Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface functionalization in combination with confinement for crystallization from undersaturated solutions†
Shankul
Vartak
 ,
Leia M.
Dwyer
and
Allan S.
Myerson
,
Leia M.
Dwyer
and
Allan S.
Myerson
 *
*
Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: myerson@mit.edu
First published on 1st October 2018
Abstract
Crystallization from undersaturated solutions was demonstrated using functionalized nanoporous silica (Zorbax® chromatographic media). The silica matrix acts as a source of bound groups within the nanopores which act to reduce the solubility within the small pore volume resulting in the formation of nanocrystals within the pores. Experiments were conducted within sealed capillaries which were monitored via X-ray powder diffraction. Experiments were conducted for a number of solutes and concentrations and clearly demonstrated a critical concentration in the undersaturated region below which crystals would not form. Concentrations above this critical concentration would form crystals. Batch experiments confirmed that the crystallization yield could be calculated from the difference between the initial concentration before the addition of Zorbax® and the critical (effective saturation) concentration.
Crystallization is an important separation and purification technique, especially in the pharmaceutical industry. The tight regulations on product quality including crystal form, particle size distribution, crystal shape, purity, and yield require fine control of the crystallization process.1 The crystallization process includes both nucleation and growth, both of which have implications for the end product of the process. For nucleation to occur, the solution must be supersaturated, meaning the concentration of solute is greater than the equilibrium concentration at that given temperature and solvent condition.2 Supersaturated conditions are generated most often by changing the solvent with the addition of an antisolvent or by cooling the solution. The addition of heterogeneous surfaces to the crystallization process also changes the dynamics of nucleation.3
Antisolvent crystallization is particularly common in pharmaceutical processes when the solute or active pharmaceutical ingredient (API) may be sensitive to changes in temperature.2 A solute molecule is dissolved in one solvent, typically at ambient temperature. The addition of an antisolvent to the solution generates supersaturation because the solute is less soluble in this new anti-solvent. The choice of antisolvent and composition of the solvent/antisolvent mixture can have implications on crystal size, shape, form, and yield.4–6 Heterogeneous surfaces can be used to facilitate the formation of crystal nuclei from supersaturated solutions. These surfaces may include vessel walls or stirring mechanisms, or seeds of the desired crystal product.7 The activation energy of nucleation is lowered in these instances due to the addition of the surface site, corresponding to a decrease in the surface area of the nuclei in classical nucleation theory. Templates whose surface chemistries or geometries (such as self-assembled monolayers, SAMs) are selected to be favorable for certain solute crystallizations have been shown to promote nucleation and growth.8–11 The selection of a surface chemistry can orient solute molecules in addition to providing a surface site, and thus lower even further the energy barrier to nucleation. Nanoparticles functionalized with groups which can act as antisolvents or co-solvents have been demonstrated to induce crystallization and dissolution reversibly, where the solution can be restored to its original state with the removal of the functionalized nanoparticles.12
Crystallization in confinement has been demonstrated as a way to produce stable pharmaceutical nanocrystals of a controlled size.13–15 In this approach, crystallization of the API is restricted to a micro- or nanoporous environment to form nanocrystals, with contributions to nucleation both from the confinement of the crystallization volume and heterogeneous surfaces of the pore. This study combines the effects of crystallization in confinement as well as the principles of heterogeneous nucleation and surface functionalization to produce nanosized crystals from undersaturated solutions. Crystallization in pores has been carried out before, however there the driving force was always present due to antisolvent addition, evaporation, or cooling crystallization as opposed to this unique combined effect of surface functionalization and confinement.
Zorbax® chromatographic media with C8-like surface groups was used, to mimic the functional group interaction of alkanes, which tend to be poor solvents for the organic APIs chosen for this study. We postulate that in the confined volumes of the pores of the matrix, the addition of these surface groups rendered a change in the solubility of API in solution in these nanoscale volumes, providing the driving force for nucleation and crystallization. This has been applied to the crystallization of several small molecule organic compounds. Given that the parent solutions are undersaturated, when held at a fixed temperature left alone or in the presence of non-functionalized control silica media which mimics the surface area of the Zorbax® media, the solutions will not crystallize because there is no driving force for nucleation. However, we demonstrate that the addition of the functionalized Zorbax® media induces crystallization. The confined nanoscale volumes of the pores have high surface areas, and thus the expected contribution of the surface functionalization interaction with the solvent in this environment is high. We believe the combined surface functionalization and confinement effect allows for the Zorbax® media to act as an antisolvent, reducing the solubility of the APIs and causing nucleation and crystallization.
