reduction method using RhCl3·3H2O as the metal precursor and poly(N-vinyl-2-pyrrolidone) (PVP) as the stabilizing agent. Ethylene glycol (EG) was used as both the solvent and the reducing agent. The detailed procedure is described in ref. 20. The particle size of the prepared samples was determined from transmission electron microscopy (TEM) images. The details of the determination of NP size from the size histograms and temperature dependences for the hydrogen pressure–composition (PC) isotherms are also described in supporting notes 1 and 2 (ESI†).| Size (TEM)/nm | Metal precursor/mmol | Solvent/mL | PVP/mmol | H/Rh |
|---|---|---|---|---|
| 2.4 ± 0.5 | RhCl3·3H2O/1.0 | EG/220 | 10.0 | 0.225 |
| 4.0 ± 0.7 | RhCl3·3H2O/2.0 | EG/220 | 10.0 | 0.185 |
| 7.1 ± 1.2 | RhCl3·3H2O/2.0 | EG/110 | 5.0 | 0.130 |
| 10.5 ± 1.5 | RhCl3·3H2O/5.0 | EG/200 | 5.0 | 0.067 |
2.2 Hard X-ray photoelectron spectroscopy (HAXPES)
HAXPES measurements were performed by employing a linearly polarized X-ray beam in the horizontal plane with a photon energy of 5.95 keV at the undulator beamline BL15XU of the National Institute for Materials Science (NIMS) at SPring-8 in Japan. The total energy resolution was estimated to be 240 meV with a pass energy of 200 eV using a standard Au sample. All HAXPES spectra were recorded at room temperature. To eliminate the charge-up phenomenon, all the NPs were mixed with carbon powder. The corresponding inelastic mean free path (IMFP: λ), which was calculated using the TPP-2M equation,33 was approximately 5.6 nm for metallic Rh. The corresponding effective information depth was approximately 17–18 nm (3λ), greater than the diameter of the largest NPs. Hence, we were able to obtain information regarding the electronic structure of the Rh NPs.2.3 High-energy X-ray diffraction (HEXRD)
High-energy XRD experiments were performed at a beamline BL04B2 of SPring-8 in Japan using X-rays of 61.37 keV in photon energy (λ = 0.202 Å). The measurements were taken in the symmetric transmission geometry. The HEXRD data were corrected for background, polarization, and absorption and were then normalized to the structure factor and Fourier transformed using the SPring-8 BL04B2 analysis software.2.4 X-ray absorption fine structure (XAFS)
XAFS measurements of the Rh K-edge (23.22 keV) were performed using a Si(311) double-crystal monochromator at beamline BL01B1 of SPring-8, Japan. All XAFS spectra were obtained in transmission mode at room temperature. The powder samples were used to fill the hole of a copper plate of 0.5 mm in thickness using Kapton tape on both sides. The inner diameter of the hole was 3.0 mm. The intensities of the incident and transmitted X-ray beams were collected using ionization chambers located in front and behind the respective samples. To extract the XAFS oscillation, |χ(k)| (where k is the photoelectron wavenumber), from the obtained absorption spectra, the extrapolated pre-edge background absorption was subtracted first. The resulting curve was then normalized by dividing the jump in the absorption at the edge. Data processing and analysis of the XAFS spectra were performed using the Athena and Artemis programs of the IFEFFIT software package.342.5 Reverse Monte Carlo (RMC) method
RMC simulations were performed using RMC_POT software35 to construct the 3-dimensional atomic configuration and to calculate the volume fraction of cavities for Rh NPs of three different sizes (2.4, 4.0, and 7.1 nm). RMC_POT provides RMC simulation on the assumption of the NPs in sphere shapes under non-periodic boundary conditions. Here, the diameter of spherical samples was determined from TEM images in Table 1. The RMC run was carried out for each system whose configuration closely resembles that of a spherical NP. The simulations were guided by the experimental structure factor S(Q) and were stopped when the RMC computed and experimental data agreed very well over the entire range of wave vectors (Q = 20 Å−1). An average coordination constraint of first neighbors was enforced the coordination number, as obtained from the results of XAFS experiments.3 Results and discussion
3.1 Electronic structure: HAXPES
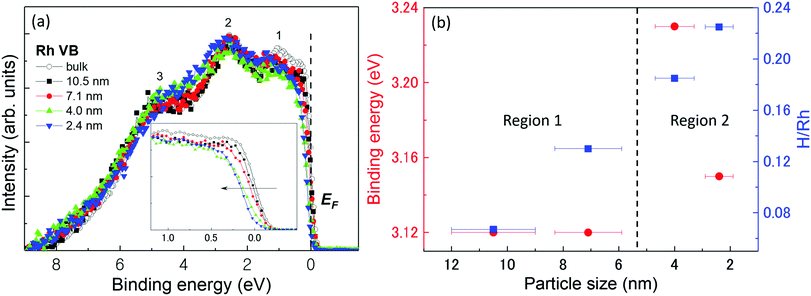
