Open Access Article
Open Access ArticleA systematic examination of classical and multi-center bonding in heteroborane clusters†
Petr
Melichar
a,
Drahomír
Hnyk
 *b and
Jindřich
Fanfrlík
*b and
Jindřich
Fanfrlík
 *a
*a
aInstitute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo nam. 2, 166 10 Prague 6, Czech Republic. E-mail: fanfrlik@uochb.cas.cz
bInstitute of Inorganic Chemistry of the Czech Academy of Sciences, v.v.i., 250 68 Husinec-Řež, Czech Republic. E-mail: hnyk@iic.cas.cz
First published on 18th December 2017
Abstract
This paper presents a systematic study of multicenter and classical bonding on a broad series of experimentally known heteroboranes covering closo, nido, arachno and hypho types of cages with incorporated tetrel, pnictogen or chalcogen heterovertices up to the third-row elements. The nature of bonding is studied using a novel quantum-chemical tool, the intrinsic atomic/bond orbital (IAO/IBO) approach, which provides a direct connection between quantum chemistry and chemical concepts. We also discuss how the computed IBO properties are related to molecular observables such as interatomic distances, molecular electrostatic potential surfaces and dipole moments.
Introduction
Bonding in boron clusters (boron hydrides, boranes) is predominantly of multicenter nature, and usually referred to as 3-center-2-electron (3c2e) bonding.1–4 Two types of 3c2e bonds, B–B–B and B–H–B triangles, occur in boranes. The bridging B–H–B triangles of B2H6 led W. N. Lipscomb to formulate the concept of multicenter bonding. The Nobel Prize was awarded to him for the explanation of the differences between boranes and analogous organic compounds. The geometries of boranes and hydrocarbons are not compatible even in the case of simple B2H6 and C2H6 molecules. B2H6 with D2h symmetry differs from the ethane structure with D3d symmetry. Conceivably, B2H6 could be fitted to the electron diffraction data only when a hydrogen-bridge-based model of D2h symmetry is considered.5Multicenter bonding is not limited to boranes. It has been shown by using high-angle X-ray diffraction that the bonding in the trimethylaluminium dimer (Al2Me6) has multicenter character.6 Multicenter bonding was also proposed for heteroborane clusters. W. N. Lipscomb et al. studied the nature of bonding in closo-carboranes at the semiempirical quantum mechanical level (the partial retention of diatomic differential overlap/PRDDO/method7).8 In Lipscomb's classical view of 3c2e bonding, n atomic orbitals are combined into 3c2e bonds by forming only n/3 bonding orbitals. Extra electrons would have to occupy antibonding orbitals. A valence pattern for a given number of atoms is thus restricted to a few well-defined possibilities. Further attention has been paid to smaller cages such as closo-1,2-Y2B3H5 (Y = CH, N, P), where it has been known that both 3c2e and 2c2e alternatives are possible.9 Since the effort to study the bonding of heteroroboranes has focused mainly on smaller closo-carboranes, the knowledge of bonding in other heteroborane architectures is very limited.
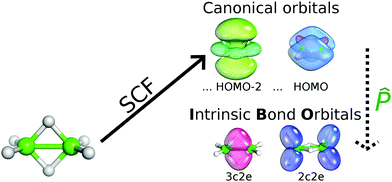
Recently, it has also been possible to analyze bonding patterns using a novel quantum-chemical tool, the intrinsic atomic/bond orbital (IAO/IBO) method.10 This method provides a direct connection between quantum chemistry and intuitive chemical concepts. It helps in determining the nature of chemical bonding from first principles calculations and computes the nature and shape of chemical bonds in terms of connecting quantitative SCF wave functions to a qualitative chemical picture (see Scheme 1).
Generally speaking, the bonding character illustrated (e.g. in the form of not assumed Lewis structures) thus naturally emerges. This procedure thus goes beyond the standard natural bond orbital (NBO) approach,11a which assumes a spherical symmetry of AOs and Lewis bonding patterns. Boldyrev has also introduced a tool known as adaptive natural density partitioning (AdNP),11b a method that analyzes the first-order reduced density matrix, which inherently contains some assumptions. However, this approach works well for various boron rings such as boron molecular Wankel motors, boron fullerenes and 2D boron sheets.11c The wave function used for IBOs is initially calculated using canonical molecular orbitals (MOs) from the SCF procedure, and their subsequent unitary transformation yields the desired IBOs, which clarifies the nature of the corresponding bonding pattern. The point is that basis set functions are not associated with any atom. In the case of expanding MOs over a minimal basis set of free-atom AOs (known from any textbook of general chemistry), the resulting wave function can easily be interpreted. However, such a wave-function would be imprecise because the free-atom AOs do not undergo any polarization from the corresponding surroundings. On that basis, an accurate wave function is calculated and a set of polarized AOs is formed in terms of splitting the free-atom AOs into contributions corresponding to a depolarized occupied space and its complement by the corresponding projection.
We have applied the novel IBO approach to a broad series of experimentally known heteroboranes covering closo, nido, arachno and hypho types of cages with incorporated tetrel, pnictogen or chalcogen heterovertices up to the third-row elements in order to study the nature of bonding in heteroborane clusters.
Classical and multicenter bonding patterns are reflected in observable molecular properties. Firstly, the different bonding arrangements of various bonding types result in various interatomic distances that are slightly longer in multicenter bonds, as exemplified further. An interatomic distance close to the sum of the covalent radii (Σrcov)1c of both atoms can thus be considered as an indication of classical bonding. However, it might be questionable whether a larger interatomic distance necessarily indicates multicenter bonding. It might also be a consequence of electron deficiency in the case of electron unsaturated boranes. In this study, the obtained IBO results are compared with the interatomic distances refined either from the experimental electron diffraction or from the combined ab initio/GIAO/NMR approach. Secondly, classical bonding follows the established electronegativity concept, which is not true for multicenter bonding.1b We have thus computed and analyzed the molecular electrostatic potential (ESP) surfaces and the dipole moments of selected representative molecules.
