Open Access Article
Open Access ArticleGraphene-like metal–organic frameworks: morphology control, optimization of thin film electrical conductivity and fast sensing applications†
Bastian
Hoppe
 *ad,
Karen D. J.
Hindricks
*ad,
Dawid P.
Warwas
*ad,
Hendrik A.
Schulze
*ad,
Alexander
Mohmeyer
*ad,
Tim J.
Pinkvos
*ad,
Saskia
Zailskas
*ad,
Marc R.
Krey
*ad,
Christopher
Belke
*bd,
Sandra
König
c,
Michael
Fröba
*ad,
Karen D. J.
Hindricks
*ad,
Dawid P.
Warwas
*ad,
Hendrik A.
Schulze
*ad,
Alexander
Mohmeyer
*ad,
Tim J.
Pinkvos
*ad,
Saskia
Zailskas
*ad,
Marc R.
Krey
*ad,
Christopher
Belke
*bd,
Sandra
König
c,
Michael
Fröba
 c,
Rolf J.
Haug
c,
Rolf J.
Haug
 *bd and
Peter
Behrens
*bd and
Peter
Behrens
 *ad
*ad
aInstitut für Anorganische Chemie, Leibniz Universität Hannover, Schneiderberg 39, Callinstraße 9, 30167 Hannover, Germany. E-mail: peter.behrens@acb.uni-hannover.de
bInstitut für Festkörperphysik, Leibniz Universität Hannover, Schneiderberg 39, Appelstraße 2, 30167 Hannover, Germany
cInstitut für Anorganische und Angewandte Chemie, Universität Hamburg, Martin-Luther-King-Platz 6, 20146 Hamburg, Germany
dLaboratory of Nano and Quantum Engineering (LNQE), Leibniz Universität Hannover, Schneiderberg 39, 30167 Hannover, Germany
First published on 25th September 2018
Abstract
The metal–organic framework Cu-2,3,6,7,10,11-hexahydroxytriphenylene (Cu3hhtp2-MOF), a copper-based graphene-like framework, is one of the few MOFs featuring inherent electrical conductivity. Here, we investigate the synthesis of this material with regard to the influence of different additives. It is shown that ammonia acts as a modulator leading to platelet-like particles in a water-based synthesis system. This material is thoroughly characterized by X-ray diffraction (XRD), electron microscopy, atomic force microscopy (AFM), physisorption, thermal behaviour, and electrical conductivity. The measured conductivity value of 0.045 S cm−1 surpasses all formerly reported measurements. The obtained platelets appear especially suitable for the preparation of different devices. As an example, we prepared thin and homogenous films by spray-coating water-based dispersions of this MOF on glass and on polymer substrates. In the films, the platelets are oriented parallel to the substrate and are in intimate contact. This leads to a high electrical conductivity combined with an easily accessible pore system. The applicability of such coatings is shown in a preliminary sensing test, showing quick and strong response and fast recovery. This work shows that control of the crystal morphology combined with suitable preparation procedures can enhance the performance of MOF-based devices.
1. Introduction
In recent years, the research on metal–organic frameworks (MOFs) has grown tremendously due to their permanent porosity and versatile opportunities to manipulate their properties (e.g. surface area, pore size, functional groups and active sites for catalysis) for different potential applications like gas storage and separation, catalysis, sensing or in biomedical applications. However, despite the large variety of MOFs described, members of this family with inherent electrical conductivity are extremely rare. To overcome this lack, non-conductive MOFs can be infiltrated by conjugated organic molecules like tetracyanoquinodimethane (TCNQ)1 or conductive organic polymers2 and thereby attain electrical conductivity. Unfortunately, this procedure reduces the accessible pore volume, and is thus detrimental to the combination of efficient sorption properties and electrical conduction.Recently, a new group of graphene-like 2D metal–organic frameworks has been reported which exhibits inherent high electrical conductivity of the metal–organic structure and an open pore system at the same time. These 2D MOFs are built up from phenylene3–5 or triphenylene5–11 linker molecules with six functional groups (–OH, –NH2 or –SH). Based on the trigonal linker molecules and square-planarly coordinated metal ions (e.g. Cu2+, Ni2+, Co2+, Pt2+), layers with a hexagonal structure are formed which topologically resemble that of graphene on a larger scale. These layers can stack to build up a one-dimensional pore system. Members of this group of graphene-like MOFs possess electrical conductivities ranging from 0.002–58.8 S cm−1 (pressed pellets)6,12 at room temperature. Thin films of Cu3bht2 (benzenehexathiol, bht) with a thickness of 400 nm show even higher values up to 1580 S cm−1.4 However, this MOF is not porous because the specific stacking of the layers leads to pore blocking. Electrodes built from graphene-like MOFs have been tested for hydrogen evolution from water,5,13,14 oxygen reduction reactions,15 sensing of volatile organic compounds (VOCs)6,7 and for energy storage in supercapacitors.9
The electronic structure of Ni3hitp2 (hexaiminotriphenylene, hitp) has been investigated by density functional theory (DFT) and ab initio calculations, stating that a monolayer of this MOF is semiconductive with bandgaps between 0.13 eV (ref. 16 and 17) and 0.25 eV.18 When a second layer is added to the simulation model, the bandgap decreases with the interlayer distance. For a slipped parallel stacking the band gap disappears at 3.8 Å. The bulk material with an experimentally determined interlayer distance of 3.3 Å does not have a bandgap and the material shows metallic conductivity. Consequently, this material should have an appreciable electrical conductivity not only parallel but also perpendicular to the stacked layers of the MOF.18 In contrast to the nickel-based MOF, not only the bulk material but also a monolayer of Cu3hitp2 is predicted to be a metallic conductor without a bandgap.17
In a final device, cooperative properties like electrical conductivity depend not only on the actual crystal and electronic structure of a compound, but also on defects, (anisotropy of) crystal morphology, and the packing and orientation of primary crystals. Thus, for generating favourable effects, the fabrication of crystal morphologies suitable for a certain fabrication procedure (“shaping”) is also of prime importance. In case of the combined application of nanoporosity and electrical conductivity, this is especially important, e.g., with respect to good contacts and favourable orientations at the crystal boundaries as well as with respect to the accessibility of the pore system and short diffusion pathways. In this respect, it remains to be noted that in most of the aforementioned studies on graphene-like MOFs, no special significance was given to control the crystal morphology, the arrangement of crystals or the fabrication of shapes suitable for device applications. In this study, we describe the synthesis of thin platelets of the graphene-like MOF copper-2,3,6,7,19,11-hexahydroxy-triphenylene (Cu3hhtp2) and the fabrication of highly homogeneous thin conducting films from stable dispersions of this material by spray-coating.