Fig. 1(a) shows the VB HAXPES spectra of bulk Rh and Rh NPs with different particle sizes. To ensure proper comparison between the spectra, the following steps were implemented: (1) all VB spectra were recorded in the same energy region (0–9 eV), (2) the background caused by carbon powder was properly subtracted, (3) the Shirley background was subtracted36 and (4) the VB area intensity was normalized. For ease of discussion, peaks 1, 2, and 3 have been tagged in the VB HAXPES spectra. According to our previous density functional theory (DFT) calculations,23 peak 1 arose from the Rh 4d density of states (DOS). Peak 2 was mainly composed of the Rh 4d partial DOS. Peak 3 was mainly composed of Rh 4d and 5s partial DOSs. The Rh 5p DOS just uniformly distributed in both peaks 2 and 3. The most apparent difference in the spectra is that the intensity of peak 1 was higher for bulk Rh than for the Rh NPs. The calculated s-DOS was most intense at the bottom of the d-band far away from the Fermi edge (EF), as shown in Supplementary Fig. 5 of ref. 23, similarly to the Ag case. This suggested that the difference between the 4d DOSs near the Fermi edge caused by the coordination number37 of bulk Rh and Rh NPs may be the reason why the bulk Rh cannot store hydrogen. This result indicates that the metal d state is an important parameter for estimating the chemical molecular adsorption barrier of noble metal surfaces. The inset of Fig. 1(a) shows an enlargement of the photoemission spectra in the vicinity of the Fermi edge. The VB DOS shifts obviously toward a higher binding energy with decreasing particle size. In particular, for the 4.0 nm and 2.4 nm diameter Rh NPs, the Fermi edge onsets in the VB HAXPES spectra were not the usual metallic Fermi edge, with steep slopes away from the Fermi edge. This phenomenon probably originated from the final state effect caused by the photoholes left behind in the NPs during the photoemission process38 and the effective number of the neighbors of a given atom decreases as the particle size decreases, consequently, the interaction of the d-electrons localized on neighboring atoms is expected to decrease.39Generally, the electronic structure change due to hydrogen absorption would be like that due to alloying reaction as a metallic bond, not covalent character within the host–guest framework. In fact, for not only Rh but also other 3d, 4d, and 5d metals, the electronic structures especially the d-band structures are very important for adsorption hydrogen.40,41 It has been shown that the activity of metal catalysts strongly depends on the coordination number of the metal atoms. Hammer and Nørskov42 proposed the so-called d-band model to explain trends in the catalytic activities of metal surfaces, films, and clusters.
The strength of the interaction and the occupancy of the resulting states, which are directly related to the potential barrier for adsorption, can be reflected by the position of the d-band center. According to their study,42 the d-band width becomes smaller and the d-band center tends to move away from EF with decreasing coordination number. Therefore, the center of gravity of each VB HAXPES spectrum, which is called the d-band-center position, was plotted as shown by the red circles in Fig. 1(b). The relative hydrogen-storage capacity of the Rh NPs is also plotted in the figure with the blue squares. It is obvious that the hydrogen-storage capacity of the NPs linearly increased as their diameter was decreased.20 However, the d-band center positions for the 10.5 nm and 7.1 nm diameter Rh NPs were almost the same. As the particle size was decreased from 7.1 nm to 4.0 nm, the center position is shifted away from EF. It then shifted towards EF from 4.0 nm to 2.4 nm. The whole behaviour of the d-band center position deviated from that predicted by the d-band-center theory.
The 4.0 nm diameter Rh NPs, the d-band center of which was the most shifted away from EF, did not have the highest hydrogen-storage capacity. This suggested that the hydrogen-storage capacity may be determined by structural parameters, not the position of the d-band center. Here, the structural parameters included lattice parameters, atomic bond length, surface area, lattice distortion, coordination number, mean-square displacement, and cavity. Notably, the d-band widths of the 4.0 nm and 2.4 nm diameter Rh NPs were slightly smaller than that of the 10.5 nm and 7.1 nm diameter Rh NPs, suggesting d-band rehybridization in these smaller NPs. Furthermore, the limitation condition may exist for d-band theory when explaining the hydrogen-storage capacity of NPs.
