Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvatomorphism of Reichardt's dye†
Sarah J.
Pike
 ,
Andrew D.
Bond
,
Andrew D.
Bond
 and
Christopher A.
Hunter
and
Christopher A.
Hunter
 *
*
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: herchelsmith.orgchem@ch.cam.ac.uk
First published on 16th May 2018
Abstract
A systematic study of the influence of solvent on the crystal packing behaviour of Reichardt's dye demonstrates that the structure of the assembly formed in the solid state depends on the nature of the solvent–solute interactions present in the solution phase. Apolar aprotic solvents lead to solvates with a hexagonal channel topology, but this supramolecular assembly is perturbed by the presence of aromatic or polar protic solvents.
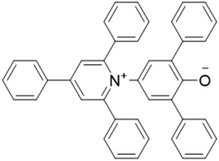
Solvatomorphism is important in determining the properties of molecular solids, including solubility, physical and chemical stability.1,2 Consequently, solvatomorphism plays a key role in a number of fields including pharmaceuticals, pigment chemistry and materials science.1–3 In supramolecular chemistry, the role of solvent is crucial in controlling both the formation of self-assembled structures and host–guest chemistry.4–6 Reichardt's dye 1 (Fig. 1) is a pyridinium N-phenoxide betaine that was used to develop the ET(30) solvent polarity scale.7 The solution behaviour of 1 has been the subject of intense interest, due to applications in thermochromism,8 solvatochromism,9 piezochromism10 and halochromism,11 and 1 has been employed to study the properties of micellar environments and binary liquid mixtures.7,11 However, reports on the solid-state behaviour of 1 are rare.12–14
The high solubility of 1 in a wide range of solvents makes it ideal for investigating the influence of crystallisation solvent on packing in the solid state. The only crystal structures of 1 that have been reported to date are the solvates of ethanol and iso-propanol, which are isostructural.12 Crystal structures of the protonated form of Reichardt's dye have also been obtained by crystallisation in the presence of nitric and sulphuric acid.14 Here, we report a systematic study of solvatomorphism of 1 based on crystallisation experiments in a wide range of different solvent systems.
Two crystallization methods were employed for the growth of single crystals: (i) slow evaporation of a solution of 1; (ii) vapour diffusion of n-hexane or diethyl ether into a solution of 1. Single crystals suitable for X-ray diffraction were obtained from the following solvents: (i) apolar aprotic (chloroform, dichloromethane, 4-methylanisole and chlorobenzene); (ii) polar aprotic (ethyl acetate, acetonitrile, 1,4-dioxane and acetone); (iii) polar protic (methanol, ethylene glycol and 1-octanol). Six distinct new packing arrangements were found (Table 1). Crystallisation experiments in pyridine, toluene and DMSO did not yield single crystals suitable for structure determination.
| Crystallisation solvent | Crystal appearance | Space group | Packing arrangement |
|---|---|---|---|
| Chloroform/Et2O | Red (plate) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
7.2 Å channel |
| Dichloromethane/Et2O | Purple (block) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
7.2 Å channel |
| Acetonitrile/Et2O | Red (block) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
7.2 Å channel |
| 1-Octanol/Et2O | Green (block) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
7.2 Å channel |
| Ethyl acetate | Green (block) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
7.2 Å channel |
| 1,4-Dioxane | Green (lath) | C2/c | 4.6 Å channel |
| Acetone | Green (block) | P21/n | No channel |
| Chlorobenzene | Green (plate) | P21/n | 4.0 Å channel |
| 4-Methylanisole | Green (plate) | P21/n | 4.0 Å channel |
| Methanol | Green (block) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
No channel |
| Ethylene glycol | Red (lath) | P21/c | No channel |
| Chloroform/H2O | Green (lath) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
No channel |
Crystallisation by vapour diffusion of diethyl ether into solutions of 1 in chloroform, dichloromethane, acetonitrile, and 1-octanol all gave the same hexagonal topology with channels running along the c axis (Fig. 2). The same structure was obtained by slow evaporation from ethyl acetate. The channels have a minimum internal diameter of 7.2 Å (ref. 15) and are filled with disordered solvent molecules.16 All of the crystal structures with the 7.2 Å channel packing arrangement were very similar. It was not possible to identify the solvent unambiguously from the diffuse electron density, but the largest density was found consistently to lie close to the surface of the channels (i.e. close to the surface of the cylindrical mesh defined in Fig. 2). Applying the SQUEEZE procedure within PLATON16 indicated a total electron count per unit cell typically close to 1200.