Methods
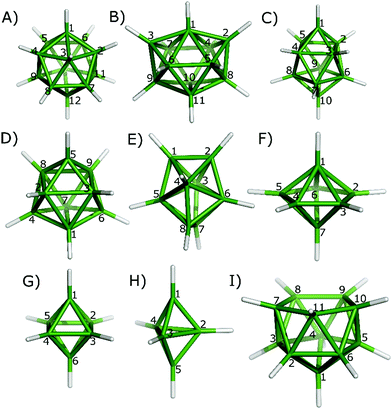
In the first step, the IBO method was validated on a series of organic and inorganic compounds with well described either covalent or multicenter bonding: ethane, elemental white phosphorus P4 and hydrogen disulfide (containing classical, 2c2e bonding), trimethylaluminium dimer,6 diborane,12closo-B12H122−,13nido-B11H114−,14arachno-B10H142−,15closo-B10H102−,13bcloso-B8H82−,16closo-B7H72−,17closo-B6H62−,18closo-B5H52−,19 and tetra-tert-butyltetrabora-tetrahedrane.20In the second step, we used the IBO method to investigate the nature of bonding in a series of experimentally known heteroboranes containing only up to third-row elements, specifically: closo-1-CB11H12−,21closo-1,2-C2B10H12,22closo-1,7-C2B10H12,22closo-1-NB11H12,23closo-1,2-P2B10H10,24closo-1,7-P2B10H10,25closo-1-SB11H11,26closo-2-CB10H11−,27closo-2,3-C2B9H11,28closo-1-CB9H10−,29closo-1,2-C2B8H10,30closo-1,6-C2B8H10,31closo-1,10-C2B8H10,32closo-1-NB9H10,33closo-2,1-PCB8H9,34closo-6,1-PCB8H9,34closo-1-SB9H9,35closo-4-CB8H9−,36closo-1,7-C2B7H9,37closo-1-CB7H8−,38closo-1,2-C2B6H8,39closo-1,7-C2B6H8,40closo-1,6-C2B6H8,39closo-2-CB6H7−,41closo-2,4-C2B5H7,42closo-1,2-C2B4H6,43closo-1,6-C2B4H6,42closo-1,5-C2B3H5,42closo-2,3-C2B3H5,42closo-1,2-C2B3H5,42nido-2,9-C2B9H12−,44nido-2,7-C2B9H12−,45nido-7,8-C2B9H12−,46nido-7,9-C2B9H12−,44nido-7,8,10-C2SB8H10,47nido-9,11,7,8-P2C2B7H9,48nido-7,8,9,10-P2C2B7H9,48nido-7,8,9,10-P3CB7H8,49nido-7,9,8,10-P2C2B7H9,48nido-7,8,9,11-P2C2B7H9,48nido-6,7-C2B7H92−,49arachno-1,8,11-NC2B8H13,50arachno-6,9-CSB8H12,51arachno-4,6,5-C2SB6H10,52hypho-2,5,12-C3B8H15−,53hypho-7,8-C2B6H13−,54hypho-7,8-NSB6H11,54hypho-7,8-S2B6H9−,54 and hypho-7,8-CSB6H11−.54 Because of the hypho vs. nido conflict present, the classification of such compounds as hypho should be treated with caution.54 The numbering of the studied cages is shown in Fig. 1.
All the calculations were performed at the DFT/B3-LYP/def2-TZVPP level of theory using the Turbomole6.655 and Gaussian0956 program packages. The outputs were examined using the IBOview program10 to visualize the IBOs (threshold of 60%) and render it. Furthermore, the electrostatic potential and the dipole moments were computed at the HF/cc-pVDZ level using the Gaussian0956 and Molekel4.357 program packages.
Results and discussion
Benchmarks
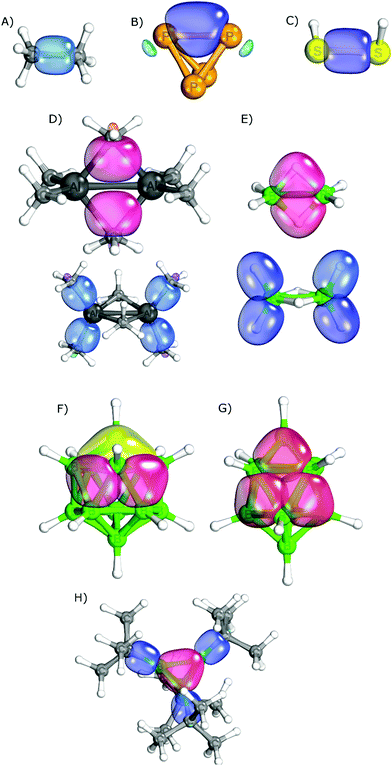
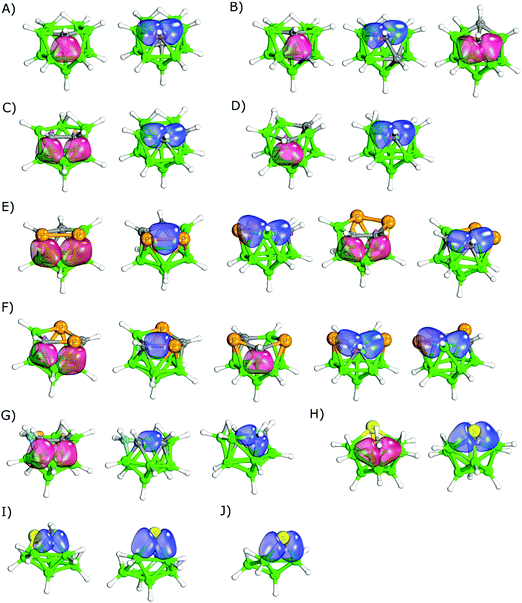
To validate the IBOs and illustrate the difference between the 2c2e and 3c2e types of bonding, we first applied the IBO approach to a series of organic/inorganic compounds with 2c2e bonding and closo-borane clusters with 3c2e bonding. In addition, we considered the bridging Al–C bonds in Al2(CH3)6 because the multicenter bonding of this molecule had been confirmed experimentally.6The IBOs for the benchmark organic/inorganic compounds and the selected boron clusters are summarized in Fig. 2. The IBOs clearly demonstrated classical bonding in ethane, elemental white tetraphosphorus P4 and disulfide hydrogen (Fig. 2A–C). The IBOs also confirmed multicenter bonding in Al2(CH3)6 (Fig. 2D) in accordance with the experimental data. In addition, the hydrogen bridging in diborane was also shown to be of multicenter nature (Fig. 2E). The interatomic distances are slightly elongated in the shown multicenter bonds. The B–H, B–B and C–Al separations are 4–14% longer than the sum of the covalent radii (Σrcov)1c of both atoms, i.e. distances of 1.334, 1.7705 and 2.1256 Å, respectively. For comparison, the classical 2c2e B–H and C–Al bonds of the same molecules are closer to Σrcov, i.e. 1.1875 and 1.9536 Å, which correspond to 101.5 and 97.2% of Σrcov, respectively. In addition, the terminal (exosceletal) H atoms involved in the classical 2c2e B–H bonds have hydridic character due to the low electronegativity of B atoms. In contrast to the terminal H atoms, the bridging H atoms of diborane have partial positive atomic charges.58
In the series of various borane clusters, the presence of multicenter bonding was confirmed (Fig. 2F and G). Besides the 3c2e bonds, this approach also revealed 4-center-2-electron (4c2e)59 bonds of the B–B–B–B type in closo-BnHn2− (n = 12, 10 and 8), nido-B11H114− and arachno-B10H142−. This unique 4c2e bonding has already been known for some borane compounds.60,61 However, this bonding has only been reported as 4c2e B–B–B–H with no evidence for 4c2e B–B–B–B bonding. The presence of the 4c2e bond in closo-B12H122− is shown in Fig. 2F. The number of 4c2e bonds increased with the size of the cluster (e.g. closo-B10H102− and closo-B12H122− had one and three 4c2e bonds, respectively).