3.2 Structural characterization: HEXRD, XAFS and RMC structure modeling
To fully understand the origin of the hydrogen-absorption ability of Rh NPs, we carried out a structural characterization using HEXRD and XAFS. Fig. 2(a) shows the XRD patterns of all the prepared Rh NPs. The full-width-at-half-maximum (FWHM) of the diffraction peaks became sharper with an increase in mean particle size, which was obtained from TEM observations.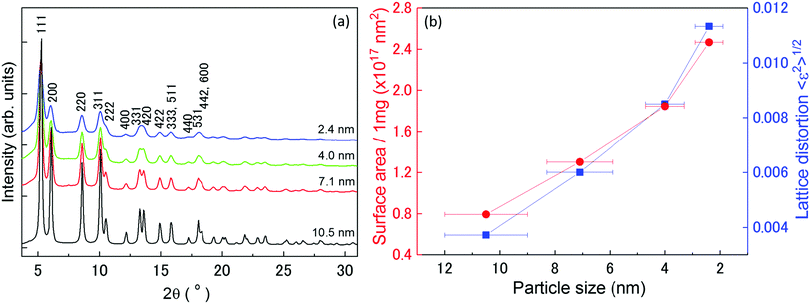 | ||
| Fig. 2 XRD patterns, surface area and lattice distortion: (a) XRD patterns of Rh NPs at room temperature. The incident X-ray energy was 61.37 keV with a wavelength of 0.202 Å. Fifteen Bragg peaks (from 111 to 600) were used to determine the average crystalline domain size of the Rh NPs, as shown in Fig. S4 (ESI†). (b) Dependence of surface area of crystalline domains (red circles) and lattice distortion (blue squares), as obtained from the Rietveld analysis, on particle size. | ||
All the XRD patterns were analysed using the Rietveld refinement method43 with the pseudo-Voigt function. The results of the Rietveld refinement analysis are shown in Fig. S3 (ESI†). The plot shows the refined XRD patterns, residuals, Bragg peak positions, and Rwp and RB agreement factors. In the refinement, the Rh fractional position and occupancy were considered to be fixed. Other parameters such as lattice constants, B factor, scale factor, and peak-shape function parameters were taken as free parameters. The B factor is defined as the mean-square displacement and is related to atomic thermal vibration. The space group associated with fcc Rh NPs is Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The evaluated average domain size, lattice parameter, the nearest neighbour distance (RNN), unit-cell volume, the number of unit cells, B factor, Debye–Waller factor
m. The evaluated average domain size, lattice parameter, the nearest neighbour distance (RNN), unit-cell volume, the number of unit cells, B factor, Debye–Waller factor 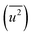 , lattice distortion, and the domain surface area of the NPs are listed in Table 2.
, lattice distortion, and the domain surface area of the NPs are listed in Table 2.
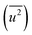 , lattice distortion, and the domain surface area of Rh NPs obtained by Rietveld refinement of the HEXRD data
, lattice distortion, and the domain surface area of Rh NPs obtained by Rietveld refinement of the HEXRD data
To reveal the origin of the hydrogen-storage properties of the Rh NPs, we plotted the particle size dependence of the surface area of the crystalline domains, lattice distortion, and mean-square displacement. Fig. 2(b) shows the relationship between the surface area of the crystalline domains (red circles)/lattice distortion ratio (blue square), obtained from Rietveld analysis and particle size, as obtained from TEM results. The surface area was calculated using the following equation: Asurface = N × 4π(D/2)2, assuming that the NPs are spherical in shape. Here, Asurface denotes the surface area of the crystalline domains, N is the number of domains per 1 mg of Rh NPs, and D is the average crystalline domain size. N is defined by the following equation: N = (number of Rh atoms per 1.0 mg)/(number of Rh atoms per domain). The details for the calculation of N are described in supporting note 5 (ESI†). The surface area of the crystalline domains increased with a decrease in NP diameter. This result indicates that the hydrogen-storage capacity is closely related to the surface area of the crystalline domains, and can be interpreted as the main reason why the 10.5 nm and 7.1 nm diameter Rh NPs had different hydrogen-storage capacities even though their VB spectra (Fig. 1(b)) were almost the same.
In the calculation of lattice distortion, based on the assumption that the absorption of hydrogen on Rh NPs occurs on all crystalline planes, not only the close-packed (111) lattice planes, the lattice distortion was determined using all 15 lattice planes (from (111) to (600)). The crystal lattice distortion 〈ε2〉1/2 of the Rh NPs at various domain sizes was calculated using the following equation:44,45
Here, K (= 0.9 if nanoparticles are assumed to be spherical) is the shape factor. Lattice distortion in Fig. 2(b) increased with a decrease in NP diameter. The hydrogen-storage capacity of the Rh NPs can be attributed to this phenomenon, together with the result of the surface area in Fig. 2(b). In other words, larger surface area and lattice distortion result in large hydrogen-storage capacity for the Rh NPs.
