Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From small molecules to polymeric catalysts in the oscillatory carbonylation reaction: multiple effects of adding HI†
Anna
Isakova
 *a,
Billy J.
Murdoch
b and
Katarina
Novakovic
a
*a,
Billy J.
Murdoch
b and
Katarina
Novakovic
a
aSchool of Engineering, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: anna.isakova@newcastle.ac.uk
bNational EPSRC XPS Users’ Service (NEXUS), School of Engineering, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK
First published on 21st March 2018
Abstract
The oscillatory palladium-catalysed carbonylation reaction opens new horizons for applications in smart materials due to the versatility of its conditions and substrates, as well as the adjustability of amplitude and period of pH oscillations. A variety of viable substrates have been demonstrated, including polymeric alkyne-terminated substrates. However, so far, there have not been any reports of polymer-based palladium catalysts in oscillatory mode. In this paper, we demonstrate pH oscillations in various systems, using commercially available palladium acetate, a triphenylphosphine palladium acetate complex and a polymer-bound palladium catalyst. While palladium acetate was able to generate oscillations under the conditions already established in our previous research on PdI2-catalysed oscillators, the other two catalysts needed the addition of HI to induce oscillations. HI forced an initial pH drop, bringing pH into the range where oscillations generally occur. Addition of HI had a significant effect on all catalysts, modifying the amplitude and period of oscillations, oscillation mode, as well as starting material conversion and product distribution.
Introduction
Oscillatory chemical reactions have been known for decades.1 Currently these reaction systems are generating profound interest in materials science as a driving force for smart materials, owing to the ability of smart materials to sense and react to changes in the environment, generated during the oscillatory process.2 A number of self-oscillating materials and architectures have been reported, based on such reactions as the Landolt clock or Belousov–Zhabotinsky reaction.3–5The palladium-catalysed oxidative carbonylation (PCOC) reaction has recently attracted attention due to its ability to exhibit oscillations in pH, redox potential, turbidity and heat of reaction.6–8 The most studied system employs phenylacetylene (PhAc) as substrate due to the facile analysis of products9 and uses PdI2 as a catalyst. Oscillations in the PhAc/PdI2 system are sustained in a batch reactor for days of monitoring with the possibility of varying the amplitude and period of oscillations by adjusting the temperature of reaction,10,11 concentration of the catalyst, substrate or even water content.12,13 Besides the sustained oscillations, the PCOC reaction has demonstrated a temperature-dependent product selectivity, which is potentially useful for industrial application due to a reduction of costs for isolation and purification,14 applicable to a number of substrates, as demonstrated by Gorodsky.15–17 Recently oscillations in pH were reported for PCOC, where an alkyne-functionalised polymeric substrate (poly(ethylene glycol)methyl ether acetylene, abbreviated as PEGA) was employed in place of PhAc, extending the opportunities of application of PCOC in smart materials.13
Although the PCOC reaction in oscillatory mode has been studied in depth by Novakovic et al., Gorodsky and Temkin et al., the evidence for other palladium catalysts’ activity displaying similar dynamics in this reaction system is lacking. While studies of similar carbonylation reactions have demonstrated the application of palladium diacetate18 and palladium chloride,19 no oscillations were reported. In this work, we studied a number of commercially-available palladium catalysts for application in PCOC (Fig. 1), in order to explore their potential for oscillatory dynamics that could lead to future applications in smart materials as part of more complex architectures. By studying the catalyst on three levels – from small molecule through a ligand–metal complex to a polymer-supported ligand–metal complex – we obtained a better insight into the working boundaries of the reaction.