In the case of tetra-tert-butyl-tetrabora-tetrahedrane, B4(t-Bu)4 (see Fig. 2H), the IBO approach clearly indicated the presence of four multicenter 3c2e bonds (B–B–B triangles). The other bonds, including the B–C, C–C and C–H bonds, were confirmed by the IBOs to be of classical 2c2e nature. The observed presence of 3c2e bonding corresponded well with the published study on B4H4 and B4(CH3)4.62
Bonding in heteroboranes
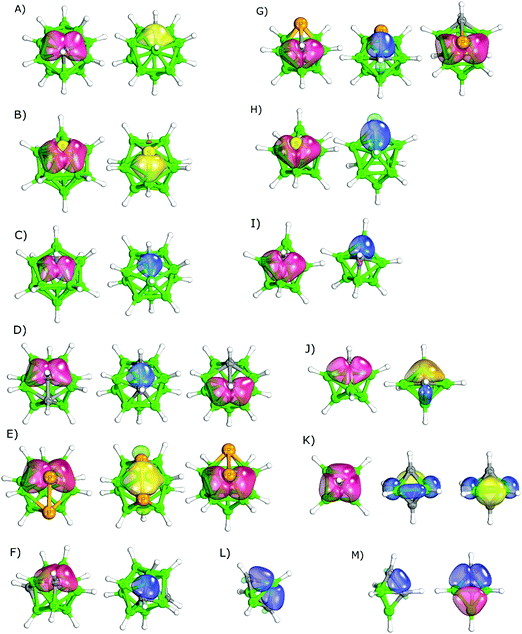
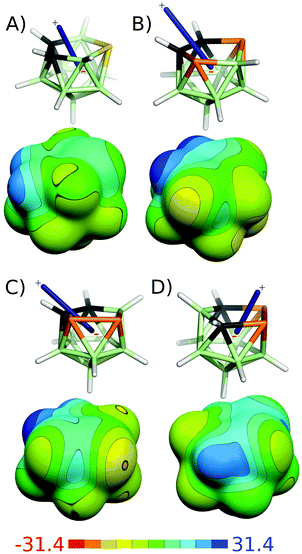
It is important to consider the differences between various boron architectures when analyzing the results for multidimensional boron compounds – e.g. closo-1-SB11H11 differs in many respects from closo-1-SB9H9.63 The IBO results of icosahedral heteroboranes are summarized in Table 1. Furthermore, the IBOs of selected closo-heteroborane clusters are shown in Fig. 3. When the icosahedral cluster only contained a single heteroatom, the heteroatom was mostly part of multicenter bonding. For instance, the C atom of closo-1-CB11H12− participated in three bonds, one of 4c2e nature (B–B–B–C) and two of 3c2e nature (B–B–C, see Fig. 3A). This bonding was thus analogous to the parent closo-B12H122−, which also contained one 4c2e and two 3c2e bonds in the same location. Similarly, the S atom of closo-1-SB11H11 (see Fig. 3B) took part in two multicenter (3c2e) bonds of B–S–B type and one bond of B–B–S–B type of 4c2e nature. Such a bonding pattern corresponded to a B–S distance of 2.010 Å (106.9% of Σrcov).26a More interestingly, it could also help in explaining the highly positive σ-holes (VS,max of 28.2 kcal mol−1) on the S atom, which are known to be important for its crystal packing.64 This VS,max value is unusually high considering the low electronegativity of boron atoms. closo-1-NB11H12 contained two 3c2e B–N–B bonds and one 2c2e B–N bond. However, it should be mentioned that the 2c2e bonding was debatable in the case of closo-1-NB11H12 (see Fig. 3C). The bond evaluated as a classical covalent bond was less localized than a typical 2c2e bond (Fig. 2) and could also be seen as a crossing between the 2c2e and 4c2e bonds. Such a bonding pattern would also better correspond to a B–N distance of 1.716 Å (110.0% of Σrcov).23| Compound | Bonding | |
|---|---|---|
| Multicenter | Classical | |
| 12-vertex | ||
| closo-1-CB11H12− | 2 × B–C–B; 1 × B–C–B–B | — |
| closo-1-SB11H11 | 1× B–S–B; 1 × B–S–B–B | — |
| closo-1-NB11H12 | 2 × B–N–B | 1 × B–N |
| closo-1,7-C2B10H12 | 6 × B–C–B | — |
| closo-1,7-P2B10H10 | 3 × B–P–B; 3 × B–B–P–B | — |
| closo-1,2-C2B10H12 | 4 × B–C–B | 1 × C–C |
| closo-1,2-P2B10H10 | 4 × B–P–B; 1 × B–P–P–B | — |
closo-1,7-C2B10H12 and closo-1,7-P2B10H10 have two heteroatoms that are not adjacent. They can thus be considered as a crossing between heteroboranes with one and more heterovertices. In contrast to closo-1-CB11H12−, the C atoms of closo-1,7-C2B10H12 did not form 4c2e B–C–B–B bonds. Instead, they only formed B–C–B 3c2e multicenter bonds. closo-1,7-P2B10H10, however, formed both 3c2e and 4c2e bonds. closo-1,2-C2B10H12 has two adjacent C atoms, which form 3c2e B–C–B bonds and a classical C–C bond (see Fig. 3D). This pair of adjacent C–C atoms is known to act as an electron donor and becomes the center of the partial positive charge in the molecule. This has already been determined experimentally.65 It might thus be considered important experimental evidence supporting the 3c2e B–C–B bonding. The C–C bond of closo-1,2-C2B10H12 was the only classical bond found by the IBO approach among the icosahedral heteroboranes besides the debatable case of closo-1-NB11H12 described above. Similar to the B–N bond of closo-1-NB11H12, the C–C bond of closo-1,2-C2B10H12 was less localized and longer (1.624 Å) than the C–C classical bonds in the validation data set, e.g. in ethane (1.540 Å).22 It also corresponds to LMO results obtained by Lipscomb et al.66 The studied icosahedral cluster with adjacent P atoms, closo-1,2-P2B10H10 (see Fig. 3E), did not form a classical bond between the P atoms; instead, it formed a multicenter B–P–P–B 4c2e bond. This bonding could be compared to B–B–B–B obtained in the parent closo-B12H122−. The P–P distance in closo-1,2-P2B10H10 is 2.310 Å (104.1% of Σrcov).24 The most positive molecular ESP surface is located between the two P atoms (VS,max = 28.2 kcal mol−1),67 which is analogous to closo-1,2-C2B10H12.