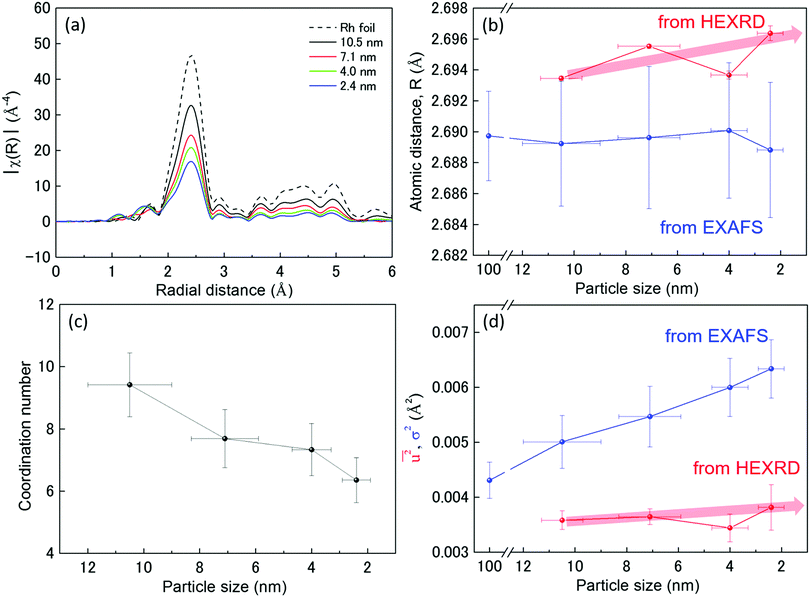
Fig. 3(a) show the Fourier transformed (FT) magnitude |χ(R)| of k3χ(k) for the experimental K-edge XAFS spectra of Rh foil and Rh NPs (Fig. S5, ESI†). All the |χ(R)| peaks in the spectrum of the Rh foil were present in the spectra of all the Rh NPs (Fig. 3(a)). This result indicates that an fcc Rh local structure existed in at least part of the 2.4 to 10.5 nm diameter Rh NPs. The behaviour of the experimental Rh K-edge XAFS spectra was reflected in the fit of the FT magnitudes |χ(R)| of k3χ(k) of these spectra, assuming that the local structure in both Rh foil and Rh NPs can be represented by a single-term contribution. The mean interatomic Rh–Rh distance (RRh–Rh), coordination number (CN), and Debye–Waller parameter (σ2) evaluated from the fitting are listed in Table 3. Rh foil was also used as a reference to obtain the value of RRh–Rh, reduction factor (So2), and σ2. This value of So2 was used in the following study of the atomic structure of the Rh NPs (Table 3), in which the CN for the Rh NPs were obtained by dividing the corresponding variable So2 × CN by So2 = 0.994. From XAFS results in Fig. 3(b), the mean interatomic Rh–Rh distance (RRh–Rh) of Rh NPs was confirmed to be equal to that of Rh foil. However, the nearest neighbour distance (RNN) and the lattice parameter from HEXRD tend to increase with decreasing particle size, except for the 4.0 nm diameter Rh NPs. This size effect on the lattice parameters was not in agreement with results from previous studies on metallic NPs.46–52 Furthermore, the abnormal change in RNN for the 4.0 nm diameter Rh NPs can be interpreted as one of the reasons why the 2.4 nm diameter Rh NPs had a higher hydrogen-storage capacity than the 4.0 nm diameter Rh NPs, even though the d-band center of the Rh 2.4 nm diameter NPs was closer to EF than that of the 4.0 nm diameter Rh NPs in Fig. 1(b).
| Sample | Rh 2.4 nm | Rh 4.0 nm | Rh 7.1 nm | Rh 10.5 nm | Rh foil |
|---|---|---|---|---|---|
| R Rh–Rh (Å) | 2.6888 ± 0.0044 | 2.690 ± 0.0044 | 2.6896 ± 0.0046 | 2.6892 ± 0.0040 | 2.6897 ± 0.0029 |
| S o 2 | 0.994 ± 0.077 | ||||
| CN | 6.355 ± 0.719 | 7.333 ± 0.840 | 7.688 ± 0.936 | 9.419 ± 1.022 | |
| σ 2 (Å2) | 0.0063 ± 0.0005 | 0.0060 ± 0.0005 | 0.0055 ± 0.0006 | 0.0050 ± 0.0005 | 0.0043 ± 0.0003 |
Fig. 3(c) shows the relationship between the coordination number (CN) and particle size. The apparent decrease in CN, compared with the 12 of the Rh bulk metal, may be associated with the increase in the number of unoccupied sites in each unit cell and localized disordering. This also indicates that the smaller particle tends to have more vacancy spaces or the larger one tends to be denser. These results, along with the results of surface area and lattice distortion in Fig. 2(b), are advantageous for the hydrogen-absorption capacity in terms of crystal structures of the Rh NPs.