Cu3hhtp2 shows the lowest conductivity values (0.002 S cm−1, pressed pellet;6 0.001–0.02 S cm−1, thin film;19,20 0.2 S cm−1, single crystal11) among the graphene-like MOFs, but it is the one which shows the strongest response towards many VOCs in sensing experiments.6 The reported synthesis by Yaghi and coworkers leads to rod-like particles within which the one-dimensional pores run along the particle elongation, an unfavourable situation with regard to the accessibility of the pore system.11 Dincă and coworkers explicitly resigned to check the influence of the particle morphology in their sensing experiments, although they found significant differences in crystal size and morphology between Cu3hhtp2, Cu3hitp2 and Ni3hitp2.6 Recently reported investigations on Cu3hib2 and Ni3hib2 (hexaiminobenzol, hib) demonstrate that reaction conditions and postsynthetic treatments can have a strong impact on the properties of these materials. Whereas water-based synthesis21 results in products with a BET surface area of about 350 m2 g−1 and a conductivity 0.7 S cm−1 (Ni3hib2) or 0.1 S cm−1 (Cu3hib2), the products from a DMSO-based synthesis22 show lower BET surface areas but enhanced conductivities of 8 S cm−1 (151 m2 g−1, Ni3hib2) and 13 S cm−1 (114 m2 g−1, Cu3hib2).
The concept of modulated synthesis is a strong tool in MOF synthesis, especially with regard to influencing size and shape of MOF crystals.23–25 Within the concept of coordination modulation, modulators are molecules with only one coordinating function. They compete with linker molecules for the coordination sites at the metal cations and thus in general slow down nucleation and growth of the crystals, often leading to crystals of high quality.26 Especially the synthesis of large single crystals for the determination of new crystal structures often requires the presence of a modulator within the synthesis mixture.27,28 In some cases, crystalline products can only be obtained when a modulator is used during the synthesis.27,29,30 Also thermodynamically unfavourable phases, e.g. ZIF-71 and [Zn(dcim)2], can become accessible by the use of a modulator,31,32 although in these systems protonation modulation also plays a role for nanocrystal synthesis.33
Here, we report on the control of crystal morphology of Cu3hhtp2 by modulated synthesis. We tested different additives and found that by the addition of pyridine or ammonia accompanied by a change of the copper salt employed, we are able to synthesize large flake-like particles of this porous and conductive MOF. The flakes or platelets produced present their pore openings on their large surfaces, as the pores run vertically to the platelet plane. Thus, they should be very suitable for applications where fast diffusion into the pore system is necessary, for example with regard to the response time of sensors. We prepared water-based dispersions of the Cu3hhtp2 platelets and used these to fabricate homogeneous thin films on glass and also flexible polycarbonate foils by simple spray-coating. In contrast to recent reports of thin film preparation by layer-by-layer spray-coating19 or the Langmuir–Blodgett transfer technique,20 our approach is easily adjustable for patterned deposition of small structures and conductor paths as well as for large area deposition. Also, the preferred flake-like morphology leads to highly textured orientation of the particles inside the film. All in all, this work demonstrates that detailed studies of MOF synthesis systems can lead to defined and uniform crystal morphologies, and that suitable morphologies can be beneficially used to fabricate well-defined shapes for potential applications.
2. Experimental
2.1 Synthesis procedures
For more controlled thin film generation on polycarbonate foils and glass substrates, an automated spray-coating robot from Walther Systemtechnik (Germersheim, Germany) was employed. The composition of the dispersion was adjusted by adding 2.6 mL of a 12 wt% ethanolic Span® 80 solution to the Cu3hhtp2-dispersion and stirring for at least 1 h. Prior to the coating, the polycarbonate foils were washed with absolute ethanol, dried with a nitrogen stream and fixed with an aluminium sample holder (see ESI† Fig. S1). The coating was carried out at a substrate temperature of 60 °C and by equipping the spray-coating robot with an SMS-02 spraying valve to coat 5 samples simultaneously at the same time. The used vaporizing pressure was 1.2 bar and the nozzle was only slightly opened to deposit only a small amount of material at each of the 50–100 repetitions. For patterned depositions, masks with different structures were used under the same conditions (see ESI† Fig. S1). After spray-coating, the sample was washed cautiously with ethanol to remove the surfactant and dried in air afterwards.
2.2 Structure simulation
The simulation of the crystal structure was performed with BIOVIA Materials Studio 2017 (San Diego, USA). To construct the structure, a copper ion was coordinated in a square-planar fashion with two linker molecules. This complex was then transferred into a unit cell with the space group P6/mmm and a geometry optimization was performed. Subsequently, the cell parameters a and b were optimized by reference to the experimental reflections at 4.7, 9.5, 12.6 and 16.5° 2θ. Afterwards, the interlayer distance was optimised to 3.16 Å according to the broad reflection at 28.2° 2θ. This procedure results in an AA stacking of the layered structure.2.3 X-ray diffraction
Powder X-ray diffraction (XRD) patterns were measured in transmission using a STOE Stadi P diffractometer (Darmstadt, Germany) with CuKα1 radiation with a wavelength of λ = 1.5406 Å and a Ge (111) monochromator. The powders were grinded before the measurement.X-ray diffraction patterns of coatings were measured in reflection using a STOE Stadi P diffractometer (Darmstadt, Germany) working in θ–θ geometry with CuKα radiation with a wavelength of λ = 1.5418 Å and a nickel foil in front of the detector.