The bonding patterns of the 11-vertex closo-carboranes were similar to those of the 12-vertex cages (compare Table 1 and Table S1, ESI†). The IBO results did not reveal any difference in bonding between closo-2-CB10H11− and the above-discussed closo-1-CB11H12−. However, some difference was found between closo-2,3-C2B9H11 and closo-1,7-C2B10H12 (separated C atoms in both clusters, numbering shown in Fig. 1). The C atoms of closo-2,3-C2B9H11 (see Fig. 3F) participated in a classical B–C bond besides the two B–C–B 3c2e bonds. On the other hand, closo-1,7-C2B10H12 only had 3c2e B–C–B bonds.
The bonding patterns obtained in the 10-vertex closo-heteroboranes (see Table 2) were also overall similar to those in the icosahedral cages. closo-1-CB9H10− and closo-1,2-C2B8H10 had analogous bonding patterns to closo-1-CB11H12− and closo-1,2-C2B10H12, respectively. It should be mentioned, however, that the C–C bond of closo-1,2-C2B8H10 is shorter than that bond in closo-1,2-C2B10H12, (1.538 vs. 1.624 Å).30 In addition, the bonding in closo-2,1-PCB8H9 (see Fig. 3G) was similar to closo-1,2-C2B10H12. Besides closo-1,2-C2B8H10, two other isomers of closo-C2B10H12 were studied, both with non-adjacent C atoms. Both clusters only formed multicenter bonds of either 3c2e or 4c2e nature. The fact that these molecules formed a 4c2e bond corresponded to the parent closo-B10H102−, in which a 4c2e bond occurred as well. The analysis of closo-1-NB9H10 revealed very similar results to the above-discussed closo-1-NB11H12. Both of these molecules had a single 2c2e B–N bond. This might be in accordance with closo-1-SB9H9, where two multicenter B–S–B bonds and one classical B–S bond were found. When closo-1-SB9H9 (see Fig. 3H) was compared to closo-1-SB11H11, one classical B–S bond was formed instead of a B–S–B–B 4c2e bond. This finding might help to rationalize the less positive σ-holes (22.4 vs. 28.2 kcal mol−1)67 and the shorter S–B distance (1.93935avs. 2.01026a Å) in closo-1-SB9H9.
| Compound | Bonding | |
|---|---|---|
| Multicenter | Classical | |
| 10-vertex | ||
| closo-1-CB9H10− | 2 × B–C–B; 1 × B–C–B–B | — |
| closo-1,2-C2B8H10 | 4 × B–C–B | 1 × C–C |
| closo-1,6-C2B8H10 | 5 × B–C–B; 1 × B–C–B–B | — |
| closo-1,10-C2B8H10 | 5 × B–C–B; 1 × B–C–B–B | — |
| closo-1-NB9H10 | 2 × B–N–B | 1 × B–N |
| closo-2,1-PCB8H9 | 2 × B–P–B; 2 × B–C–B | 1 × C–P |
| closo-6,1-PCB8H9 | 3 × B–C–B; 2 × B–P–B; 1 × B–P–B–B | — |
| closo-1-SB9H9 | 2 × B–S–B | 1 × B–S |
| 8-vertex | ||
| closo-1-CB7H8− | 2 × B–C–B | 1 × B–C |
| closo-1,2-C2B6H8 | 4 × B–C–B | 1 × C–C |
| closo-1,7-C2B6H8 | 4 × B–C–B; 2 × B–C–B–B | — |
| closo-1,6-C2B6H8 | 4 × B–C–B; 2 × B–C–B–B | — |
Two closo-carborane cages with 9 vertices were considered (Table S1, ESI†): closo-4-CB8H9− and closo-1,7-C2B7H9. Only B–C–B 3c2e bonds were found in these molecules. The absence of 4c2e bonding in closo-4-CB8H9− could be caused by differences in the architecture of the cage. Otherwise, the data were in agreement with the results obtained for the larger closo-cages, discussed above.
The bonding patterns observed across the 8- and 7-vertex closo-carbaboranes were overall similar to the bigger ones (see Tables 1 and 2). However, one significant difference was found. While the IBOs did not show any C–B classical bonding in the bigger closo-CBn−1Hn− (n = 9–12) cages, the C atom of closo-CBn−1Hn− (n = 7–8) formed a classical 2c2e B–C bond as well as multicenter bonds.
The 6- and 5-vertex closo-dicarbaboranes showed different trends from the bigger ones. Classical bonding was still more frequent in the smallest cages. In addition to the C–C and B–C bonds, the classical B–B bond was also found in closo-1,6-C2B4H6 and closo-2,3-C2B3H5 (see Table 3 and Fig. 3). Furthermore, the IBOs even disproved the presence of any multicenter bonding in closo-1,5-C2B3H5. All bonds appeared to be B–C classical bonds. Such a bonding pattern would correspond to the localized molecular orbital (LMO) structures,8 which were already used to question the presence of the multicenter nature of the C–B–C bonding in closo-1,5-C2B3H5.68 Note that the small cluster dimensions are problematic cases in terms of 11B NMR computations.20b In addition, the interatomic distances and charge distribution should reflect the classical bonding pattern. The short C–B separation (1.553 Å,20b 97.1% of Σrcov) supports the classical bonding pattern. It should also be mentioned that the B–B separation in these molecules is 1.844 Å20b (108.5% of Σrcov) and the IBO did not show any bonding between these boron atoms. In contrast to the other closo carboranes, the most positive ESP of closo-1,5-C2B3H5 is not on the top of the CH vertex; instead, it is on the B atom (see Fig. 4). This finding shows that the classical electronegativity concept is valid in this molecule, which supports classical bonding in this molecule. The IBO results of closo-1,2-C2B4H6 did not differ from the IBO results of the bigger closo-1,2-C2Bn−2Hn (n = 12, 10, 8), in contrast to the other smaller compounds. The C atom participated in four B–C–B bonds and one C–C bond in all of these closo-1,2-C2Bn−2Hn (n = 12, 10, 8, 6) molecules. The IBO results of closo-1,2-C2B4H6 nicely resembled the LMO structures on the PRDDO level obtained using the Boys criteria.8
| Compound | Bonding | |
|---|---|---|
| Multicenter | Classical | |
| 6-vertex | ||