Experimental part
Materials
Materials studied were used as received: palladium acetate (PdAc), bis(acetato)bis(triphenylphosphine)palladium(II) (L-Cat), di(acetato)dicyclohexylphenylphosphinepalladium(II), polymer-bound FibreCat® (Pd ∼5%, extent of labeling: 0.4–0.6 mmol g−1 PPH3 ligand content loading, P-Cat), palladium(II) iodide (≥99.99% trace metals basis), hydroiodic acid (HI, ≥57%), phenylacetylene (PhAc, 98%), triphenylphosphine (≥95.0% GC), methanol (HPLC Plus, ≥99.9) all Sigma Aldrich; naphthalene (extra pure), potassium iodide (≥99% GPR RECTAPUR®), VWR Chemicals; buffer solutions: pH 2.00 (glycine), pH 7 (phosphate) and pH 10 (borate) (all NIST Standard, ready to use for pH measurement, Fisher Chemical). Pure air and carbon monoxide were supplied by BOC. Na2PdAc2Cl2 was synthesised by mixing 2 equiv. of NaCl with 1 equiv. of PdAc in methanol for 18 h.The reaction
The reaction was performed in a flat-bottom Erlenmeyer flask (100 ml) at constant stirring, using the HEL Micronote system to record pH and temperature within the bulk of the reaction. Prior to the reaction, probes were calibrated at room temperature (17–20 °C) against buffer solutions of pH 2, 7 and 10. The stated amounts of KI (4.150 g, 25 mmol), catalyst (30 mg PdAc, 98.5 mg L-Cat or 285 mg P-Cat) and naphthalene (256 mg, used as an internal standard for subsequent GC-MS analysis) were all charged into the flask and dissolved (or suspended for P-Cat) in 100 ml HPLC grade methanol. The pH monitoring was initiated while the solids were dissolving with the stabilisation of pH taken as indication of complete dissolution of KI. At that point, purging through the solution with CO (15 ml min−1) and air (15 ml min−1) commenced. After 10–20 min (or after stabilisation of pH following an initial drop), phenylacetylene (1.38 ml, 12.57 mmol) was added. The system was monitored continuously for changes in pH and temperature of the reaction. It should be noted that the pH electrode was calibrated against aqueous buffer solutions while reaction pH was recorded in methanol solution. As the standard pH scale is based on measurements in water, pH values recorded in non-aqueous solutions need to be adjusted prior to being converted to hydrogen ion concentrations. It has been reported that in 100% methanol, pH values can be adjusted by adding 2.3 to the observed pH measurements to get the equivalent pH value in water.20 As the focus of this paper is comparison of the oscillatory trend achieved in pH, it was not needed to adjust pH values since the trend remains the same in both recorded and adjusted pH values. A detailed study of the effect a methanol–water solvent composition has on recorded pH and actual concentration of acid present in solution has been previously published for the PdI2/PhAc oscillatory carbonylation system.12Samples were taken at the end of the experimental runs (unless otherwise stated within the main text) and filtered over silica prior to being diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with methanol. The samples were analysed for starting material conversion and product distribution by a Varian Saturn 2200 gas chromatograph fitted with a VF-5ms column (30 m) and equipped with a mass spectrometry detector (GC-MS). The method was as follows: injector temperature 150 °C; helium flow rate 1 ml min−1; oven temperature 100–195 °C over 35 min in 5 steps.
2 with methanol. The samples were analysed for starting material conversion and product distribution by a Varian Saturn 2200 gas chromatograph fitted with a VF-5ms column (30 m) and equipped with a mass spectrometry detector (GC-MS). The method was as follows: injector temperature 150 °C; helium flow rate 1 ml min−1; oven temperature 100–195 °C over 35 min in 5 steps.
X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectroscopy (XPS) was performed on solid catalysts in a Thermo Scientific K-Alpha spectrometer, using a monochromated Al K-Alpha source (1486 eV). The flood gun was used for charge compensation.Absorbance
Absorbance was measured using a FLUOStar Omega microplate reader using a multiwell plate (200 μl) in a wavelength range from 275 to 700 nm with a resolution of 1 nm. Samples (1 ml) were withdrawn at certain time points throughout the experiment as stated within the main text and diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with HPLC grade methanol.
2 with HPLC grade methanol.