Zorbax® functionalized chromatographic media was obtained from Agilent in bulk packing form. This was porous silica functionalized with a C8-like group (n-octyldimethylsilane). The average listed grain size of the beads was 7 μm and the average pore diameter was 7 nm with a nominal surface area of 160–180 m2 g−1. Thermogravimetric analysis (TGA) studies were performed on the Zorbax® to determine the mass loss from C8 groups on heating and in turn calculate the density of the functional groups on the pore surface (see the ESI,† for details). The functional group coverage is of the order of 5 μmole m−2. As a control, unfunctionalized controlled pore glass (CPG) was purchased from Prime Synthesis (Aston, PA, USA). This was a fumed silica with controlled pore size of approximately 12 nm. The average grain size of this material was 100 μm.
First, capillary X-ray powder diffraction (XRPD) studies were performed to demonstrate that functionalization on the porous matrix is necessary for crystallization to occur. Three systems of active pharmaceutical ingredients (APIs) were studied- diphenhydramine hydrochloride, aspirin and nicotinamide- in isopropyl alcohol as solvent. The solubility of each system at 25 °C was determined and is listed in Table 1 (see ESI† for a discussion of solubility determination and the procedure for the capillary experiments). Next, solutions of about 85% of the solubility were prepared (actual concentrations listed in Table 1) and loaded into glass capillary columns in three sets of experiments- without any matrix, with unfunctionalized CPG, and with the functionalized Zorbax®. The capillaries were sealed to prevent solvent evaporation in order to eliminate any external influences on concentration. After a period of 1 hour to allow for any potential crystallization to occur, each capillary was mounted in a capillary spinner apparatus of the XRPD instrument and scanned. For comparison, the three dry commercial APIs were also scanned using capillary XRPD.
| API | Solubility at 25 °C (mg mL−1) | Concentration tested with capillary XRPD (mg mL−1) |
|---|---|---|
| Diphenhydramine hydrochloride | 38.0 | 30.0 |
| Aspirin | 90.0 | 75.0 |
| Nicotinamide | 46.5 | 40.0 |
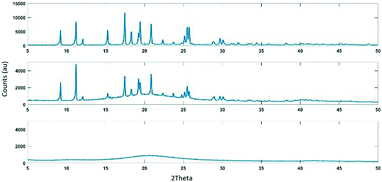
For each of the three systems, no crystallization was observed in the capillaries filled simply with the API solutions without any matrix. This was expected since the solutions were undersaturated and there were no potential influences on saturation. Furthermore, the capillaries with unfunctionalized CPG displayed just an amorphous background signal from the silica matrix but no presence of crystals. These results demonstrate that confinement effects from the pores alone, without any functionalization, cannot induce crystallization in the undersaturated solutions studied.
However, all three systems displayed the presence of crystals in capillaries containing the functionalized Zorbax®. The results for the first system, diphenhydramine hydrochloride are shown in Fig. 1 (for the other two systems, see the ESI†). This demonstrates that the C8 functionalization on the Zorbax was successful in bringing about crystallization even from the undersaturated solutions.
It was hypothesized that there exists an effective solubility of the API in the presence of the functionalized nanoporous silica, and this solubility is lower than that of the API in pure solvent. This is responsible for the antisolvent-like behaviour of the silica matrix. Provided that there is enough matrix available for crystallization, crystals would grow within the pores till the API concentration in the mother liquor equilibrates at the effective solubility value. Hence, feed solutions with API concentrations above the effective solubility would crystallize with the addition of Zorbax®, and those with concentrations below this value wouldn't crystallize. In other words, there exists a critical minimum concentration below which no crystallization can occur, even in the presence of the functionalized Zorbax®.