| closo-1,2-C2B4H6 | 4 × B–C–B | 1 × C–C |
| closo-1,6-C2B4H6 | 6 × B–C–B; 1 × C–B–B–C | 3 × B–B |
| 5-vertex | ||
| closo-1,5-C2B3H5 | — | 6 × B–C |
| closo-2,3-C2B3H5 | 2 × B–C–B; 1 × B–C–C–B | 2 × B–C; 2 × B–B |
| closo-1,2-C2B3H5 | 2 × B–C–B | 1 × C–C; 2 × B–C |
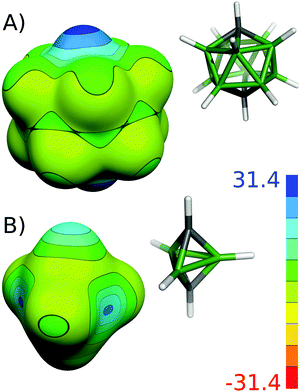 | ||
| Fig. 4 The molecular diagrams and electrostatic potential on the 0.001 a.u. molecular surface for closo-1,12-C2B10H11 (A) and closo-1,5-C2B3H5 (B). The color range of the ESP is in kcal mol−1. | ||
The bonding results for nido- are summarized in Table 4. The calculated IBOs for the selected heteroborane clusters are included in Fig. 5 to illustrate their bonding. The results show that the nature of bonding is heavily dependent on the position of the heteroatom in the nido-cages. Covalent bonds were found exclusively in the open face. For example, nido-2,9-C2B9H12 and nido-2,7-C2B9H12, which had one C atom located in the open face and one in the closed face, only formed the classical B–C bonds on the open belt, whereas the carbon in the closed face only participated in bonding of 3c2e nature. Moreover, although nido-2,7-C2B9H12 had adjacent carbon atoms, the classical C–C bonding did not occur. Instead, C–C–B bonding of 3c2e nature was formed. On the other hand, when a nido-cage contained at least one heteroatom in the open pentagonal belt, evidence for classical covalent bonding was found. nido-Carboranes with two C atoms in the face formed B–C–B, B–C and C–C bonds. nido-7,8-C2B9H12 contained adjacent C atoms and thus formed one C–C and two B–C bonds of classical 2c2e nature. nido-7,9-C2B9H12 with separated C atoms formed four B–C bonds of 2c2e nature, all in the open face. The 3c2e bonds were formed in B–C–B triangles, when both B atoms were located under the C atom.
| Compound | Bonding | |
|---|---|---|
| Multicenter | Classical | |
| nido- | ||
| nido-2,9-C2B9H12− | 4 × B–C–B | 2 × B–C |
| nido-2,7-C2B9H12− | 1 × B–C–C; 2 × B–C–B | 2 × B–C |
| nido-7,8-C2B9H12− | 2 × B–C–B | 1 × C–C; 2 × B–C |
| nido-7,9-C2B9H12− | 2 × B–C–B | 4 × B–C |
| nido-7,8,10-C2SB8H10 | 1 × B–S–B | 2 × B–S; 2 × B–C; 1 × C–C |
| nido-9,11,7,8-P2C2B7H9 | 2 × B–C–B; 2 × B–P–B | 2 × B–P; 2 × C–P; 1 × C–C |
| nido-8,9,7,11-P2C2B7H9 | 2 × B–P–B; 2 × B–C–B | 1 × P–P; 1 × C–C; 1 × C–P; 1 × B–C; 1 × P–B |
| nido-7,8,9,10-P3CB7H8 | 3 × B–P–B; 1 × B–C–B | 2 × P–P; 1 × P–C; 1 × B–C; 1 × B–P |
| nido-7,9,8,10-P2C2B7H9 | 2 × B–P–B; 2 × B–C–B | 3 × P–C; 1 × B–P; 1 × B–C |
| nido-7,8,9,11-P2C2B7H9 | 2 × B–P–B; 2 × B–C–B | 1 × P–P; 2 × P–C; 2 × B–C |
| nido-6,7-C2B7H92− | 2 × B–C–B | 1 × C–C; 2 × B–C |
| arachno- | ||
| arachno-1,8,11-NC2B8H13 | 3 × B–C–B | 1 × C–C; 2 × N–B |
| arachno-6,9-CSB8H12 | 2 × B–C–B | 2 × B–S |
| arachno-4,6,5-C2SB6H10 | 4 × B–C–B | 2 × B–S |
| hypho- | ||
| hypho-C3B8H15− | 4 × B–C–B | 2 × B–C |
| hypho-7,8-C2B6H13− | — | 4 × B–C |
| hypho-7,8-NSB6H11 | — | 2 × B–S; 2 × N–B |
| hypho-7,8-S2B6H9− | — | 4 × S–B |
| hypho-7,8-SCB6H11− | — | 2 × B–S; 2 × B–C |
The bonding patterns of the nido-cages with other incorporated heteroatoms were very similar to the already-described nido-carboranes. Any heteroatom incorporated into the open face was a source of classical bonding. This resulted in a variety of bonding. For example, the open belts of nido-7,8,10-C2SB8H10, nido-7,8,9,10-P3CB7H8, nido-7,8,9,10-P2C2B7H9 and nido-9,11,7,8-P2C2B7H9 were formed exclusively via the classical covalent bonds. There was evidence for S–B, P–P, C–C, C–P, B–C and P–B classical bonds. The length of these bonds ranged from 98.3 to 102.0% of Σrcov, thus supporting the classical bonding pattern.47–49 Besides the classical bonds in the open face, the nido-heteroboranes also contained multicenter bonds of 3c2e nature with the participating heteroatom in agreement with the above-described nido-carboranes. In nido-8,9,7,11-P2C2B7H9, the 3c2e B–P–B bonds were formed in the triangles 3–4–8 and 4–5–9 (the numbering of this cage is shown in Fig. 1I). Similarly, B–C–B 3c2e bonds were formed in the 2–6–11 and 2–3–7 triangles. The interatomic distances ranged from 103.9 to 108.7% of Σrcov in these triangles.48nido-9,11,7,8-P2C2B7H9 had an analogous bonding pattern even though the P atoms were not adjacent.
When the two heteroatoms were adjacent to each other, two 3c2e bonds of B–Z–B (Z = various heteroatoms) were formed. Here, one boron atom was shared by both of the triangles. This one lay on the under-belt under the middle of the Z–Z covalent bonding. When the heteroatoms were not located adjacent to each other, the 3c-2e triangles were still formed. The heteroatoms and boron atoms lying under them are involved in this kind of bonding. The computed dipole moments and molecular ESP surfaces of the representative nido clusters show that the heterovertices located in the open pentagonal face act as an electron donor and become the center of a partial positive charge of the molecule (Fig. 6), which supports the existence of the B–Z–B 3c2e bonds. Moreover, the crystal packing of nido-7,8,9,11-Sb2C2B7H9 is predominantly dictated by the very strong Sb2⋯H–B σ-hole interaction.48b
We also applied the IAO method to nido-6,7-C2B7H9 as a representative of the 9-vertex nido-heteroboranes. Two B–C–B bonds of 3c2e nature and B–C and C–C 2c2e bonds suggested that bonding in the 9-vertex is not different from the 11-vertex nido-clusters.