2.4 Scanning electron microscopy
Low-magnification scanning electron microscopy (SEM), mainly used for particle morphology screening, was carried out with a JEOL JSM-6610LV (Tokyo, Japan) with a tungsten filament, a working distance of 10 mm and an acceleration voltage between 10 and 20 kV. The bulk material was dispersed in ethanol and dropped onto polished carbon. High-resolution SEM (HR-SEM) was carried out on a JEOL JSM-6700F (Tokyo, Japan) with a field emission cathode, a working distance of 3 mm and an acceleration voltage of 2 kV. The diluted dispersions in water were dropped on acetone-cleaned gold-sputtered silicon wafers.2.5 Transmission electron microscopy
Transmission electron microscopy (TEM) was performed using a FEI Tecnai G2 F20 TMP (CS = 2 mm, CC = 2 mm, Hillsboro, USA) with a 200 kV field emission gun. 10 μL of diluted dispersions were drop-casted on Quantifoil 400 mesh carbon-coated copper grids (Jena, Germany) and images were taken in bright-field mode. Distances were measured with NIH ImageJ (Bethesda, USA).2.6 Atomic force microscopy
For atomic force microscopy, 10 μL of a diluted water-based dispersion was drop-casted on a silica-coated silicon wafer and spin-coated after a latency period of 20 minutes. The measurements were performed with a Veeco Dimension V (Plainview, USA) equipped with a NanoWorld Pointprobe silicon cantilever (Neuchâtel, Switzerland) in tapping mode. The measurement was performed with a scan size of 10 μm at 0.5 Hz and a scan angle of 0°. Height determinations of individual particles were performed with the implemented software Nanoscope 7.30. Topographic images were prepared with Gwyddion 2.49 (Czech Metrology Institute, Brno).2.7 Confocal microscopy
In addition to the SEM investigations, confocal microscopy is used for the determination of coating thickness as well as for the evaluation of coating quality. Topographical imaging was performed using a Leica DCM3D scanning confocal microscope (Wetzlar, Germany) and the associated software LeicaScan version 3.2.3. Overview images were taken in the microscopic mode at a tenfold magnification with white light. Using the confocal image and the surface topography mode, the 3D view of the surface was constructed at a fifty-fold magnification, utilizing the EPI50x 0.9-L lens. Images were taken over the desired distance within z-direction with a step size of 0.2 μm, starting at the bottom. A lower threshold of 2% was used. Topographic images and height distributions using one-dimensional statistic functions were prepared with Gwyddion 2.49 of the Czech Metrology Institute (Brno, Czech).2.8 Physisorption experiments
Argon physisorption isotherms were measured with a Quantachrome Autosorb-1 (Odelzhausen, Germany). The samples were Soxhlet-extracted and activated prior to the measurement for 24 h at 80 °C in vacuum. For the determination of the BET specific surface area the “Micropore BET Assistant” implemented in the ASiQwin 2.0 software form Quantachrome was used. The pore size distribution was calculated by fitting the NLDFT Quantachrome kernel “Ar at 87K zeolites/silica” to the experimental data.Water physisorption isotherms were measured with a Quantachrome Aquadyne DVS-2 (Odelzhausen, Germany) at 25 °C. The samples were Soxhlet-extracted and activated prior to the measurement for 24 h at 80 °C in a dry nitrogen stream (100 mL min−1).
2.9 Zeta potential measurement
MOF dispersions (2.4 mg ml−1) were characterized by pH-dependent zeta potential measurements using a Malvern Zetasizer Nano ZS (Kassel, Germany) equipped with a Malvern multi purpose titrator MPT-2 (Kassel, Germany). 0.1 M HCl and NaOH solutions were used to adjust the pH of the dispersion.2.10 X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy measurements were performed with a Leybold Heraeus EA 10/100 (Cologne, Germany) spectrometer. A hemispheric analyzer with a diameter of 100 mm and non-monochromatic MgKα radiation of 1253.6 eV was used for the measurement. The films were deposited on gold-coated silicon wafers using automated spray-coating with Span® 80 as surfactant and were cleaned thoroughly by Soxhlet extraction with ethanol for 4 hours before the measurement.2.11 Thermal gravimetric analysis
To investigate the thermal stability of the MOF, the simultaneous thermogravimetry and differential scanning calorimeter Netzsch STA 409 PC (Selb, Germany) was used. Experiments were performed in the range of 40–400 °C with a heating ramp of 5 °C min−1 in an oxygen/argon (20/80) atmosphere. Furthermore, the device is coupled with a Netzsch QMS 403 D Aeolos quadrupole mass spectrometer (Selb, Germany) to obtain additional information on the combustion products.2.12 Measurements of electrical conductivity
Conductivity measurements of pressed pellet samples were performed with the van der Pauw method using a Keithley 2100 multimeter (Beaverton, USA) and steel probes. With the van der Pauw method34,35 the resistances of contacts and wires are excluded and only the resistance of the material is observed. The samples were measured at room temperature under argon atmosphere in a glovebox after activation in vacuum for at least 24 hours. Pellets (diameter of 8 mm, thickness between 0.13–0.41 mm) were pressed using a hydraulic press for 1 minute at a weight of 1 ton.Conductivity measurements of thin films were performed using a self-made linear 4-point-probe setup with a Keithley 2100 multimeter (Beaverton, USA). The samples were measured at room temperature under argon atmosphere in a glovebox after activation in vacuum for at least 24 hours.
2.13 Sensing experiments
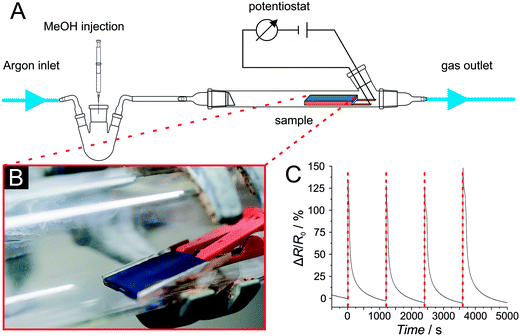
With regard to a possible cross-sensitivity, investigations on the effect of water on the electrical conductivity of Cu3hhtp2 were performed first. Pressed pellets were placed between two brass electrodes inside of a PTFE cell. The cell was placed in an argon-filled desiccator to avoid influences of moisture from the environment. A constant potential of 500 mV was applied to the electrodes using an Ametek Princeton Applied Research Versastat 4 potentiostat (Berwyn, USA). Prior to the measurement the setup was flushed with dry argon. During the measurement, a wet argon stream which had passed through water was used to achieve a water-saturated gas stream.Sensing experiments with coatings were performed with coated glass slides (2 × 2 cm2) in a laboratory quartz glass tube. Prior to the measurement the glass slides were mounted in a holder, bonded to copper electrodes with conductive silver ink and placed in the gas stream. A constant potential of 500 mV was applied to the electrodes using an Ametek Princeton Applied Research Versastat 4 potentiostat. To remove adsorbed guests, especially adsorbed water, the setup was flushed with argon for at least 1 hour before the experiment. For the measurement, 20 μL of MeOH was injected into the argon stream, using a syringe and employing a glass flask with a septum; the glass flask is placed in front of the quartz glass tube setup.