In order to test this hypothesis, solutions of successively lower concentrations were loaded into capillaries containing the functionalized Zorbax® and tested for the presence of crystals. As hypothesized, for each system, crystallization failed to occur below a certain critical concentration. Results for diphenhydramine hydrochloride are shown in Fig. 1 (see ESI† for more details). This critical value lies between the lowest concentration studied which displays crystallization and the highest concentration studied which fails to display any crystallization.
This hypothesis was validated further by performing batch experiments and determining the mother liquor concentration to verify if it matched the critical value from the capillary XRPD experiments. For each system, 10 mL saturated solutions with Zorbax® added were stirred for 6 h at 25 °C. The amount of Zorbax® added was 1 g for the diphenhydramine hydrochloride and the nicotinamide systems and 1.5 g for the aspirin system. The higher amount was chosen for the aspirin system to allow for sufficient matrix in view of the higher mass to be crystallized compared to the other two systems. At the end of the batch run, the solids were filtered out and the mother liquor concentration was determined via HPLC (see ESI† for details). In each system, the critical value lied in the range predicted from the capillary XRPD experiments as shown in Table 2, validating the hypothesis.
| API | Mother liquor concentration (mg mL−1) | Concentration range from XRPD (mg mL−1) |
|---|---|---|
| Diphenhydramine hydrochloride | 26.4 | 25–27 |
| Aspirin | 66.8 | 65–70 |
| Nicotinamide | 33.2 | 30–35 |
To examine if there is any surface crystallization in addition to crystals confined within the Zorbax® nanopores, the differential scanning calorimetry (DSC) scan of the post-crystallization Zorbax® matrix for the diphenhydramine hydrochloride system was compared with the scan of bulk API. Since the melting point of crystals decreases with size, the presence of nanocrystals can be established by a peak at a lower melting point compared to that of the bulk API.14–17 Furthermore, in the case of surface crystals in addition to the confined nanocrystals, there would be two peaks in the DSC scan- one at the lower melting point corresponding to the nanocrystals and the other at the same melting point as the bulk API corresponding to the surface crystals.1 The presence of a single peak at a lower melting point for the post-crystallization Zorbax® sample as shown in Fig. 2 proves that there is no observable surface crystallization and all crystals are confined to the nanopores. The significant depression in melting point indicates the nanoscale dimensions of the confined crystals (see ESI† for details).
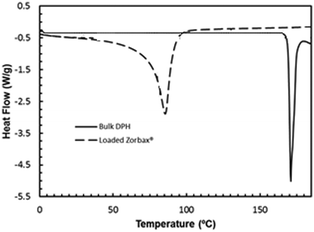 | ||
| Fig. 2 DSC scans show the presence of nanocrystalline DPH with a depressed melting point from batch experiments with Zorbax®. | ||
For the diphenhydramine hydrochloride system, the single step batch yield at 25 °C starting with a saturated feed solution (concentration 38 mg mL−1) and ending with a mother liquor of concentration 26.4 mg mL−1 is about 30% and corresponds to a loading of 116 mg API per g Zorbax®. While the single step yield may be low, the mother liquor can be concentrated by evaporation and subjected to re-crystallization with Zorbax® added. This can improve the overall yield of the process. After each crystallization step, pure solvent preferably at a higher temperature can be used to dissolve the crystals from the Zorbax® matrix followed by filtration of the matrix and subsequent recovery of the API. This strategy can be employed in synthesis for intermediates which require a solvent switch or in the purification of an API or intermediate where an intermediate is crystallized and then dissolved in a different solvent. As a practical example, a preparative chromatography column filled with the functionalized nanoporous silica can be operated in a continuous mode for the purification process.
We believe this work successfully demonstrates that a surface-functionalized nanoporous media may be used to provide surface functionality in the confined nano-volumes of the pores which results in a reduced solubility within the confined volume resulting in the formation of nanocrystals within the pores. We believe the most relevant application of this principle would be in use as a purification technique, where an impure API or intermediate solution may be flowed through a column with chosen antisolvent-like functionality for the API alone to selectively crystallize API within the pores of the matrix while enriching the flowthrough in impurity. An eluent solution could be then flowed over the column to recapture a purer API solution.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Novartis-MIT Center for Continuous Manufacturing as well as the DARPA Grant No. W911NF-16-2-0023.Notes and references
- L. Dwyer, V. Michaelis, M. O'Mahony, R. Griffin and A. Myerson, Confined Crystallization of Fenofibrate in Nanoporous Silica, CrystEngComm, 2015, 17(41), 7922–7929, 10.1039/C5CE01148E.