The IBO results of the arachno-clusters are summarized in Table 4 and some of them have also been selected for Fig. 5, where the IBO results are illustrated. The results were similar to those of the nido-clusters. The C atoms were bonded via 3c2e B–C–B bonds in the arachno-clusters. In the case of arachno-1,8,11-NC2B8H13 with adjacent carbon atoms, the classical C–C bond was formed. At the same time, two B–C–B multicenter bonds were formed as well (see Fig. 5G). The other heteroatoms only formed classical bonds, e.g. the S atom of arachno-4,6,5-C2SB6H10 or the N atom of arachno-1,8,11-NC2B8H13.
The calculated IBOs for hypho-clusters are summarized in Table 4. Their IBOs are shown in Fig. 5. Bonding of the heteroatoms was dominated by classical bonding in the studied hypho-clusters. With the exception of hypho-C3B8H15, the IBOs did not reveal any bonds of multicenter nature with the exception of the B–B–B and B–B–H bonds. Each of the heteroatoms formed two covalent bonds with a boron atom of 2c2e nature.
In contrast to closo-1-SB11H11, the S atom of hypho-7,8-NSB6H11 is incorporated into the hypho-cluster network exclusively via classical B–S bonds and consequently acts as an electron acceptor. This S atom thus has a negative electrostatic potential (ESP) surface without areas of positive ESP (see Fig. 7).
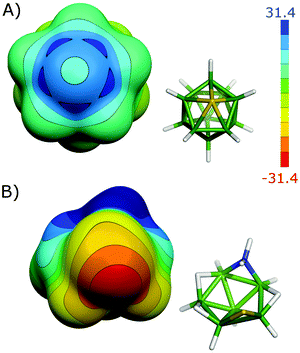 | ||
| Fig. 7 The molecular diagrams and electrostatic potential on the 0.001 a.u. molecular surface of closo-1-SB11H11 (A) and hypho-7,8-NSB6H11 (B). The color range of the ESP is in kcal mol−1. | ||
Table S2 (ESI†) shows the results for a selected series of representative compounds obtained by the most widely used orbital localization scheme, the NBO methodology. The NBO and IBO results are overall similar with many small differences. The majority of the differences are due to the different localization techniques used. While IBO only minimizes the spread of bond orbitals over the atoms (i.e. maximizes their localization), the NBOs explicitly search for the predefined Lewis structures most closely matching the given wave function. Consequently, 4c2e bonds naturally emerged in the IBO analysis of many heteroborane clusters. On the other hand, the NBO analysis was limited to 2c2e and 3c2e bonds. Other nonsystematic differences could be found between the IBO and NBO methods. However, an exhaustive comparison of the IBO with other methods is beyond the scope of this study.
Conclusions
The nature of bonding has been systematically studied on a broad series of heteroboranes using a novel quantum chemical tool, the intrinsic bond orbital (IBO) approach. The results have shown that the bonding of heteroatoms in icosahedral clusters is mainly of multicenter nature. The role of classical bonding increases with the decreasing size of the closo-heteroborane cages. An extreme case is closo-1,5-C2B3H5, where the IBOs have disproved the presence of any multicenter bonding. The nature of bonding in the nido- and arachno-cages heavily depends on the position of the heteroatom, and covalent bonds have been found exclusively in the open face in nido- and arachno-cages. However, classical bonds are more common in clusters with open faces than in closo-heteroboranes.It has also been shown in the studied architectures that the nature of bonding is reflected in the molecular observables such as the molecular electrostatic potential surface. Roughly speaking, where electron distribution is opposed to the classical electronegativity complex, such an electron distribution might be attributed to multicenter bonding. On the other hand, the classical bonding indicates the classical electronegativity concept. Vector analyses of the experimental and computed dipole moments support such an observation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the research project RVO 61388963 of the Czech Academy of Sciences. We acknowledge the financial support from the Czech Science Foundation (17-08045S).Notes and references
- (a) W. N. Lipscomb, Boron Hydrides, Benjamin, New York, 1963 Search PubMed; (b) more recent examples are outlined in D. Hnyk and D. A. Wann, Boron – the Fifth Element, in Challenges and Advances in Computational Chemistry and Physics, ed. D. Hnyk and M. McKee, Springer, Heidelberg, New York, Dordrecht and London, 2015, ch. 2, vol. 20 Search PubMed; (c) the nature of a chemical bond is reflected in bond lengths, see P. Pykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 186–197 CrossRef PubMed.
- R. C. Bochicchio, R. Ponec and F. Uhlík, Inorg. Chem., 1997, 36, 5363–5368 CrossRef CAS.
- R. M. Lobayan, R. C. Bochicchio, A. Torre and L. Lain, J. Chem. Theory Comput., 2009, 5, 2030–2043 CrossRef CAS PubMed.
- K. A. Lyssenko, M. Y. Antipin and V. N. Lebedev, Inorg. Chem., 1998, 37, 5834–5843 CrossRef CAS.
- K. Hedberg and V. Schomaker, J. Am. Chem. Soc., 1951, 73, 1482–1487 CrossRef CAS.
- H. Stammler, S. Blomeyer, R. J. F. Berger and N. W. Mitzel, Angew. Chem., Int. Ed., 2015, 54, 13816–13820 CrossRef CAS PubMed.
- Although PRDDO keeps a partially differential overlap and does not neglect it completely unlike CNDO, this approach is still empirically-based.
- D. A. Dixon, D. A. Kleier, T. A. Halgren, J. H. Hall and W. N. Lipscomb, J. Am. Chem. Soc., 1977, 99, 6226–6237 CrossRef CAS.