3. Results and discussion
3.1 Structure simulation
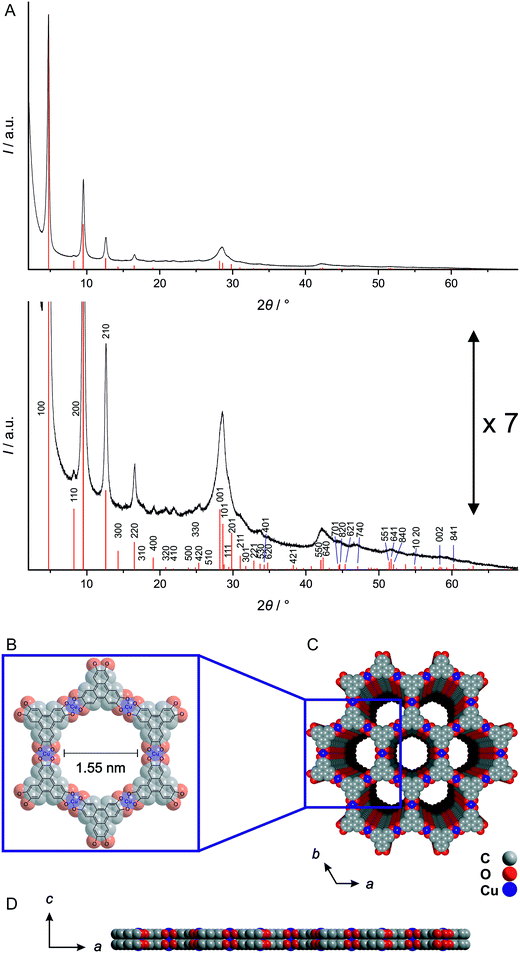
Single crystals and correspondingly single crystal structures of 2D layered materials are often difficult to obtain.36–38 Also for the graphene-like MOFs, true single crystals suitable for single crystal X-ray diffraction studies have not been obtained so far. Nevertheless, some valuable statements about the structure of the Cu3hhtp2-MOF can be made by close inspection of the X-ray diffractogram, using a combination of simulations and arguments from the theory of diffraction of two-dimensional materials.36,38,39 These statements are based on the X-ray diffraction pattern displayed in Fig. 1A which stems from a material of comparatively high crystallinity, consisting of platelet-like crystals and obtained within this work by modulation of the synthesis with 0.3 mmol H6hhtph and 50 eq. ammonia (see below).Fig. 1A shows the indexed experimental X-ray diffraction pattern together with diffraction lines calculated from a simulated model. The experimental hk0 reflections, especially those in the 2θ region up to ca. 26° 2θ, are relatively sharp. Their positions and intensities show good agreement with the simulated diffraction data depicted as line diagram. This certifies the basic correctness of the structure within the a, b plane (depicted in Fig. 1B) and indicates that within this plane, the degree of disorder is low. Also, there is no significant asymmetric broadening of the diffraction peaks and thus no indication for excessive disorder in the stacking of the basic layers. Reflections of the hkl family which relate to this disorder are weak, but some can be discerned as distinct peaks in the shoulders of other reflections, e.g. (211) at 31.0° 2θ. The simulated line pattern was calculated on the basis of an AA stacking of the layers as depicted in Fig. 1C and D.
The first non-hk0 reflection appears at 28.2° 2θ. It is the (001) reflection related to the order along the c axis, i.e. perpendicular to the layers. Although it overlaps with the (101) reflection, it is obvious that this reflection is considerably broadened. There are several possible reasons for such a broadening. First of all, it can be associated with Scherrer broadening caused by the small thickness of the particles produced by the ammonia-modulated synthesis used by us (see below), i.e. their short coherence length along the c axis. Secondly, structural reasons are possible. Disorder of the stacking of the layers (translational or rotational) would lead to slightly varying interlayer distances with correspondingly varying d001 distances; however, significant stacking disorder can be ruled out on the basis of the symmetric appearance of hk0 reflections. It cannot be ruled out, however, that the layers are (irregularly) corrugated or curved which would also result in varying d100 distances. The open and soft metal–organic framework is probably much more susceptible to such corrugation than more stiff layers, like, e.g., graphene layers.
From the position of the broadened 001 reflection, a mean interlayer distance of 3.16 Å can be calculated. This value is considerably lower than in graphite and the values given for other related graphene-analogue MOFs7,8,11 and COFs.40 Probably, most of the structures described in the literature were not refined for this parameter. For example, the Cu3hitp2-MOF described in ref. 8 seems to have a shorter interlayer distance than given by the presented structure simulation. In fact, the broad 001 reflection appears at slightly higher 2θ values in comparison to the calculated value of 27.8° 2θ (representing an interlayer distance of 3.3 Å).7 The short interlayer distance observed on the Cu3hhtp2-MOF might be indicative of attractive metal–metal interactions between the copper cations in adjacent layers which can occur in the proposed AA stacking.
Summarizing, the Cu3hhtp2-MOF platelet crystals synthesized and inspected in this work appear to possess a high crystallinity, with no significant stacking or turbostratic disorder. The diffraction data are in agreement with an AA stacking which might be stabilized by attractive metal–metal interactions. The stacking is important as it defines the pore shape; for an AA stacking, unobtruded one-dimensional channels with a diameter of 1.55 nm result.
3.2 Influence of additives on morphology
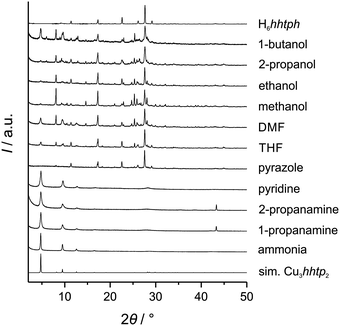
In order to study the influence of additives on the crystal morphology of the Cu3hhtp2-MOF, we modified the synthesis described by Hmadeh et al.11 Reactions were carried out at 80 °C in water. Instead of using copper(II) trifluoroacetylacetonate, with a rather strongly coordinating anion, as precursor, and NMP as additive, the synthesis was carried out with copper(II) nitrate trihydrate. We employed different additives in different amounts, among them alcohols, amines and azines as well as DMF and THF. The additives with a nitrogen-based ligand function were used because it was expected that they could coordinate to Cu2+ cations in a square-planar environment as present in the Cu3hhtp2 layer structure and thus might effect a strong modulating action. Using coordination modulation, we hoped to reduce the amount of stacking of the layers and thereby suppress the crystal growth along the c-axis.In general, the use of alcohols, DMF and THF leads to only very low yields of the MOF. The products consist mainly of unreacted H6hhtph linker. Using amines or pyridine, it is possible to receive the desired phase of the Cu3hhtp2-MOF as the main product (Fig. 2). Probably, this is mainly caused by the improved solubility of the linker at higher pH. The formation of elemental copper was observed when amines with longer alkyl chains were used. The best products with regard to crystallinity were received with pyridine and ammonia.