- R. Ganapathy and A. Sood, Crystallization: Brought to the Surface, Nat. Phys., 2017, 13(5), 421–422, DOI:10.1038/nphys4057.
- R. Hiremath, J. Basile, S. Varney and J. Swift, Controlling Molecular Crystal Polymorphism with Self-Assembled Monolayer Templates, J. Am. Chem. Soc., 2005, 127(51), 18321–18327, DOI:10.1021/ja0565119.
- Q. Jiang and M. Ward, Crystallization under Nanoscale Confinement, Chem. Soc. Rev., 2014, 43(7), 2066–2079, 10.1039/C3CS60234F.
- S. Kulkarni, C. Weber, A. Myerson and J. ter Horst, Self-Association during Heterogeneous Nucleation onto Well-Defined Templates, Langmuir, 2014, 30(41), 12368–12375, DOI:10.1021/la5024828.
- A. Lee, A. Ulman and A. Myerson, Crystallization of Amino Acids on Self-Assembled Monolayers of Rigid Thiols on Gold, Langmuir, 2002, 18(15), 5886–5898, DOI:10.1021/la025704w.
- M. Matsumoto, M. Ohno, Y. Wada, T. Sato, M. Okada and T. Hiaki, Enhanced Production of α-Form Indomethacin Using the Antisolvent Crystallization Method Assisted by N2 Fine Bubbles, J. Cryst. Growth, 2017, 469, 91–96, DOI:10.1016/j.jcrysgro.2016.09.042.
- A. Myerson, Handbook of Industrial Crystallization, Butterworth-Heinemann, 2002 Search PubMed.
- M. O'Mahony, A. Leung, S. Ferguson, B. Trout and A. Myerson, A Process for the Formation of Nanocrystals of Active Pharmaceutical Ingredients with Poor Aqueous Solubility in a Nanoporous Substrate, Org. Process Res. Dev., 2015, 19(9), 1109–1118, DOI:10.1021/op500262q.
- L. Padrela, J. Zeglinski and K. Ryan, Insight into the Role of Additives in Controlling Polymorphic Outcome: A CO2-Antisolvent Crystallization Process of Carbamazepine, Cryst. Growth Des., 2017, 17(9), 4544–4553, DOI:10.1021/acs.cgd.7b00163.
- S. Rohani, S. Horne and K. Murthy, Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent, Org. Process Res. Dev., 2005, 9(6), 858–872, DOI:10.1021/op050049v.
- S. Kulkarni and A. Myerson, Reversible control of solubility using functionalized nanoparticles, Chem. Commun., 2017, 53, 1429–1432, 10.1039/c6cc09390f.
- A. Singh, I. Lee, K. Kim and A. Myerson, Crystal Growth on Self-Assembled Monolayers, CrystEngComm, 2011, 13(1), 24–32, 10.1039/C0CE00030B.
- T. Tierney, A. Rasmuson and S. Hudson, Size and Shape Control of Micron-Sized Salicylic Acid Crystals during Antisolvent Crystallization, Org. Process Res. Dev., 2017, 21(11), 1732–1740, DOI:10.1021/acs.oprd.7b00181.
- P. Vekilov, Nucleation, Cryst. Growth Des., 2010, 10(12), 5007–5019, DOI:10.1021/cg1011633.
- Y. Xue, Q. Zhao and C. Luan, The Thermodynamic Relations between the Melting Point and the Size of Crystals, J. Colloid Interface Sci., 2001, 243, 388–390, DOI:10.1006/jcis.2001.7837.
- S. Shimizu, K. Agrawal, M. O'Mahony, L. Drahushuk, N. Manohar, A. Myerson and M. Strano, Understanding and Analyzing Freezing Point Transitions of Confined Fluids within Nanopores, Langmuir, 2015, 31, 10113–10118, DOI:10.1021/acs.langmuir.5b02149.
Footnote |
| † Electronic supplementary information (ESI) available: Indicating materials, methods, and operating conditions. See DOI: 10.1039/c8ce01543k |
| This journal is © The Royal Society of Chemistry 2018 |