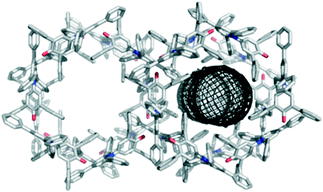 | ||
| Fig. 2 Packing arrangement in crystals obtained by vapour diffusion of diethyl ether into a solution of 1 in chloroform displaying the 7.2 Å hexagonal channel structure as viewed along the c axis. The channels contain disordered solvent,16 and the dimensions of the channel are illustrated using the mesh image calculated using CAVER (right).15 | ||
The porous packing arrangement is built from one key intermolecular interaction, in which the O atom of the C–O− group accepts three C–H⋯O H-bonds from aromatic protons,18 and one phenyl ring makes an aromatic stacking interaction with the pyridinium ring (see ESI† for details).17 The interaction introduces a 120° angle between the central cores (along the C2 axis) of the molecules. Each molecule makes two sets of such interactions, symmetrically related by a 2-fold rotation about its central core, producing a “trigonal node” that enables formation of the hexagonal structure. The pyridinium ring of each molecule is sandwiched between two phenyl rings from two different dye molecules.
The hexagonal packing arrangement is surprisingly robust. When the solvent was allowed to evaporate entirely following crystallisation from chloroform/Et2O, the integrity of the crystals was maintained, and the same crystal structure was obtained, except that the electron density due to solvent in the channels was effectively absent. This is the first example of a solid-state structure of Reichardt's dye that does not contain solvent molecules. Interestingly, the colour of the crystals changed from red to green when the solvent was evaporated. Table 1 shows that three different colours were observed for crystals that all have the hexagonal packing arrangement shown in Fig. 2. Reichardt's dye 1 is solvatochromic, and clearly the sensitivity of the absorption spectrum to environment also extends to the solid state.13b
Solvents that compete with the interactions stabilising the hexagonal channel structure shown in Fig. 2 would be expected to promote different crystalline forms of 1. A clear example was found for the aromatic solvents chlorobenzene and 4-methylanisole, which produce isostructural solvate crystals. Intermolecular interactions between the dye molecules in the solvate structure are identical to those in the hexagonal channel structure, and the structures retain 1-D similarity with the O atom of the C–O− group accepting C–H⋯O H-bonds from aromatic protons18 (see ESI†). However, the symmetry-related intermolecular interaction that produces the trigonal node in the hexagonal structure is not present. Instead, the dye molecules lie in layers that are stacked to generate channels of internal diameter 4.0 Å, which are filled with solvent molecules (Fig. 3). For chlorobenzene, the solvent molecules are disordered across inversion centres in clearly defined sites, one of which is sandwiched between the pyridinium rings of dye molecules. This interaction is clearly a competitor to the phenyl-pyridinium aromatic interactions that build the trigonal nodes of the hexagonal structure. For the 4-methylanisole solvate, it is harder to unravel the observed crystallographic disorder, but the electron density within the channels indicates that the solvent molecules adopt positions comparable to those of chlorobenzene.
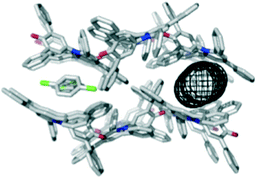 | ||
| Fig. 3 Packing arrangement in single crystals of 1 obtained by slow evaporation of chlorobenzene displaying the 4.0 Å channel structure as viewed along the a axis. The channels are filled with disordered solvent molecules, and the dimensions of the channel are illustrated using the mesh image calculated using CAVER (right).15 An identical structure is formed with 4-methylanisole. | ||
The polar aprotic solvents 1,4-dioxane and acetone gave different solvate crystal structures that are built from common 2-D sections. The principal intermolecular interaction between dye molecules is closely related to that in the hexagonal channel structure, retaining the C–H⋯O H-bonds from the phenyl rings to the C–O− group. However, the relative orientation of one molecule is mirrored compared to the arrangement in the hexagonal structure, and the accompanying aromatic interaction between the phenyl and pyridinium rings is not present (see ESI†). The packing arrangement illustrated in Fig. 4 shows 1,4-dioxane molecules occupying channels with a minimum diameter of 4.6 Å.15 The dioxane molecules sit directly above the pyridinium rings of the dye molecules, with a lone pair on O clearly directed towards N+. The acetone solvate contains dye molecules forming identical 2-D sections, but stacked to form a more condensed structure (where the bumps in each 2-D section fit into the hollows of its neighbour; see ESI†). The acetone molecules again cap the pyridinium ring in each dye molecule via O⋯N+ interactions, but they occupy discrete pockets in the structure rather than continuous channels.