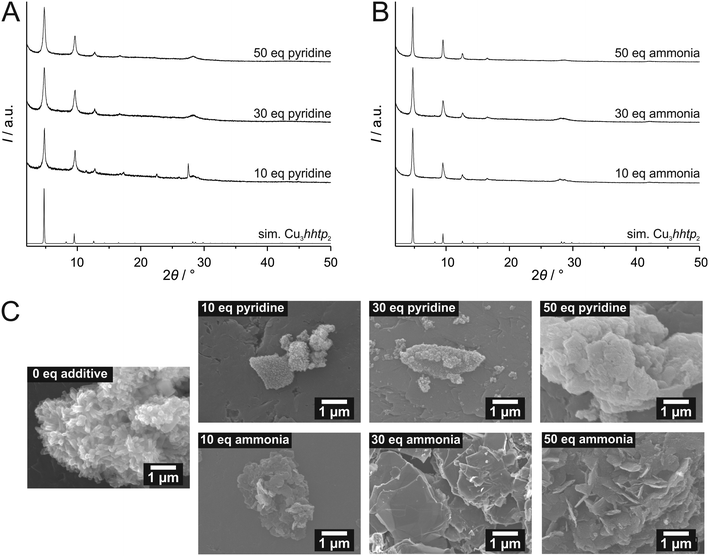
To further investigate the influence of the additives pyridine and ammonia, we used different concentrations of these modulators. All products showed a dark blue to black colour when dried. Except for the sample prepared with 10 equivalents of pyridine, which shows additional reflections assigned to unreacted linker molecules, the XRD patterns agree with phase-pure Cu3hhtp2-MOF (Fig. 3A and B). SEM images (Fig. 3C) show that the products from syntheses with pyridine do not show the desired crystal morphology. Especially with low amounts of pyridine, small agglomerated particles with uncharacteristic morphologies can be observed. This product bears some similarities to the product from the non-modulated original synthesis, which consists of small rod-like, strongly aggregated particles.11 Only when 50 equivalents of pyridine were used during the synthesis, the product appears to have a more flake-like shape. In contrast, all samples synthesized in the presence of ammonia show a well-defined flake-like morphology. Individual platelets have a diameter of several micrometers and are randomly stacked on each other. Because of the very broad size distribution, it is hard to discern a trend in the particle diameter as a function of the amounts of ammonia employed during the synthesis. The thickness of individual flakes can be estimated from those particles in the SEM pictures which stand on an edge to be less than 20 nm. This is in good agreement with the very broad 001 reflection at 28.2° 2θ. Especially the sample synthesized in the presence of 50 equivalents of ammonia shows platelets with a regular morphology and clean surfaces. This result can be explained by coordination modulation, assuming that ammonia molecules bind at free coordination sites of Cu2+ ions which are in a square-planar environment of the hhtp ligands. For the growth of crystals along the c direction, these ammonia ligands have to be removed first, explaining the small elongation of the crystals along this direction.
Apart from a coordination–modulation effect, the amine-containing additives also increase the pH of the synthesis solution. To exclude this pH increase as a possible reason for the change in morphology we conducted further experiments where no amine-containing additives were added, but where the pH of the synthesis solution was adjusted to higher values by the addition of NaOH. Investigation of the products by PXRD (see ESI† Fig. S6) and SEM (see ESI† Fig. S7) showed that no flake-like morphologies of the Cu3hhtp2-MOF could be obtained in this way. At higher pH values some Cu2O is formed; at pH 14 the oxide is the major product according to PXRD. This is line with the observation that only ammonia and (to a lesser extent) pyridine exert a modulating effect, whereas pyrazole and 1- and 2-propanamine, which also increase the pH, do not.
3.3 Characterisation of platelet-like nanoparticles
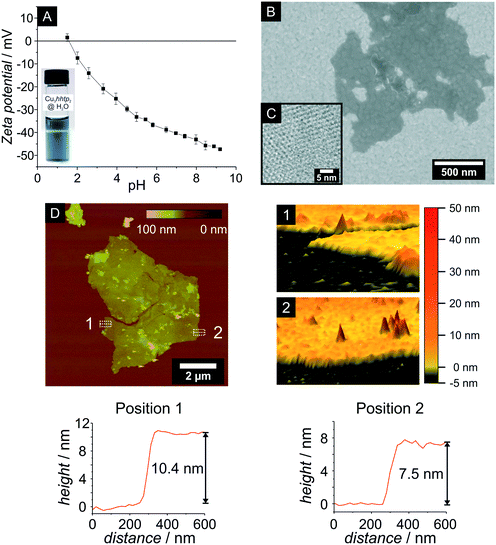
The thin platelets obtained from the ammonia-augmented synthesis are very interesting for several reasons. Firstly, within these platelets the pores run perpendicular to the basal plane, resulting in many short pores and many pore entrances; thus, the pore system can be easily accessed, diffusion paths are short, and sorption as well as desorption kinetics should be fast. Secondly, nanoparticles of defined morphology are well-suited precursors for various fabrication methods used to generate specific shapes and devices. Thirdly, the yield of this synthesis is easily scalable to at least 120 mg per batch. Therefore, we set out to characterize these particles in more detail.The nanoplatelets of the Cu3hhtp2-MOF are easily dispersed in water; pH-dependent zeta potential measurements (Fig. 4A) reveal that the particles are negatively charged above the isoelectric point at pH 2 and have a zeta potential below −30 mV above pH values of ca. 4.5. This explains the very high stability of dispersions at neutral or basic conditions.
SEM and AFM images (Fig. 4B and D) show thin particles with a non-uniform geometry and diameters extending up to a few micrometers. The thickness of these very thin particles, as determined by AFM height measurements, is about 7.5–10.4 nm. Considering an interlayer distance of 0.316 nm, this height corresponds to 24–33 stacked layers of Cu3hhtp2. The hexagonal honeycomb pattern observed in TEM images (Fig. 4C) also confirms the orientation of the 1D pore system perpendicular to the surface of the particles. The determination of the pore size from the TEM picture is somewhat ambiguous because of the damage caused by the electron beam. Nevertheless, a pore size between 1.4 and 1.6 nm can be observed, which is in very good agreement with the crystallographic pore size of 1.55 nm.
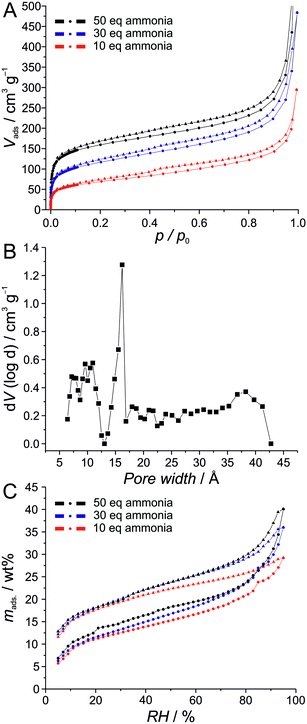
The diffractograms shown in Fig. 3B show no significant changes in the crystallinity with the amount of ammonia added during the synthesis. Therefore, we set out to also compare the sorption properties of the products of these syntheses and performed argon and water physisorption measurements to investigate the influence of added ammonia. The argon physisorption isotherms (Fig. 5A) show clearly that higher BET surface areas are obtained with higher amounts of added ammonia. The sample prepared with 50 equivalents ammonia exhibits a BET surface area of 512 m2 g−1. The pore size of ca. 1.6 nm (Fig. 5B), determined by NLDFT fitting of the sorption data, agrees well with the crystallographically expected value of 1.55 nm. The small hysteresis in the mesopore regime (p/p0 = 0.1–0.9) as well as the strong increase of adsorbed volume at p/p0 > 0.9 are probably caused by disordered interparticular pore volume.