Fig. 3(d) shows the dependence of the mean-square displacement of the mean structure (red circles, 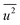 ) and local structure (blue circles, σ2) on particle size. The value of
) and local structure (blue circles, σ2) on particle size. The value of 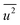 (Debye–Waller factor) is estimated from B, defined as53
(Debye–Waller factor) is estimated from B, defined as53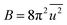 , in Table 2 and regarded as indicative of the relative thermal vibrational motion of different parts of the structure relative to the mean structure (in the bulk). Atoms with a small
, in Table 2 and regarded as indicative of the relative thermal vibrational motion of different parts of the structure relative to the mean structure (in the bulk). Atoms with a small 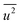 belong to parts of the structure that are well ordered. In contrast, atoms with a large
belong to parts of the structure that are well ordered. In contrast, atoms with a large 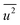 generally belong to parts of the structure that are very flexible or more sensitive to the ambient environment. In contrast, strictly speaking, σ2 is not a Debye–Waller factor. The Debye–Waller factor is a term commonly used in diffraction to describe the attenuation of the scattered intensity caused by the displacement of atoms away from a lattice point. For XAFS, the analogous Debye–Waller factor would describe the attenuation of χ(k) caused by the thermal and static disorder in the bond length or local structure. The value of
generally belong to parts of the structure that are very flexible or more sensitive to the ambient environment. In contrast, strictly speaking, σ2 is not a Debye–Waller factor. The Debye–Waller factor is a term commonly used in diffraction to describe the attenuation of the scattered intensity caused by the displacement of atoms away from a lattice point. For XAFS, the analogous Debye–Waller factor would describe the attenuation of χ(k) caused by the thermal and static disorder in the bond length or local structure. The value of 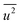 , obtained from HEXRD, slightly increased with decreasing particle size, except for the 4.0 nm diameter Rh NPs. The value of σ2, obtained from XAFS, also increased with a decrease in particle size. These results mean that the mean-square displacement/disorder greatly affects the hydrogen-storage capacity of Rh NPs. It has been noted that the value of σ2 is considerably larger than that of
, obtained from HEXRD, slightly increased with decreasing particle size, except for the 4.0 nm diameter Rh NPs. The value of σ2, obtained from XAFS, also increased with a decrease in particle size. These results mean that the mean-square displacement/disorder greatly affects the hydrogen-storage capacity of Rh NPs. It has been noted that the value of σ2 is considerably larger than that of 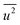 for the same particle size. This result suggests that the hydrogen-absorption capability of Rh NPs improves more as the mean-square displacement in the local structure increases than that in the mean crystal structure.
for the same particle size. This result suggests that the hydrogen-absorption capability of Rh NPs improves more as the mean-square displacement in the local structure increases than that in the mean crystal structure.
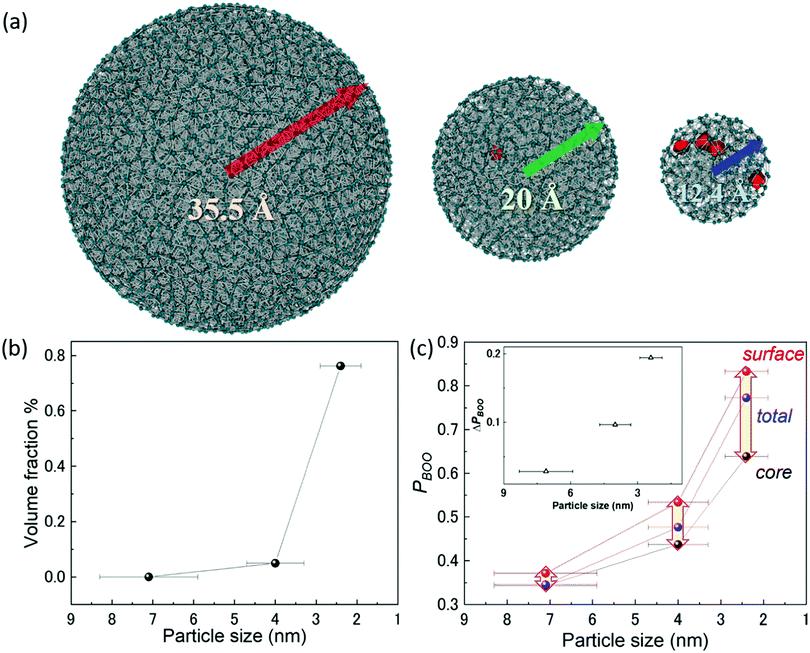
To fully reveal the size effect of the hydrogen-storage capacity of Rh NPs, the cavities and bond-orientational order (BOO) parameter54,55 within Rh NPs were calculated with RMC structure modeling based on high-energy X-ray diffraction data. It is well known that hydrogen is more likely to be trapped on grain boundary or dislocations in metals.56 It is also expected that hydrogen can be absorbed on cavities in their entire volume. Notably, RMC structure modeling is suitable to investigate the local structure of isolated and finite-sized spherical NPs and to determine the 3-dimensional atomic positions.32 The RMC experimental and simulated structure factor data sets for Rh NPs (2.4, 4.0, and 7.1 nm) are shown in Fig. S6 (ESI†). The RMC configuration models of Rh NPs reproduced the experimental total structure factor S(Q) with a good agreement. Fig. 4(a) shows the atomic configuration and cavity distribution obtained from RMC structure modeling. Here, the cavities were presented in red. The position and volume size of