Results and discussion
Single mode oscillations in PdAc
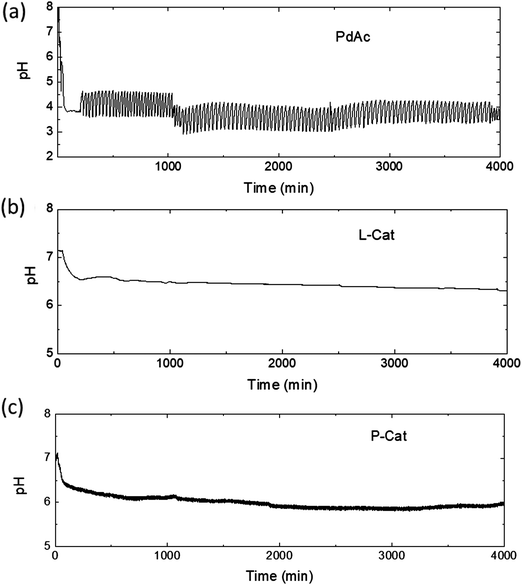
Three commercially available palladium catalysts were studied: small molecule palladium diacetate salt (denoted as PdAc), triphenylphosphine ligand palladium acetate (L-Cat) and a polymer-bound P-Cat (Fig. 1). PdAc and L-Cat are soluble in saturated KI solution in methanol, whereas polymer-bound palladium catalyst P-Cat is crosslinked and insoluble, thus providing an insight into the possibility of heterogeneous catalysis in the PCOC reaction. The changes in pH were monitored for 4000 min during which the reaction demonstrated oscillations (using PdAc) or stable values of pH (using L-Cat and P-Cat), Fig. 2.It can be observed from Fig. 2a that palladium acetate catalysis proceeded as has generally been reported for the PdI2 catalysed systems in oscillatory mode.10,14 When the system was purged with CO/air, an initial drop in pH from 7.9 to 6.4 was observed, indicating the incorporation of CO and methanol molecules into the structure of the catalyst with a corresponding release of H+ ions (Scheme 1a).14,21,22 Previous studies by other groups have suggested conversion of Pd2+ into Pd+ species at this initial stage of the reaction, however, the evidence for Pd+ species was not solid.6,23 A further pH drop from 6.4 to 3.8 occurred upon addition of the substrate and indicated the initial substrate conversion according to the generally accepted reaction mechanism.14
While general features are similar to those when using PdI2 as catalyst, previously investigated by our group, the oscillations in the PdAc system started significantly earlier, after 180 min (compared to well over 1000 min for PdI2).10 The reaction system employing PdAc catalyst exhibited a faster pH drop after addition of phenylacetylene with pH oscillations occurring shortly after. The period of oscillations for the PdAc catalyst was also shorter than when PdI2 was used (32 min and 60–70 min, respectively). This may be associated with the concentration difference (1.336 × 10−3 M of PdAc against 2.644 × 10−3 M PdI2 used in a previous study). When PdAc was used at a higher concentration of 2.644 × 10−3 M, similar to that of PdI2 reported previously, the oscillations started even earlier (104 min) but severe clogging of gas lines occurred which disrupted the oscillations (see ESI,† Fig. S1). The much shorter induction period with PdAc compared to PdI2 is most likely associated with the higher solubility of PdAc in KI solution.
Furthermore, the pattern of pH oscillations was different from that generally observed in PdI2 systems. pH oscillations in the PdAc reaction were highly regular with an amplitude of 0.98 (±0.09) pH units and a period of 31 (±4) min. This regularity is frequently seen in oscillatory redox systems in a continuous-flow stirred tank reactor (CSTR),24–27 but rarely observed in a batch reactor. The variations in amplitude, associated with substrate consumption and solvent evaporation, are not observed for the PdAc run, indicating the very slow conversion of substrate to products and making it one of the most stable batch oscillators reported to continue for more than 4000 min in the same mode. The unchanged mode of pH oscillations also indicates that a single oscillator is responsible for the recorded pattern.25
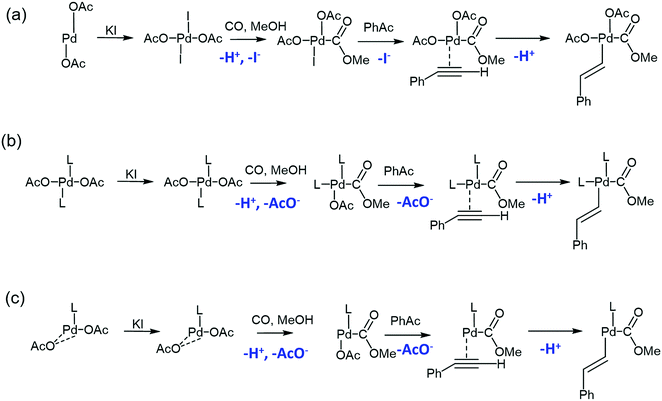
On the other hand, the PdAc-based catalysts L-Cat and P-Cat exhibited no oscillations. A much smaller pH drop occurred upon CO/air purging (from 7.1 to 6.9), and after the addition of substrate, pH decreased gradually to 6.5 and 6.1, respectively, where it plateaued (Fig. 2b and c). A ligand exchange model was proposed for all three catalysts (PdAc, L-Cat and P-Cat) to suggest how the ligands affected the initial change of pH during CO and air purging (Scheme 1).