- (a) P. Schleyer, G. Subramanian and A. Dransfeld, J. Am. Chem. Soc., 1996, 118, 9988–9989 CrossRef CAS; (b) G. D. Graham, D. S. Marynick and W. N. Lipscomb, J. Am. Chem. Soc., 1980, 102, 2939–2945 CrossRef CAS; (c) E. D. Jemmis, J. Am. Chem. Soc., 1982, 104, 7017–7020 CrossRef CAS; (d) R. F. W. Bader and D. A. Legare, Can. J. Chem., 1992, 70, 657–676 CrossRef CAS; (e) E. D. Jemmis, G. Subramanian, I. H. Srivastava and S. R. Gadre, J. Phys. Chem., 1994, 98, 6445–6451 CrossRef CAS; (f) K. Takano, M. Izuho and H. Hosoya, J. Phys. Chem., 1992, 96, 6962–6969 CrossRef CAS; (g) E. D. Jemmis and G. Subramanian, J. Phys. Chem., 1994, 98, 9222–9226 CrossRef CAS; (h) Y. Sahin, C. Präsang, M. Hofmann, G. Geiseler, W. Massa and A. Berndt, Angew. Chem., 2005, 117, 1670–1673 CrossRef; (i) D. R. Armstrong, M. A. Fox and K. Wade, J. Organomet. Chem., 2012, 721, 130–136 CrossRef; (j) V. Dyczmons, M. Horn, P. Botschwina and A. Meller, THEOCHEM, 1998, 1, 137–149 CrossRef; (k) R. M. Lobayan, R. C. Bochicchio, A. Torre and L. Lain, J. Chem. Theory Comput., 2011, 7, 979–987 CrossRef CAS PubMed; (l) A. Torre, L. Lain, R. Bochicchio and R. Ponec, J. Comput. Chem., 1999, 20, 1085–1090 CrossRef CAS.
- (a) G. Knizia, J. Chem. Theory Comput., 2013, 9, 4834–4843 CrossRef CAS PubMed; (b) G. Knizia and J. E. M. N. Klein, Angew. Chem., Int. Ed., 2015, 54, 5518–5522 CrossRef CAS PubMed.
- (a) E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735 CrossRef; (b) D. Yu Zubarev and A. Boldyrev, Phys. Chem. Chem. Phys., 2008, 10, 5207–5217 RSC . This work references other “empirically-based” bonding tools; (c) I. A. Popov and A. I. Boldyrev, Boron – the Fifth Element, in Challenges and Advances in Computational Chemistry and Physics, ed. D. Hnyk and M. McKee, Springer, Heidelberg, New York, Dordrecht and London, 2015, ch. 1, vol. 20 Search PubMed.
- S. H. Bauer, J. Am. Chem. Soc., 1937, 59, 1096–1103 CrossRef CAS.
- (a) I. B. Sivaeva, V. I. Bregadze and S. Sjöberg, Collect. Czech. Chem. Commun., 2002, 67, 679–727 CrossRef and the references therein; (b) W. H. Knoth, H. C. Miller, J. C. Sauer, J. H. Balthis, Y. T. Chia and E. L. Muetterties, Inorg. Chem., 1964, 3, 159–167 CrossRef CAS.
- For the structure of B11H14− with three hydrogen bridges, see: J. Fritchie, Inorg. Chem., 1967, 6, 1199–1203 CrossRef.
- M. Hofmann and P. Schleyer, Inorg. Chem., 1998, 37, 5557–5565 CrossRef CAS PubMed.
- J. W. Bausch, G. K. S. Prakash and R. E. Williams, Inorg. Chem., 1992, 31, 3763–3768 CrossRef CAS ; see also the references therein.
- F. Schlüter and E. Bernhardt, Inorg. Chem., 2011, 50, 2580–2589 CrossRef PubMed.
- T. Schaper and Wilhelm Preetz, Inorg. Chem., 1998, 37, 363–365 CrossRef CAS.
- D. J. Wales, Encyclopedia of Inorganic Chemistry, Electronic Structure of Clusters, Wiley, Berlin, 2006 Search PubMed.
- (a) D. Hnyk, Polyhedron, 1997, 16, 603–606 CrossRef CAS; (b) M. Bühl, J. Gauss, M. Hofmann and P. Schleyer, J. Am. Chem. Soc., 1993, 115, 12385–12390 CrossRef.
- J. Pecyna, B. Ringstrand, S. Domagała, P. Kaszyński and K. Woźniak, Inorg. Chem., 2014, 53, 12617–12626 CrossRef CAS PubMed.
- A. R. Turner, H. E. Robertson, K. B. Borisenko, D. W. H. Rankin and M. A. Fox, Dalton Trans., 2005, 1310–1318 RSC.
- D. Hnyk, M. Bühl, P. Schleyer, H. V. Volden, S. Gundersen, J. Müller and P. Paetzold, Inorg. Chem., 1993, 32, 2442–2445 CrossRef CAS.
- R. McLellan, N. M. Boag, K. Dodds, D. Ellis, S. A. Macgregor, D. McKay, S. L. Masters, R. Noble-Eddy, N. P. Platt, D. W. H. Rankin, H. E. Robertson, G. M. Rosaira and A. J. Welch, Dalton Trans., 2011, 40, 7181–7192 RSC.
- J. L. Little, J. G. Kester, J. C. Huffman and L. J. Todd, Inorg. Chem., 1989, 28, 1087–1091 CrossRef CAS.
- (a) D. Hnyk, E. Vajda, M. Bühl and P. R. Schleyer, Inorg. Chem., 1992, 31, 2464 CrossRef CAS; (b) H. Møllendal, S. Samdal, J. Holub and D. Hnyk, Inorg. Chem., 2003, 42, 3043–3046 CrossRef PubMed.
- Of the five possible isomers of closo-CB10H11−, only closo-2-CB10H11− exists: W. H. Knoth, J. Am. Chem. Soc., 1967, 89, 1274–1275 CrossRef CAS . This was also analyzed in P. R. Schleyer and K. Najafian, Inorg. Chem., 1998, 37, 3454–3470 CrossRef PubMed.
- I. D. Mackie, H. E. Robertson, D. W. H. Rankin, M. A. Fox and J. M. Malget, Inorg. Chem., 2004, 43, 5387–5392 CrossRef CAS PubMed.
- B. Ringstranda, D. Batemana, R. K. Shoemaker and Z. Janoušek, Collect. Czech. Chem. Commun., 2009, 74, 419–431 CrossRef.
- D. Hnyk, D. W. H. Rankin, H. E. Robertson, M. Hofmann, P. v. R. Schleyer and M. Bühl, Inorg. Chem., 1994, 33, 4781–4786 CrossRef CAS.
- P. M. Garrett, G. S. Ditta and M. F. Hawthorne, Inorg. Chem., 1970, 9, 947–1948 CrossRef.
- J. Holub, T. Jelínek and Z. Janoušek, Collect. Czech. Chem. Commun., 2002, 67, 949–952 CrossRef CAS.
- L. Schneider, U. Englert and P. Paetzold, Z. Anorg. Allg. Chem., 1994, 620, 1191–1193 CrossRef CAS.
- J. Holub, M. Bakardjiev, B. Štíbr, D. Hnyk, O. L. Tok and B. Wrackmeyer, Inorg. Chem., 2002, 41, 2817–2819 CrossRef CAS PubMed.