In contrast to this, the sorption properties of water are less strongly affected by the amount of ammonia added during the synthesis, although (and as in the case of argon sorption), the sample prepared with 10 equivalents of ammonia shows the lowest uptake. On the other hand, the samples prepared with 30 and 50 equivalents exhibit very similar sorption properties with a maximum water uptake of ca. 40 wt%. Water uptake starts at very small pressures. The material can thus be considered to be strongly hydrophilic.
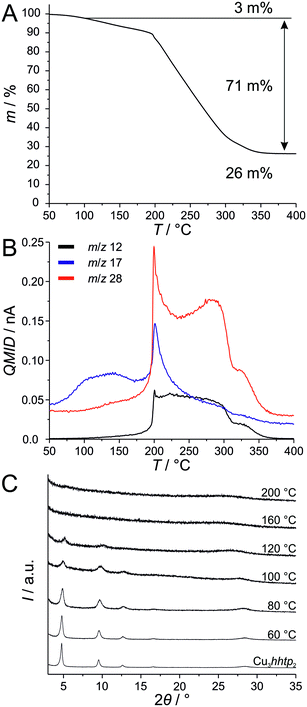
To check the thermal stability of the Cu3hhtp2-MOF coupled thermogravimetric, differential thermal analysis and mass spectroscopy measurements were performed on a Soxhlet-extracted sample which was prepared in the presence of 50 equivalents ammonia (Fig. 6). The experiment was carried out in an oxygen/argon (20/80) atmosphere. The first step is correlated to the evaporation of guest molecules from the framework. These guests are adsorbed water (or remaining ammonia), as confirmed by the signal for m/z 17 in mass spectroscopy. Above 80 °C, organic compounds start to slowly combust in an exothermal reaction. This value is lower than the reported thermal stabilities of the related MOFs Ni3hitp2 (300 °C),8 Co3hhtp2 (250 °C)11 and Ni3hhtp2 (200 °C).11 The mass signal of 28 can be related to CO22+ or C2H4+˙ species and derives most probably from organic parts of the framework. Additionally, m/z 12 is related to C+ species which can also be associated with the combustion of organic compounds. Combustion intensifies above 200 °C and is completed at 350 °C. The residue was proven to be CuO by XRD. Subtracting the 3 mass-% of guest species liberated in the first step, the amount of organic components corresponds to 73.1 mass-% and the CuO residue to 26.9 mass-%; this fits very well to the theoretical values of 72.7 mass-% organic and 27.3 mass-% CuO. Further investigations on the initial reaction of the framework to heating, carried out by XRD (Fig. 6C), prove the thermal stability of Cu3hhtp2 up to 80 °C. Upon further heating to 200 °C, only amorphous material can be observed.
3.4 Spray-coating of thin films
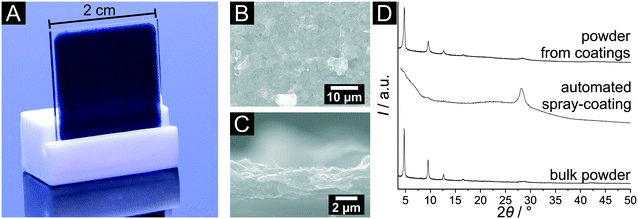
For many envisaged applications of MOFs, processing and shaping of the material is a prerequisite. Nanoparticles with uniform morphology lend themselves for many controlled shaping processes. To demonstrate the advantages of the Cu3hhtp2-MOF nanoplatelets described in this work, we chose to describe the generation of thin films by simple spray-coating procedures from water-based dispersions. We used an automated setup and added the surfactant Span® 80 in order to obtain crack-free and homogeneous coatings. As substrates we used glass slides and polymer foils.Fig. 7 depicts the results for the spray-coating experiments on glass slides. Fig. 7A shows a homogeneously covered glass substrate with the typical dark blue colour. SEM pictures show a complete and crack-free covering of the substrate (Fig. 7B). From SEM cross-sections as the one depicted in Fig. 7C, layer thicknesses can be determined. These range from 1 μm to 3 μm, depending on the number of coating cycles (50–100). In both SEM images, a strong texture of the nanoplatelets can be observed. With a strong preference, the flakes are oriented parallel to the substrate surface. Also, the nanoparticles are obviously in close contact. The strong preferred orientation of the Cu3hhtp2 particles was confirmed by XRD measurements (Fig. 7D). In comparison to the bulk material, the relative intensity of the (001) reflection is strongly increased and the other reflections are virtually absent. This is not due to a degradation of the material: powder samples received from these coatings still show the characteristic XRD pattern of the Cu3hhtp2-MOF and are again comparable to the bulk sample. The preferred orientation, with the conducting planes all lying parallel, and the good contacts between the particles, both promise favourable pathways for charge carriers and thus should support high electrical conductivity. Additionally, with the preferred orientation of the primary particles, the pore channels are arranged perpendicular to the coating surface. This in turn leads to an easily accessible pore system as it is preferred in applications like sensing, leading to strong and fast responses.
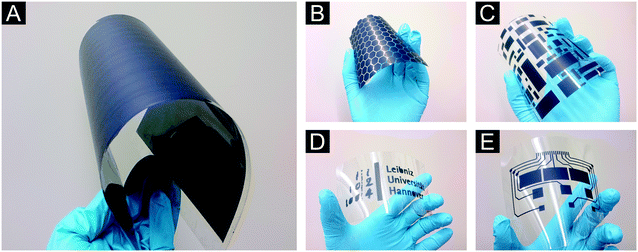
To extend the range of possible applications of our Cu3hhtp2-MOF nanoplatelets to flexible electronics and also to show the scalability of our approach to larger samples, we used thin polycarbonate foils as substrates. By the use of different stencils to generate patterned conducting pathways, we are also able to further demonstrate the flexibility of this method. Using the automated spray-coating setup and large polycarbonate foils in conjunction with these stencils, we fabricated the specimen shown in Fig. 8. The maximum coating area is only limited by the dimension of the automated spray-coating device. Additionally, very small and fine structures or even switch board circuits paths can be generated without adapting the coating parameters. The same coating quality is obtained in all cases (see ESI† Fig. S1D–G). It should be noted that coatings on polycarbonate without using a surfactant are not nearly as homogeneous.