Absorbance measurements of the reaction mixtures employing different catalysts were performed (see ESI,† Fig. S2). When PdAc was used, absorbance measurements detected the I3− (generated from I2 and I−) species upon incorporation of CO into the catalyst, which demonstrates that I− was released, in agreement with our tentative scheme. Furthermore, the oscillatory behaviour in pH when PdAc was used was accompanied by oscillatory changes in the concentrations of the Pd2+ and I3− species. Within one oscillation, during the pH rise the species Pd2+ and I2 were mostly observed, while during the pH drop, the Pd2+ peak absorbance decreased and the I3− peak was predominantly observed. Also, not detectable by UV-Vis but visible to the naked eye, particles of metallic palladium were present during the pH drop. Absorbance measurements of the other two catalysts, however, demonstrated that incorporation of CO into the catalyst did not result in the formation of I3−, in agreement with Scheme 1.
Mixed-mode oscillations after addition of HI
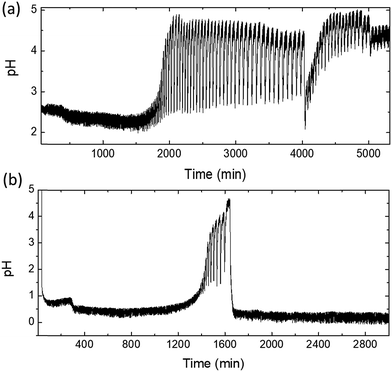
For phosphine-bound Pd catalysts (L-Cat and P-Cat), pH did not reach the values where oscillations typically occurred in previous oscillatory carbonylation reactions.7,10,13,17 Therefore, three catalysts were examined further in a new set of experiments where pH was artificially decreased by the addition of HI after the addition of substrate (Fig. 3). Although when PdAc was used, addition of HI was not needed for oscillations to start, PdAc was included in this study for comparison.PdAc exhibited delayed oscillations (Fig. 3a), when compared to the same catalyst without external addition of HI (Fig. 2a). The first series of oscillations with a relatively small amplitude and a period of 20–30 min were observed after 1200 min, which then developed into a series of oscillations with continuously increasing amplitude. The maximum observed amplitude was 1.91 pH. This time the pattern of oscillations was irregular and complex, reminiscent of previously observed mixed-mode oscillations, particularly so-called ‘bursting’ oscillations.28 Similar complex oscillations have been reported previously for the oscillatory Briggs–Rauscher reaction in a batch set-up.29 In that system, intermittent-bursting oscillations were characterised by the number of oscillations per burst, the duration of periods of bursts as well as the periods of gaps between them. Due to the constant change in concentration of reactant per s in a batch set-up, a CSTR is considered to be better suited for studying complex oscillatory behaviour.30 The complex trends in oscillatory patterns are associated with the existence of two qualitatively different behaviours replacing one another during the course of the reaction, even when all the control parameters remain the same.29 In such systems, the reaction networks (subnetworks) responsible for occurrence of small and large amplitude oscillations alternate.31 While our system was a batch reaction, observed oscillations (Fig. 3a) appeared to consist of two overlaid patterns, one with a short period of 20–30 min and another larger period of 140–290 min.
The heterogeneous polymeric P-Cat demonstrated oscillations after a long induction period of approximately 4000 min with the development of a similar, complex oscillatory pattern with progressively increasing amplitude and period (Fig. 3c). The large period took over 330–348 min, whereas the shorter period was of 20–53 min.
Catalyst L-Cat did not exhibit oscillations after addition of HI (Fig. 3b), although pH gradually decreased over the course of reaction from 6.9 to 3.8, where oscillations were generally observed. The expected rapid large drop of pH when HI was added did not occur. This was associated with an immediate consumption of protons by the triphenylphosphine ligands of the complex (see test runs in Fig. S3, ESI†), caused by their reversible dissociation. Increasing the amount of added hydroiodic acid ten-fold saw a more pronounced initial pH drop, similar to the other catalysts and the subsequent development of pH oscillations. The increase in added HI compensated for the HI consumed by the triphenylphosphine ligands during their dissociation. The oscillations in pH in this instance appeared after an induction period of approximately 4180 min (similar to the induction time using the P-Cat catalyst after the addition of HI (Fig. 3c), Fig. 4), during which the pH slowly rose from 2 to 2.8. The oscillations had a short period of 10–15 min and a small amplitude of 0.10–0.15 units until the pH started rising after 9200 min and the amplitude increased to 0.5 units.