- (a) D. Hnyk, D. A. Wann, J. Holub, S. Samdal and D. W. H. Rankin, Dalton Trans., 2011, 40, 5734–5737 RSC; (b) H. Møllendal, S. Samdal, J. Holub and D. Hnyk, Inorg. Chem., 2002, 41, 4574–4578 CrossRef; (c) J. MacCurtain, P. Brint and T. R. Spalding, J. Chem. Soc., Dalton Trans., 1985, 2591–2594 RSC.
- T. Jelínek, B. Štíbr, J. Holub, M. Bakardjiev, D. Hnyk, D. L. Ormsby, C. A. Kilner, M. Thornton-Pett, H. J. Schanz, B. Wrackmeyer and J. D. Kennedy, Chem. Commun., 2001, 1756–1757 RSC.
- B. M. Gimarc, B. Dai and J. J. Ott, J. Comput. Chem., 1989, 10, 14–16 CrossRef CAS.
- T. Jelínek, B. Štíbr, J. Plešek, J. D. Kennedy and M. Thornton-Pett, J. Chem. Soc., Dalton Trans., 1995, 431–437 RSC.
- B. M. Gimarc and J. J. Ott, J. Am. Chem. Soc., 1987, 109, 1388–1392 CrossRef CAS.
- G. B. Dunks and M. F. Hawthorne, Inorg. Chem., 1968, 7, 1038–1039 CrossRef CAS.
- B. Štíbr, O. L. Tok, W. Milius, M. Bakardjiev, J. Holub, D. Hnyk and B. Wrackmeyer, Chem. – Eur. J., 2008, 14, 6529–6533 CrossRef PubMed.
- E. D. Jemmis, E. G. Jayasree and P. Parameswaran, Chem. Soc. Rev., 2006, 35, 157–168 RSC and the references therein.
- R. M. Minyaev, V. I. Minkin, T. N. Gribanova and A. G. Starikov, Russ. Chem. Bull., Int. Ed., 2004, 53, 1159–1167 CrossRef CAS.
- M. A. Fox, A. E. Goeta, A. K. Hughes and A. L. Johnson, J. Chem. Soc., Dalton Trans., 2002, 2132–2141 RSC.
- F. A. Kiani and M. Hofmann, Inorg. Chem., 2004, 43, 8561–8571 CrossRef CAS PubMed.
- M. A. Fox, A. E. Goeta, J. A. K. Howard, A. K. Hughes, A. L. Johnson, D. A. Keen, K. Wade and Ch. C. Wilson, Inorg. Chem., 2001, 40, 173–175 CrossRef CAS PubMed.
- D. Hnyk, M. Hofmann, P. Schleyer, M. Bühl and D. Rankin, J. Phys. Chem., 1996, 100, 3435–3440 CrossRef CAS.
- (a) J. Holub, T. Jelínek, D. Hnyk, Z. Plzák, I. Císařová, M. Bakardjiev and B. Štíbr, Chem. – Eur. J., 2001, 7, 1546–1554 CrossRef CAS PubMed; (b) J. Holub, P. Melichar, Z. Růžičková, J. Vrána, D. A. Wann, J. Fanfrlík, D. Hnyk and A. Růžička, Dalton Trans., 2017, 46, 13714–13719 RSC.
- M. Bakardjiev, J. Holub, B. Štíbr, D. Hnyk and B. Wrackmeyer, Inorg. Chem., 2005, 44, 5826–5832 CrossRef CAS PubMed.
- J. Plešek, B. Štíbr, D. Hnyk, T. Jelínek, S. Heřmánek, J. D. Kennedy, M. Hofmann and P. R. Schleyer, Inorg. Chem., 1998, 37, 3902–3909 CrossRef.
- D. Hnyk, J. Holub, S. A. Hayes, M. F. Robinson, D. A. Wann, H. E. Robertson and D. W. H. Rankin, Inorg. Chem., 2006, 45, 8442–8446 CrossRef CAS PubMed.
- D. Hnyk, M. Hofmann and P. R. Schleyer, Collect. Czech. Chem. Commun., 1999, 64, 993–1000 CrossRef CAS.
- M. G. S. Londesborough, Z. Janoušek, B. Štíbr, D. Hnyk, J. Plešek and I. Císařová, Dalton Trans., 2007, 1221–1228 RSC.
- J. P. F. Nunes, J. Holub, D. W. H. Rankin, D. A. Wann and D. Hnyk, Dalton Trans., 2015, 44, 11819–11826 RSC.
- TURBOMOLE Version 6.6, 2014, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- (a) P. Flükiger, H. P. Lüthi, S. Portmann and J. Weber, MOLEKEL 4.3, Swiss Center for Scientific Computing, Manno (Switzerland), 2000 Search PubMed; (b) S. Portmann and H. P. Lüthi. MOLEKEL: CHIMIA, 2007, vol. 28, 555 Search PubMed.
- J. Fanfrlík, A. Pecina, J. Řezáč, R. Sedlak, D. Hnyk, M. Lepšík and P. Hobza, Phys. Chem. Chem. Phys., 2017, 19, 18194–18200 RSC.
- J. S. Miller and J. J. Novoa, Acc. Chem. Res., 2007, 40, 189–196 CrossRef CAS PubMed.
- H. Jacobsen, Dalton Trans., 2009, 4252–4258 RSC.
- M. L. McKee, M. Bühl, O. P. Charkin and P. R. Schleyer, Inorg. Chem., 1993, 32, 4549–4554 CrossRef CAS.
- J. E. D. Bene, I. Alkorta and J. Elguero, J. Phys. Chem. A, 2016, 120, 5745–5751 CrossRef PubMed.
- J. Fanfrlik and D. Hnyk, CrystEngComm, 2016, 18, 8982–8987 RSC.
- J. Fanfrlík, A. Přáda, Z. Padělková, A. Pecina, J. Macháček, M. Lepšík, J. Holub, A. Růžička, D. Hnyk and P. Hobza, Angew. Chem., Int. Ed., 2014, 53, 10139–10142 CrossRef PubMed.
- D. Hnyk, V. Všetečka, L. Drož and O. Exner, Collect. Czech. Chem. Commun., 2001, 66, 1375–1379 CrossRef CAS.
- W. N. Lipscomb, in Boron Hydride Chemistry, ed. E. L. Muetterties, Academic Press, New York, 1975 Search PubMed.
- A. Pecina, M. Lepsik, D. Hnyk, P. Hobza and J. Fanfrlík, J. Phys. Chem. A, 2015, 119, 1388–1395 CrossRef CAS PubMed.
- T. Kar and K. Jug, Int. J. Quantum Chem., 1995, 53, 407–412 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The summarized IBO data for 7-, 9-, and 11-vertex closo-heteroboranes and the NBO results for selected representative boranes. See DOI: 10.1039/c7cp07422k |
| This journal is © the Owner Societies 2018 |