cavities were determined from a Dirichlet–Voronoi construction using the atomic positions and the cavity centers.57 From this cavity analysis, we confirm that there was no cavity within the 7.1 nm diameter Rh NPs. In contrast, the volume fractions of the cavities for the 2.4 nm and 4.0 nm Rh NPs were 0.76 and 0.05%, respectively. Namely, the volume fraction of the cavities increased with a decrease in NP diameter (Fig. 4(b)). This result indicates that the total cavity volume contributes to the hydrogen-storage capacity, and the cavities can be induced by the increase of unoccupied sites and localized disordering. Fig. 4(c) shows the relationship between the parameter PBOO and particle size for a core part and a surface part of Rh NPs. This PBOO value means the deviation of the local structure from the ideal crystal structure.55 If PBOO is zero, the local structure is regarded as ideal. The details of the calculation of BOO and PBOO are described in supporting note 7 (ESI†). Here, the whole volume of Rh NPs was divided into a core part and a surface part on the basis of the distance of 0.33 nm from the surface. The parameter PBOO increased with a decrease in NP diameter. This result indicates that the localized disordering of Rh NPs increased with a decrease in NP diameter and was in agreement with the result shown in Fig. 3(c). It has been noted that PsurfaceBOO is bigger than PcoreBOO in a same size and the difference between PsurfaceBOO and PcoreBOO increased with a decrease in NP diameter (see the inset of Fig. 4(c)). This result indicates that localized disordering is distributed over the surface part than the core part a lot; the effect on localized disordering at the surface part increased with a decrease in NP diameter. It is expected that the probability that hydrogen can be trapped on the surface part of Rh NPs increases with a decrease in NP diameter. The structural information obtained in the present work will contribute to the design and improvement of the functionality of nanosized metals.
4 Conclusions
We have investigated the size dependence of the hydrogen-storage properties of Rh nanoparticles (NPs) using various synchrotron-based X-ray techniques including HAXPES, HEXRD, XAFS and also analyzed the cavities inside Rh NPs using the RMC method. The principal findings from these measurements are as follows. (1) In the VB HAXPES spectra, the intensity of peak 1 (the occupied d-DOS) was higher for bulk Rh than for Rh NPs. This indicates that the unoccupied d-DOS of Rh NPs may be more than that of bulk Rh. (2) The electronic structures of the 2.4 nm and 4.0 nm diameter Rh NPs were very different from those of the other sizes. The narrowing of the d-band width with decreasing particle size suggested that the d-band rehybridization occurred in these smaller NPs. (3) With decreasing particle size, the domain surface area, lattice distortion, vacancy spaces, and mean-square displacement increased and the coordination number decreased. These results can explain the hydrogen-storage capacity of the Rh NPs. (4) The hydrogen-storage capability of the Rh NPs increased to a greater extent with the mean-square displacement in the local structure than that in the mean crystal structure. (5) The volume fraction of the cavities inside the Rh NPs increased with decreasing particle size. The cavities can be induced by the increase of unoccupied sites in each unit cell and localized disordering. From the result of PBOO, the effect on localized disordering at the surface part increased with a decrease in NP diameter. As a result, it is expected that the probability that hydrogen absorbed on the surface part of the Rh NPs increases with a decrease in NP diameter. It is hoped that these findings will be helpful in understanding the correlation between hydrogen-storage properties and electronic and crystal structure parameters in these new functional NPs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by ACCEL (JPMJAC1501) of the Japan Science and Technology Agency (JST). The HAXPES spectra were obtained at the NIMS synchrotron X-ray station of SPring-8 under Proposal No. 2013A4908. The HEXRD and XAFS measurements were carried out at SPring-8 with the approval of the Japan Synchrotron Radiation Institute under Proposal No. 2016A1292 and 2016B1033. This work was also partly supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (OS: 15K04616).References
- P. K. Capon, A. Burgun, C. J. Coghlan, R. S. Crees, C. J. Doonan and C. J. Sumby, CrystEngComm, 2016, 18, 7003–7010 RSC.