 | ||
| Fig. 4 Oscillations in pH recorded in PCOC of PhAc in methanol using catalyst L-Cat, after addition of 0.228 mmol of HI to achieve the pH range where oscillations normally occurred. | ||
The origin of mixed-mode oscillations
Mixed-mode oscillations in pH have been observed and explained before in other systems,26,30,32 however, due to the general complexity of the PCOC mechanism (which is not 100% established), it is difficult to confidently assign the mixed-mode oscillations in PdAc and P-Cat after addition of HI to certain reaction networks, especially since they are also observed in certain conditions (at 10 and 20 °C), when PdI2 is used without any HI addition. Thus, further studies, including the transition to a CSTR reaction set-up, are needed to clarify the underlying mechanisms of the mixed-mode patterns.To expose the effect of two simultaneous processes with differing catalysis rates on the resulting oscillatory patterns, Na2PdAc2Cl2 catalyst was employed in place of PdAc in an otherwise identical experimental setup to that used in the experiment shown in Fig. 2a (no HI added, same concentrations of KI, PhAc substrate and gas purging rates of 15 ml min−1). Na2PdAc2Cl2 was selected since in saturated KI solution two catalytic species, Na2PdAc2Cl2 and K2PdAc2I2, would become available:
| Na2PdAc2Cl2 + 2KI ⇄ K2PdAc2I2 + 2NaCl. |
As can be seen in Fig. 5a, complex mixed-mode oscillations were indeed recorded. Oscillations resembled two oscillatory patterns overlaying each other: large oscillations with a period of 417–523 min and small oscillations with a period of 10–25 min. The captured oscillations are similar to the phenomena observed when PdAc and P-Cat were used with the addition of HI (Fig. 3a and c, now enlarged in Fig. 5b and c).
 | ||
| Fig. 5 Enlarged area where mixed-mode oscillations were observed (a) Na2PdAc2Cl2; (b) PdAc + HI and (c) P-Cat + HI. | ||
The presence of two catalytic species in the PdAc and P-Cat systems may not be the main/only driver for the existence of multiple subnetworks causing switching between large and small amplitude cycles so we also investigated PCOC employing a mixture of PdAc and PdI2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar content). No HI was externally added to this system. The total content of catalyst in mol% was identical to that in PdAc only reaction (1.336 × 10−3 M). As can be seen from Fig. 6a, in the mixed catalyst system, oscillations started later (after 1400 min) and initially had a small amplitude of 0.4–0.5 and period of 35 (±6) min which rapidly increased in amplitude to 2.4 (±0.3) and a period of 46 (±5) min. At approximately 4000 min, the periodic oscillations were terminated by a sudden drop in pH. Following this event, small amplitude “climbing” oscillations developed. The increase in pH and amplitude over time is a characteristic oscillatory shape in the PCOC reaction employing PdI2,10,12,33 although the majority of catalytic species in this reaction was PdAc. While the oscillatory patterns using PdAc + PdI2 had a less obvious mixed-mode behaviour than in PdAc + HI, it is possible to trace the evolution of an overlaid PdI2-induced pattern. When PdI2 and PdAc were used in a 1
1 molar content). No HI was externally added to this system. The total content of catalyst in mol% was identical to that in PdAc only reaction (1.336 × 10−3 M). As can be seen from Fig. 6a, in the mixed catalyst system, oscillations started later (after 1400 min) and initially had a small amplitude of 0.4–0.5 and period of 35 (±6) min which rapidly increased in amplitude to 2.4 (±0.3) and a period of 46 (±5) min. At approximately 4000 min, the periodic oscillations were terminated by a sudden drop in pH. Following this event, small amplitude “climbing” oscillations developed. The increase in pH and amplitude over time is a characteristic oscillatory shape in the PCOC reaction employing PdI2,10,12,33 although the majority of catalytic species in this reaction was PdAc. While the oscillatory patterns using PdAc + PdI2 had a less obvious mixed-mode behaviour than in PdAc + HI, it is possible to trace the evolution of an overlaid PdI2-induced pattern. When PdI2 and PdAc were used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, only one series of oscillations was observed before the system consumed all substrate (Fig. 6b) and stopped oscillating. Oscillations started after 1200 min and kept growing in amplitude until they reached 4.4 pH units (the last pH fall) while the period also increased from 14 min to 77 min.