3.5 Electrical and electronic properties
The electrical properties of the Cu3hhtp2-MOF nanoplatelet material were measured on bulk materials as pressed pellets of Soxhlet-extracted samples and on thin film coatings described in the preceding section. The pellets were measured using the van der Pauw setup (Fig. 9A) and give a value for the electrical conductivity of 0.045 S cm−1. This value surpasses all reported measurements of thin films19,20 or pressed pellets6 of the Cu3hhtp2-MOF thus far; for a specimen denoted as single crystal,11 a value of 0.2 S cm−1 was reported.The electrical conductivity of coatings produced from our nanoplatelets on glass and polycarbonate show enhanced electric conductivities of about 0.023 S cm−1 at room temperature. This is in a good agreement with recently reported results of coatings produced with a layer-by-layer method19 and surpasses the values of coatings produced at the liquid–liquid interface.20 In contrast to these approaches, with automated spray-coating we can easily increase the coating area and retain the good quality of the products. To proof the usability of our coatings in flexible electronics, we show in Fig. 9B a shining LED which is contacted by Cu3hhtp2-MOF coatings on polycarbonate; this setup even works when the foil is twisted and bent.
By carrying out temperature-dependent measurements of the electrical conductivity, further information like the activation energy and the fundamental band gap can be gathered (see ESI† Fig. S2). The low thermal activation energy of EA = 0.15 eV and the band gap of Eg = 0.301 eV reveal good prerequisites for the electrical conductivity of the material. In addition, the activation energy is lower here in comparison to EA = 0.24 eV measured on samples prepared by liquid–liquid interface synthesis.20
XPS measurements (see ESI† Fig. S3) carried out on Soxhlet-extracted coatings on gold substrates show no hints of remaining ammonia or other nitrogen species in the sample. In agreement with recently reported measurements20 we can confirm, that only Cu2+ species can be detected. This is in line with the assumption that for neutral Cu3hhtp2 the triphenylene linker molecule is in a semichinon state41 with a loosely bound radical.
3.6 Sensing applications
The novel materials described in this paper, namely the nanoplatelets of Cu3hhtp2-MOF and the coatings derived therefrom, have several properties which favour their application in electric devices, as, e.g., sensors or supercapacitors. These assets are high electrical conductivity, an easily accessible pore system and facile processing for the fabrication of device-suited shapes. Here, we tested the usefulness of this material in sensing applications.Water sorption measurement had shown that the Cu3hhtp2-MOF is rather hydrophilic. As many sensing experiments are influenced by cross-sensitivity to water, we first of all tested the effect of water on the conductivity of this material. For this purpose, a water-saturated argon stream was passed through a measurement cell where a pellet of the MOF is placed between two brass electrodes; a constant potential of 500 mV was applied to the system. Under dry conditions, an Ohmic behaviour of the system can be observed. When a water-saturated stream of argon is passed through the cell, the uptake of water during this process leads to a very strong increase in resistance up to 370% (see ESI† Fig. S4). A strong influence of water on the output signal of a sensing device relying on Cu3hhtp2-MOF has therefore to be taken into account.
For preliminary investigations on the sensing properties of Cu3hhtp2-MOF-based coatings, we placed coated glass slides in a simple sensing setup (Fig. 10). Using this setup, we observed very fast responses to methanol in the gas phase. The process is also reversible. The observed increase in resistance can be cycled reproducibly many times. Similar results were obtained with coated polycarbonate foils (see ESI† Fig. S5), showing the suitability also for flexible sensing devices. Note also that the signal intensities remain constant (which is also true for further cycles) so that we can conclude that the material is stable under the conditions of the sensing setup. Nevertheless, in addition we exposed the material also to liquid methanol for 24 h. Under these much harsher conditions, some degradation can be observed (see ESI† Fig. S8). Nevertheless, we can verify that the coatings produced from the nanoplatelet dispersion are very attractive for sensing applications.
4. Conclusions
In this work we investigated the influence of additives used within a water-based synthesis system for the preparation of Cu3hhtp2-MOF on the crystallinity and morphology of the products. With ammonia acting as a coordination modulator, the Cu3hhtp2-MOF can be synthesized phase-pure and with a flake-like morphology on a large scale. These nanoparticles were thoroughly characterized. Conductivity measurements on pressed pellets reveal a conductivity of 0.045 S cm−1, an enhanced value in comparison to recent reports.6,19,20 Noteworthy, we find that the thermal stability of the Cu3hhtp2-MOF is limited to ca. 80 °C in synthetic air (O2/Ar: 20/80). The flakes or platelets have diameters in the micrometer range and very small thicknesses of about 7.5–10.4 nm, corresponding to ca. 24–33 stacked layers. These nanoplatelets form very stable dispersions in water, opening interesting pathways for fabrication and shaping.Using these aqueous dispersions, coatings of the novel nanoplatelets were prepared on glass slides and on polycarbonate foils by spray-coating, allowing also patterned deposition of the MOF coatings. Several properties of these coatings make them promising for sensing applications: i. good interparticle contacts, leading to enhanced electrical conductivity; ii. high degree of texture within the coatings with the nanoplatelets lying parallel to the substrate surface, thus exposing many pore entrances offering short diffusion pathways. In sensors, these properties can lead to well-defined signals and fast response and regeneration times. The sensing behaviour of the coatings was tested in a preliminary set-up. Upon offering methanol in a gas stream, reproducible signals with fast response and recovery were observed.
In conclusion, this work shows that apart from the composition and the structure of a MOF, crystal morphology is an important parameter with regard to shaping a MOF for a certain application. We expect the nanoplatelets and the coatings described here to be also suitable for other applications in MOF-based flexible electronic devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The TEM investigations were performed at the Laboratory of Nano and Quantum Engineering (LNQE). The XPS investigations were performed at the Institut für Festkörperphysik of the Leibniz University Hannover (Group Prof. H. Pfnür).Notes and references
- A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. El Gabaly, H. P. Yoon, F. Léonard and M. D. Allendorf, Science, 2013, 343, 66 CrossRef PubMed.
- T. C. Wang, I. Hod, C. O. Audu, N. A. Vermeulen and S. T. Nguyen, ACS Appl. Mater. Interfaces, 2017, 9, 12584 CrossRef CAS PubMed.
- R. Sakamoto, T. Kambe, S. Tsukada, K. Takada, K. Hoshiko, Y. Kitagawa, M. Okumura and H. Nishihara, Inorg. Chem., 2013, 52, 7411–7416 CrossRef CAS PubMed.
- X. Huang, P. Sheng, Z. Tu, F. Zhang, J. Wang, H. Geng, Y. Zou, C. Di, Y. Yi, Y. Sun, W. Xu and D. Zhu, Nat. Commun., 2015, 6, 7408 CrossRef CAS PubMed.
- A. J. Clough, J. W. Yoo, M. H. Mecklenburg and S. C. Marinescu, J. Am. Chem. Soc., 2015, 137, 118–121 CrossRef CAS PubMed.
- M. G. Campbell, S. F. Liu, T. M. Swager and M. Dincă, J. Am. Chem. Soc., 2015, 137, 13780–13783 CrossRef CAS PubMed.
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager and M. Dincă, Angew. Chem., Int. Ed., 2015, 54, 4349–4352 CrossRef CAS PubMed.
