Radiolytic formation of the carbon dioxide radical anion in acetonitrile revealed by transient IR spectroscopy†
Abstract
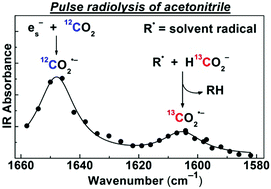
The solvated electron in CH3CN is scavenged by CO2 with a rate constant of 3.2 × 1010 M−1 s−1 to produce the carbon dioxide radical anion (CO2˙−), a strong and versatile reductant. Using pulse radiolysis with time-resolved IR detection, this radical is unambiguously identified by its absorption band at 1650 cm−1 corresponding to the antisymmetric CO2˙− stretch. This assignment is confirmed by 13C isotopic labelling experiments and DFT calculations. In neat CH3CN, CO2˙− decays on a ∼10 μs time scale via recombination with solvent-derived radicals (R˙) and solvated protons. Upon addition of formate (HCO2−), the radiation yield of CO2˙− is substantially increased due to H-atom abstraction by R˙ from HCO2− (R˙ + HCO2− → RH + CO2˙−), which occurs in two kinetically separated steps. The rapid step involves the stronger H-abstracting CN˙, CH3˙, and possibly, H˙ primary radicals, while the slower step is due to the less reactive, but more abundant radical, CH2CN˙. The removal of solvent radicals by HCO2− also results in over a hundredfold increase in the CO2˙− lifetime. CO2˙− scavenging experiments suggest that at 50 mM HCO2−, about 60% of the solvent-derived radicals are engaged in CO2˙− generation. Even under CO2 saturation, no formation of the radical adduct, (CO2)2˙−, could be detected on the microsecond time scale.


 Please wait while we load your content...
Please wait while we load your content...