1 ratio, only one series of oscillations was observed before the system consumed all substrate (Fig. 6b) and stopped oscillating. Oscillations started after 1200 min and kept growing in amplitude until they reached 4.4 pH units (the last pH fall) while the period also increased from 14 min to 77 min.
The existence of two catalytic species in PdAc and P-Cat was not straightforward, but since the mixed-mode oscillations were observed only after addition of HI, it is possible to suggest that HI was involved in the generation of PdI2 from a pool of metallic palladium within the pristine catalyst, as follows:
| HI + O2 → I2 + H2O |
| Pd0 + I2 → PdI2. |
Generation of additional PdI2 within the reaction mixture would facilitate the existence of two catalysts: K2PdAc2I2 and K2PdI4, one feasible explanation for multiple reaction subnetworks. The presence of metallic palladium in pristine P-Cat and PdAc was confirmed by XPS (Fig. S4, ESI†). The PdAc catalyst exhibited a double set of Pd 3d 5/2 and 3/2 peaks, indicating that both metallic palladium and Pd2+ were present in the catalyst, with the majority being Pd2+ species. P-Cat only exhibited a set of peaks for Pd0 with a shoulder into higher binding energies, indicating the presence of a smaller concentration of Pd2+ species. The source of metallic palladium impurities in these commercially available catalysts is unclear. Furthermore, it appeared that due to the absence of metallic palladium in L-Cat (as seen from XPS, Fig. S4, ESI†), the addition of HI did not lead to the generation of PdI2 which occurred with PdAc and P-Cat. Therefore, simple periodic oscillations and lack of mixed-mode patterns in this case could be characteristic of a system with a single catalytic species. Further studies involving a longer reaction monitoring time as well as the transition from batch to a CSTR set-up are needed to further understand the observed behaviours.
Starting material conversion and product distribution
Regarding product generation, both PdAc and P-Cat had a similar product distribution in both HI and non-HI modes – with almost 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 Z-isomer (5) and DMO (3) and only a trace amount of E-isomer (4, see Table 1). In HI mode, however, the product conversion increased significantly from 7.3% and 3.5% to 37.4% and 29.5% (for PdAc and P-Cat, respectively). Moreover, methyl cinnamate (1), generally considered as an intermediate, was observed in substantial amounts after addition of HI, suggesting that HI affected the mechanism of the reaction and altered the stoichiometry of the processes.
50 Z-isomer (5) and DMO (3) and only a trace amount of E-isomer (4, see Table 1). In HI mode, however, the product conversion increased significantly from 7.3% and 3.5% to 37.4% and 29.5% (for PdAc and P-Cat, respectively). Moreover, methyl cinnamate (1), generally considered as an intermediate, was observed in substantial amounts after addition of HI, suggesting that HI affected the mechanism of the reaction and altered the stoichiometry of the processes.
| mmol | E-Isomer (4) | Z-Isomer (5) | DMO (3) | MeCin (1) | MeAt (2) | PhAc conversion (%) |
|---|---|---|---|---|---|---|
| Without HI addition | ||||||
| PdAc 4000 min | 0.16 | 3.50 | 3.34 | — | — | 7.3 |
| L-Cat 4000 min | 0.28 | 6.37 | 0.63 | — | 0.13 | 9.0 |
| P-Cat 4000 min | 0.13 | 1.67 | 1.51 | 3.5 | ||
| After addition of 0.0228 mmol HI (1×) | ||||||
| PdAc 5600 min | 0.45 | 18.34 | 15.15 | 3.43 | 37.4 | |
| L-Cat 4600 min | 0.29 | 11.14 | 0.56 | 0.21 | 1.19 | 14.0 |
| P-Cat 6800 min | 0.32 | 12.26 | 10.35 | 5.17 | 29.5 | |
| After addition of 0.228 mmol HI (10×) | ||||||
| L-Cat 4000 min | 1.03 | 14.00 | 9.79 | 0.18 | 1.91 | 26.9 |
L-Cat 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 min 000 min |
3.74 | 16.55 | 9.87 | 0.19 | 1.98 | 32.3 |
L-Cat in both modes had a preferential product – Z-isomer (5), which was likely to be associated with steric hindrance from bulky triphenylphosphine ligands. Substrate conversion after 4000 min largely depended on the amount of HI added. Thus, after the addition of 0.228 mmol of HI, substrate conversion was 26.9%, which was higher than the conversion when only 0.0228 mmol of HI was added (14% at 4600 min) and without any HI (9.0% at 4000 min) (Table 1). This speaks for the ability of HI to improve the catalytic activity of L-Cat. At the time when conversion was measured (4000 min, Fig. 4) the oscillations had not started, indicating that the conversion process happened independently of the observable oscillations. Indeed, although the oscillations occurred after 4180 min, the additional conversion of PhAc was only 5.4% (32.3% at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 min), Table 1. This conversion was associated with an increase in formation of E and Z-isomers, whereas the concentrations of other products did not change significantly.