- J. Goldsmith, A. G. Wong-Foy, M. J. Cafarella and D. J. Siegel, Chem. Mater., 2013, 25, 3373–3382 CrossRef.
- Y. Liu, J. F. Eubank, A. J. Cairns, J. Eckert, V. C. Kravtsov, R. Luebke and M. Eddaoudi, Angew. Chem., Int. Ed., 2007, 46, 3278–3283 CrossRef PubMed.
- Y. Xia, Z. Yang and Y. Zhu, J. Mater. Chem. A, 2013, 1, 9365–9381 Search PubMed.
- R. Ströbel, J. Garche, P. Moseley, L. Jörissen and G. Wolf, J. Power Sources, 2006, 159, 781–801 CrossRef.
- M. M. Li, C. C. Yang, C. C. Wang, Z. Wen, Y. F. Zhu, M. Zhao, J. C. Li, W. T. Zheng, J. S. Lian and Q. Jiang, Sci. Rep., 2016, 6, 27601 CrossRef PubMed.
- M. D. Ward, Science, 2003, 300, 1104–1105 CrossRef PubMed.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef PubMed.
- F. A. Lewis, The Palladium Hydrogen System, Academic Press, London, 1967 Search PubMed.
- Hydrogen in Metals, ed. G. Alefeld and J. Völkl, Springer, Berlin, 1978 Search PubMed.
- S. K. Konda and A. Chen, Mater. Today, 2016, 19, 100–108 CrossRef.
- H. Kobayashi, M. Yamauchi, H. Kitagawa, Y. Kubota, K. Kato and M. Takata, J. Am. Chem. Soc., 2008, 130, 1818–1819 CrossRef PubMed.
- H. Kobayashi, M. Yamauchi, H. Kitagawa, Y. Kubota, K. Kato and M. Takata, J. Am. Chem. Soc., 2008, 130, 1828–1829 CrossRef PubMed.
- H. Kobayashi, H. Morita, M. Yamauchi, R. Ikeda, H. Kitagawa, Y. Kubota, K. Kato and M. Takata, J. Am. Chem. Soc., 2011, 133, 11034–11037 CrossRef PubMed.
- D. W. Goodman and C. H. F. Peden, J. Phys. Chem., 1986, 90, 4839–4843 CrossRef.
- E. Bergene, O. Tronstad and A. Holmen, J. Catal., 1996, 160, 141–147 CrossRef.
- A. Zaluska, L. Zaluski and J. Ström-Olsen, J. Alloys Compd., 1999, 288, 217–225 CrossRef.
- R. Kubo, J. Phys. Soc. Jpn., 1962, 17, 975–986 CrossRef.
- A. Henglein, Chem. Rev., 1989, 89, 1861–1873 CrossRef.
- K. Kusada, H. Kobayashi, R. Ikeda, H. Morita and H. Kitagawa, Chem. Lett., 2013, 42, 55–56 CrossRef.
- M. P. Seah and W. A. Dench, Surf. Interface Anal., 1979, 1, 2–11 CrossRef.
- H. S. Kim, M. Kang, M. W. Song, J. W. Park and B. R. Min, React. Kinet. Catal. Lett., 2004, 81, 251–257 CrossRef.
- A. Yang, O. Sakata, K. Kusada, T. Yayama, H. Yoshikawa, T. Ishimoto, M. Koyama, H. Kobayashi and H. Kitagawa, Appl. Phys. Lett., 2014, 105, 153109 CrossRef.
- K. Kobayashi, M. Yabashi, Y. Takata, T. Tokushima, S. Shin, K. Tamasaku, D. Miwa, T. Ishikawa, H. Nohira, T. Hattori, Y. Sugita, O. Nakatsuka, A. Sakai and S. Zaima, Appl. Phys. Lett., 2003, 83, 1005–1007 CrossRef.
- K. Kobayashi, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 601, 32–47 CrossRef.
- N. Bedford, C. Dablemont, G. Viau, P. Chupas and V. Petkov, J. Phys. Chem. C, 2007, 111, 18214–18219 Search PubMed.
- C. Song, O. Sakata, L. S. R. Kumara, S. Kohara, A. Yang, K. Kusada, H. Kobayashi and H. Kitagawa, Sci. Rep., 2016, 6, 31400 CrossRef PubMed.
- Y. F. Nishimura, T. Hamaguchi, S. Yamaguchi, H. Takagi, K. Dohmae, T. Nonaka and Y. Nagai, J. Phys.: Conf. Ser., 2016, 712, 012067 CrossRef.
