Methyl substitution enhanced photoisomerization of trans,trans-1,4-diphenyl-1,3-butadiene: direct ab initio trajectory surface hopping dynamic simulations†
Abstract
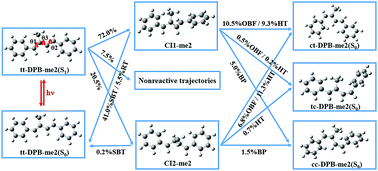
Single methyl group substitution on the p-position of the phenyl ring (tt-DPB-me1) or the conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (tt-DPB-me2) has been found to enhance the photoisomerization efficiency for two trans,trans-1,4-diphenyl-1,3-butadiene (tt-DPB) derivatives by performing direct ab initio trajectory surface hopping dynamics simulations. With implementation of the Zhu–Nakamura global switching algorithm, on-the-fly trajectory surface hopping dynamics simulations based on the ground state and first excited state potential energies and their gradients calculated by the two state averaged complete active space self-consistent field method with basis set 6-31G were propagated up to 3000 femtoseconds. Four-hundred sampling trajectories have been performed for both tt-DPB-me1 and tt-DPB-me2, and five distinctive photoisomerization pathways were observed for both of them. Among which, One Bond Flipping (OBF) and Hula-Twist (HT) are the dominant photoisomerization mechanisms. The lifetime of the S1 state is estimated to be 1423.0 fs (819.0 fs), and the photoisomerization quantum yields are 0.088 (0.378) in tt → ct, 0.070 (0.015) in tt → tc and 0.073 (0.065) in tt → cc for tt-DPB-me1 (tt-DPB-me2). By analyzing the dynamics simulation data, it can be concluded that closer methyl substitution with respect to the central C
C bond (tt-DPB-me2) has been found to enhance the photoisomerization efficiency for two trans,trans-1,4-diphenyl-1,3-butadiene (tt-DPB) derivatives by performing direct ab initio trajectory surface hopping dynamics simulations. With implementation of the Zhu–Nakamura global switching algorithm, on-the-fly trajectory surface hopping dynamics simulations based on the ground state and first excited state potential energies and their gradients calculated by the two state averaged complete active space self-consistent field method with basis set 6-31G were propagated up to 3000 femtoseconds. Four-hundred sampling trajectories have been performed for both tt-DPB-me1 and tt-DPB-me2, and five distinctive photoisomerization pathways were observed for both of them. Among which, One Bond Flipping (OBF) and Hula-Twist (HT) are the dominant photoisomerization mechanisms. The lifetime of the S1 state is estimated to be 1423.0 fs (819.0 fs), and the photoisomerization quantum yields are 0.088 (0.378) in tt → ct, 0.070 (0.015) in tt → tc and 0.073 (0.065) in tt → cc for tt-DPB-me1 (tt-DPB-me2). By analyzing the dynamics simulation data, it can be concluded that closer methyl substitution with respect to the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond results in a higher percentage of the corresponding photoisomerization products. The present simulation results are in agreement with the ultrafast spectroscopy measurements, which unveil the photoisomerization mechanisms of tt-DPB derivatives and present useful physical insights on how to tune the photoisomerization of the substituted DPB.
C double bond results in a higher percentage of the corresponding photoisomerization products. The present simulation results are in agreement with the ultrafast spectroscopy measurements, which unveil the photoisomerization mechanisms of tt-DPB derivatives and present useful physical insights on how to tune the photoisomerization of the substituted DPB.


 Please wait while we load your content...
Please wait while we load your content...