000 min), Table 1. This conversion was associated with an increase in formation of E and Z-isomers, whereas the concentrations of other products did not change significantly.
Conclusions
In this report, we demonstrate oscillatory pH behaviour in systems catalysed by various palladium-based catalysts – from a small molecule palladium acetate to polymer bound phosphine-liganded palladium catalyst. While palladium acetate worked along the lines of the previously reported PdI2, giving well-defined highly regular oscillations in the pH region of 3–4.5 pH, the other catalysts only exhibited oscillations after the initial pH drop was forced by addition of hydroiodic acid. Addition of HI not only helped to achieve the oscillations in L-Cat and P-Cat but also dramatically increased phenylacetylene conversion. Moreover, after the addition of HI, PdAc and P-Cat demonstrated mixed-mode oscillations. Further studies are required to identify the reaction subnetworks responsible for the large and small amplitude cycles. However, based on the evidence collected here, it was postulated that the observed complex behaviour was a result of HI induced conversion of metallic palladium into PdI2 meaning that two competing catalysts, PdAc and PdI2 were present in the system. The overall effect of externally added HI was complex: (i) it decreased the pH to the range where oscillations in PCOC system are generally observed; (ii) it generated additional Pd2+ from metallic palladium, as observed from XPS; (iii) it led to the observation of complex mixed-mode oscillatory patterns and (iv) it participated in the formation of mono-esters which were typically considered intermediates and not observed among the final products. This property of highly acidic HI to initiate oscillations when using a catalyst that would not otherwise give rise to oscillations is a promising feature for the targeted pH-mediated drug delivery and release.Conflicts of interest
No conflicts of interest to declare.Acknowledgements
This work was supported by UK Engineering and Physical Sciences Research Council (EPSRC) grant number EP/N033655/1. X-ray photoelectron spectroscopy (XPS) data were acquired at the National EPSRC XPS Users’ Service, an EPSRC Mid-Range Facility. Data supporting this publication is openly available under an ‘Open Data Commons Open Database License’. Additional metadata are available at: http://dx.doi.org/10.17634/153345-1. Please contact Newcastle Research Data Service at rdm@ncl.ac.uk for access instructions.References
- A. M. Zhabotinsky, Chaos, 1991, 1, 379–386 CrossRef PubMed.
- A. Isakova and K. Novakovic, Eur. Polym. J., 2017, 95, 430–439 CrossRef CAS.
- P. Topham, J. R. Howse, C. J. Crook, S. P. Armes, R. A. L. Jones and A. J. Ryan, Macromolecules, 2007, 40, 4393–4395 CrossRef CAS.
- R. Tamate, A. Mizutani Akimoto and R. Yoshida, Chem. Rec., 2016, 16, 1852–1867 CrossRef CAS PubMed.
- Y. S. Kim, R. Tamate, A. M. Akimoto, R. Yoshida, J. Groen, H. W. H. van Roekel, T. F. A. de Greef, W. T. S. Huck and T. Aida, Mater. Horiz., 2017, 4, 38–54 RSC.
- Alexander V. Malashkevich, A. Lev, G. Bruk and O. N. Temkin, J. Phys. Chem. A, 1997, 101, 9825–9827 CrossRef CAS.
- V. R. Khabibulin, A. V. Kulik, I. V. Oshanina, L. G. Bruk, O. N. Temkin, V. M. Nosova, Y. A. Ustynyuk, V. K. Bel'skii, A. I. Stash, K. A. Lysenko and M. Y. Antipin, Kinet. Catal., 2007, 48, 228–244 CrossRef CAS.