- D. Sheberla, L. Sun, M. A. Blood-Forsythe, S. Er, C. R. Wade, C. K. Brozek, A. Aspuru-Guzik and M. Dincă, J. Am. Chem. Soc., 2014, 136, 8859–8862 CrossRef CAS PubMed.
- D. Sheberla, J. C. Bachman, J. S. Elias, C.-J. Sun, Y. Shao-Horn and M. Dincă, Nat. Mater., 2017, 16, 220–224 CrossRef CAS PubMed.
- J. Cui and Z. Xu, Chem. Commun., 2014, 50, 3986–3988 RSC.
- M. Hmadeh, Z. Lu, Z. Liu, F. Gándara, H. Furukawa, S. Wan, V. Augustyn, R. Chang, L. Liao, F. Zhou, E. Perre, V. Ozolins, K. Suenaga, X. Duan, B. Dunn, Y. Yamamto, O. Terasaki and O. M. Yaghi, Chem. Mater., 2012, 24, 3511–3513 CrossRef CAS.
- L. Sun, B. Liao, D. Sheberla, D. Kraemer, J. Zhou, E. A. Stach, D. Zakharov, V. Stavila, A. A. Talin, Y. Ge, M. D. Allendorf, G. Chen, F. Léonard and M. Dincă, Joule, 2017, 1, 168–177 CrossRef CAS.
- R. Dong, M. Pfeffermann, H. Liang, Z. Zheng, X. Zhu, J. Zhang and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 12058–12063 CrossRef CAS PubMed.
- L. Wang, D. C. Tranca, J. Zhang, Y. Qi, S. Sfaelou, T. Zhang, R. Dong, X. Zhuang, Z. Zheng and G. Seifert, Small, 2017, 13, 1–8 Search PubMed.
- E. M. Miner, T. Fukushima, D. Sheberla, L. Sun, Y. Surendranath and M. Dincă, Nat. Commun., 2016, 7, 10942 CrossRef CAS PubMed.
- D. Y. Tie and Z. Chen, RSC Adv., 2015, 5, 55186–55190 RSC.
- S. Chen, J. Dai and X. C. Zeng, Phys. Chem. Chem. Phys., 2015, 17, 5954–5958 RSC.
- M. E. Foster, K. Sohlberg, C. D. Spataru and M. D. Allendorf, J. Phys. Chem. C, 2016, 120, 15001–15008 CrossRef CAS.
- M.-S. Yao, X.-J. Lv, Z.-H. Fu, W.-H. Li, W.-H. Deng, G.-D. Wu and G. Xu, Angew. Chem., Int. Ed., 2017, 3, 16510–16514 CrossRef PubMed.
- V. Rubio-Giménez, M. Galbiati, J. Castells-Gil, N. Almora-Barrios, J. Navarro-Sánchez, G. Escorcia-Ariza, M. Mattera, T. Arnold, J. Rawle, S. Tatay, E. Coronado and C. Martí-Gastaldo, Adv. Mater., 2018, 30, 1704291 CrossRef PubMed.
- D. Feng, T. Lei, M. R. Lukatskaya, J. Park, Z. Huang, M. Lee, L. Shaw, S. Chen, A. A. Yakovenko, A. Kulkarni, J. Xiao, K. Fredrickson, J. B. Tok, X. Zou, Y. Cui and Z. Bao, Nat. Energy, 2018, 3, 30–36 CrossRef CAS.
- J. H. Dou, L. Sun, Y. Ge, W. Li, C. H. Hendon, J. Li, S. Gul, J. Yano, E. A. Stach and M. Dincă, J. Am. Chem. Soc., 2017, 139, 13608–13611 CrossRef CAS PubMed.
- T. Tsuruoka, S. Furukawa, Y. Takashima, K. Yoshida, S. Isoda and S. Kitagawa, Angew. Chem., Int. Ed., 2009, 48, 4739–4743 CrossRef CAS PubMed.
- S. Diring, S. Furukawa, Y. Takashima, T. Tsuruoka and S. Kitagawa, Chem. Mater., 2010, 22, 4531–4538 CrossRef CAS.
- A. Umemura, S. Diring, S. Furukawa, H. Uehara, T. Tsuruoka and S. Kitagawa, J. Am. Chem. Soc., 2011, 133, 15506–15513 CrossRef CAS PubMed.
- G. Zahn, P. Zerner, J. Lippke, F. L. Kempf, S. Lilienthal, C. A. Schröder, A. M. Schneider and P. Behrens, CrystEngComm, 2014, 16, 9198–9207 RSC.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem. – Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- O. V. Gutov, S. Molina, E. C. Escudero-Adán and A. Shafir, Chem. – Eur. J., 2016, 22, 13582–13587 CrossRef CAS PubMed.
- G. Zahn, H. A. Schulze, J. Lippke, S. König, U. Sazama, M. Fröba and P. Behrens, Microporous Mesoporous Mater., 2015, 203, 186–194 CrossRef CAS.
- A. Schaate, S. Dühnen, G. Platz, S. Lilienthal, A. M. Schneider and P. Behrens, Eur. J. Inorg. Chem., 2012, 790–796 CrossRef CAS.
- M. E. Schweinefuß, S. Springer, I. A. Baburin, T. Hikov, K. Huber, S. Leoni and M. Wiebcke, Dalton Trans., 2014, 43, 3528–3536 RSC.
- S. Springer, I. A. Baburin, T. Heinemeyer, J. G. Schiffmann, L. van Wüllen, S. Leoni and M. Wiebcke, CrystEngComm, 2016, 18, 2477–2489 RSC.
- S. Springer, A. Satalov, J. Lippke and M. Wiebcke, Microporous Mesoporous Mater., 2014, 216, 161–170 CrossRef.
- L. J. van der Pauw, Philips Tech. Rev., 1958, 20, 220–224 Search PubMed.
- A. A. Ramadan, R. D. Gould and A. Ashour, Thin Solid Films, 1994, 239, 272–275 CrossRef CAS.
- V. A. Drits and C. Tchoubar, X-Ray Diffraction by Disordered Lamellar Structures, Springer, Berlin Heidelberg, 1990 Search PubMed.
- M. Fröba, P. Behrens, H. P. Eickhoff and W. Metz, Carbon, 1991, 29, 909–913 CrossRef.
- Z. Q. Li, C. J. Lu, Z. P. Xia, Y. Zhou and Z. Luo, Carbon, 2007, 45, 1686–1695 CrossRef CAS.
- H. Jagodzinski, Acta Crystallogr., 1949, 2, 201–207 CrossRef CAS.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- C. S. Grange, A. J. H. M. Meijer and M. D. Ward, Dalton Trans., 2010, 39, 200–211 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ce01264d |
| This journal is © The Royal Society of Chemistry 2018 |