- V. V. Srabionyan, A. L. Bugaev, V. V. Pryadchenko, L. A. Avakyan, J. A. van Bokhoven and L. A. Bugaev, J. Phys. Chem. Solids, 2014, 75, 470–476 CrossRef.
- Y. Oumellal, K. Provost, C. M. Ghimbeu, A. M. de Yuso and C. Zlotea, Nanotechnology, 2016, 27, 465401 CrossRef PubMed.
- C.-M. Lin, T.-L. Hung, Y.-H. Huang, K.-T. Wu, M.-T. Tang, C.-H. Lee, C. T. Chen and Y. Y. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125426 CrossRef.
- L. S. R. Kumara, O. Sakata, S. Kohara, A. Yang, C. Song, K. Kusada, H. Kobayashi and H. Kitagawa, Phys. Chem. Chem. Phys., 2016, 18, 30622–30629 RSC.
- S. Tanuma, C. J. Powell and D. R. Penn, Surf. Interface Anal., 1988, 11, 577–589 CrossRef.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef PubMed.
- O. Gereben and V. Petkov, J. Phys.: Condens. Matter, 2013, 25, 454211 CrossRef PubMed.
- D. A. Shirley, Phys. Rev. B: Solid State, 1972, 5, 4709–4714 CrossRef.
- B. Hvolbak, T. V. Janssens, B. S. Clausen, H. Falsig, C. H. Christensen and J. K. Nørskov, Nano Today, 2007, 2, 14–18 CrossRef.
- A. Tanaka, Y. Takeda, T. Nagasawa, H. Sasaki, Y. Kuriyama, S. Suzuki and S. Sato, Surf. Sci., 2003, 532–535, 281–286 CrossRef.
- I. Konomi, S. Hyodo and T. Motohiro, J. Catal., 2000, 192, 11–17 CrossRef.
- R. Griessen and A. Driessen, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 30, 4372–4381 CrossRef.
- B. Hammer and J. K. Nørskov, Nature, 1995, 376, 238 CrossRef.
- B. Hammer and J. K. Nørskov, Adv. Catal., 2000, 45, 71–129 Search PubMed.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef.
- X. Zhong, B. Yang, X. Zhang, J. Jia and G. Yi, Particuology, 2012, 10, 365–370 CrossRef.
- A. Gaber, M. A. Abdel-Rahim, A. Y. Abdel-Latief and M. N. Abdel-Salam, Int. J. Electrochem. Sci., 2014, 9, 81–95 Search PubMed.
- W. H. Qi, M. P. Wang and Y. C. Su, J. Mater. Sci. Lett., 2002, 21, 877–878 CrossRef.
- W. H. Qi and M. P. Wang, J. Nanopart. Res., 2005, 7, 51–57 CrossRef.
- E. Z. da Silva and A. Antonelli, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 17057–17060 CrossRef.
- R. Lamber, S. Wetjen and N. I. Jaeger, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 10968–10971 CrossRef.
- P. A. Montano, G. K. Shenoy, E. E. Alp, W. Schulze and J. Urban, Phys. Rev. Lett., 1986, 56, 2076–2079 CrossRef PubMed.
- P. A. Montano, W. Schulze, B. Tesche, G. K. Shenoy and T. I. Morrison, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 30, 672–677 CrossRef.
- G. Apai, J. F. Hamilton, J. Stohr and A. Thompson, Phys. Rev. Lett., 1979, 43, 165–169 CrossRef.
- R. W. James, The Optical Principles of the Diffraction of X-rays, Ox Bow Press, Woodbridge, Conn., 1962 Search PubMed.
- P. J. Steinhardt, D. R. Nelson and M. Ronchetti, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 28, 784–805 CrossRef.
- O. Seo, O. Sakata, J. M. Kim, S. Hiroi, C. Song, L. S. R. Kumara, K. Ohara, S. Dekura, K. Kusada, H. Kobayashi and H. Kitagawa, Appl. Phys. Lett., 2017, 111, 253101 CrossRef.
- W. Xiao and W. Geng, J. Nucl. Mater., 2012, 430, 132–136 CrossRef.
- I. Heimbach, F. Rhiem, F. Beule, D. Knodt, J. Heinen and R. O. Jones, J. Comput. Chem., 2017, 38, 389–394 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Rietveld analysis, crystalline domain size, X-ray absorption fine structure (XAFS) spectra, particle size distributions of Rh NPs, reverse Monte Carlo (RMC) simulation, bond-orientational order (BOO) parameter, and hydrogen pressure–composition (PC) isotherms (Fig. S1–S6 and Table S1). See DOI: 10.1039/c8cp01678j |
| This journal is © the Owner Societies 2018 |