- K. Novakovic, C. Grosjean, S. K. Scott, A. Whiting, M. J. Willis and A. R. Wright, Chem. Phys. Lett., 2007, 435, 142–147 CrossRef CAS.
- C. Grosjean, K. Novakovic, S. K. Scott, A. Whiting, M. J. Willis and A. R. Wright, J. Mol. Catal. A: Chem., 2008, 284, 33–39 CrossRef CAS.
- K. Novakovic, A. Mukherjee, M. Willis, A. Wright, S. Scott and A. R. Wright, Phys. Chem. Chem. Phys., 2009, 11, 9044 RSC.
- J. Parker and K. Novakovic, React. Kinet., Mech. Catal., 2016, 118, 73–85 CrossRef CAS.
- J. Parker and K. Novakovic, React. Kinet., Mech. Catal., 2018, 123, 113–124 CrossRef CAS.
- L. Donlon and K. Novakovic, Chem. Commun., 2014, 50, 15506–15508 RSC.
- J. Parker and K. Novakovic, ChemPhysChem, 2017, 18, 1981–1986 CrossRef CAS PubMed.
- S. N. Gorodsky, Org. Chem. Int., 2012, 1–6 Search PubMed.
- S. N. Gorodsky, Kinet. Catal., 2012, 53, 493–496 CrossRef CAS.
- S. N. Gorodsky, React. Kinet., Mech. Catal., 2018, 1–20 Search PubMed.
- Y. Sakurai, S. Sakaguchi and Y. Ishii, Tetrahedron Lett., 1999, 40, 1701–1704 CrossRef CAS.
- R. F. Heck, J. Am. Chem. Soc., 1972, 94, 2712–2716 CrossRef CAS.
- S. P. Porras and E. Kenndler, J. Chromatogr. A, 2004, 1037, 455–465 CrossRef CAS PubMed.
- B. Gabriele, L. Veltri, G. Salerno, M. Costa and G. P. Chiusoli, Eur. J. Org. Chem., 2003, 1722–1728 CrossRef CAS.
- S. N. Gorodskii, A. N. Zakharov, A. V. Kulik, L. G. Bruk and O. N. Temkin, Kinet. Catal., 2001, 42, 251–263 CrossRef CAS.
- V. N. Zudin, V. D. Chinakov, V. M. Nekipelov, V. A. Rogov, V. A. Likholobov and Y. I. Yermakov, J. Mol. Catal., 1989, 52, 27–48 CrossRef CAS.
- G. Rabai, M. Orban and I. R. Epstein, Acc. Chem. Res., 1990, 23, 258–263 CrossRef CAS.
- T. G. Szántó and G. Rábai, J. Phys. Chem. A, 2005, 109, 5398–5402 CrossRef PubMed.
- G. Rábai and I. Hanazaki, J. Phys. Chem. A, 1999, 103, 7268–7273 CrossRef.
- E. C. Edblom, M. Orban and I. R. Epstein, J. Am. Chem. Soc., 1986, 108, 2826–2830 CrossRef CAS.
- M. J. B. Hauser, A. Strich, R. Bakos, Z. Nagy-Ungvarai and S. C. Müller, Faraday Discuss., 2002, 120, 229–236 RSC.
- Ž. D. Čupić, L. Z. Kolar-Anić, S. R. Anić, S. R. Maćešić, J. P. Maksimović, M. S. Pavlović, M. C. Milenković, I. N. M. Bubanja, E. Greco and S. D. Furrow, Helv. Chim. Acta, 2014, 97, 321–333 CrossRef.
- I. N. Bubanja, S. Maćešić, A. Ivanović-Šašić, Ž. Čupić, S. Anić and L. Kolar-Anić, Phys. Chem. Chem. Phys., 2016, 18, 9770–9778 RSC.
- B. D. Aguda, R. Larter and B. L. Clarke, J. Chem. Phys., 1989, 90, 4168–4175 CrossRef CAS.
- V. Vukojević, S. Anić and L. Kolar-Anić, J. Phys. Chem. A, 2000, 104, 10731–10739 CrossRef.
- J. Parker and K. Novakovic, Ind. Eng. Chem. Res., 2013, 52, 2520–2527 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp07747e |
| This journal is © the Owner Societies 2018 |