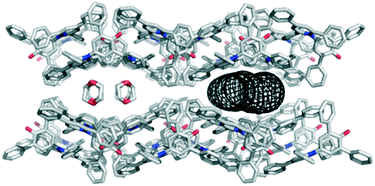 | ||
| Fig. 4 Packing arrangement in single crystals of 1 obtained by vapour diffusion of hexane into a solution of 1 in 1,4-dioxane displaying the 4.6 Å channel structure as viewed along the c axis. The channels are filled with disordered solvent molecules, and the dimensions of the channel are illustrated using the mesh image calculated using CAVER (right).15 | ||
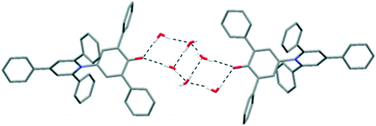
When polar protic solvents were used for crystallisation, the resulting crystal structures contained intermolecular O–H⋯O H-bonds to the C–O− group of 1. This does not necessarily prevent the C–O− group from interacting with other dye molecules in the manner described previously. Indeed, the isostructural ethanol and isopropanol solvates12 contain the same 2-D sections that are seen in the 1,4-dioxane and acetone solvates, and the alcohol solvate structures are very closely comparable to the acetone solvate. The difference arises in the relative positions of adjacent 2-D sections of dye molecules, with the alcohol molecules being accommodated in pockets constructed between the pendant phenyl groups; there are no interactions between the solvent and the pyridinium rings as seen for dioxane and acetone. The pyridinium rings are instead involved in a centrosymmetric pairing of dye molecules (see ESI†).
Further structures including O–H⋯O H-bonds were obtained when crystals were grown by vapour diffusion of hexane into a solution of 1 in ethylene glycol or chloroform. In the latter case, adventitious water was incorporated into the structure, and the unit cell contains four molecules of 1, three molecules of chloroform and four molecules of water. In the ethylene glycol solvate, the solvent molecules crowd around the C–O− group, so there are no interactions between dye molecules of the type seen in the hexagonal structure (Fig. 5). Similarly, in the CHCl3/H2O solvate, one molecule of 1 is also involved in an intermolecular C–H⋯O H-bond with a chloroform molecule (see ESI†).19 Two molecules of 1 form intermolecular H-bonds with isolated water molecules and the fourth molecule of 1 interacts with a cluster of six water molecules (Fig. 6).20 In this structure, the CHCl3 molecules also approach the pyridinium ring of 1via Cl⋯N+ interactions, as seen for dioxane and acetone. A closely-related structure was obtained by crystallisation from methanol. In this case, the X-ray structure clearly resolves the O atoms of the solvent molecules, but there is no indication of any electron density corresponding to the Me groups; thus, the structure appears to contain only water (see ESI†).
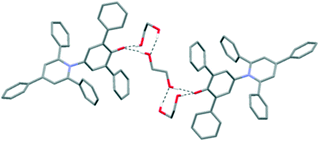 | ||
| Fig. 5 Packing arrangement in single crystals of 1 grown by vapour diffusion of hexane into a solution of 1 in ethylene glycol. Intermolecular H-bonds are shown as dotted black lines. | ||
Reichardt's dye clearly has a rich solid-state chemistry. The rugged topology of the molecule makes packing difficult and results in crystal structures that must incorporate solvents in order to fill space. The packing arrangement depends on the nature of the solvent, leading to extensive solvatomorphism. The frequently obtained crystal structure has 7.2 Å diameter hexagonal channels that are occupied by crystallographically-diffuse solvent. The structure is stabilised by aromatic interactions and C–H⋯O H-bonds between dye molecules, and is robust to removal of the solvent. Aromatic or polar protic solvents that can compete with these interactions generally lead to different packing arrangements. Six different structures were obtained by crystallisation from different solvents, including two other channel topologies of different diameters (4.6 and 4.0 Å). The results suggest that solvent–solute interactions that are present in the solution phase can be used to influence crystal packing in the solid state.
This work was funded by the EPSRC.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are grateful to Dr. Mark Williamson for helpful discussions about calculation of the channel dimensions.Notes and references
- E. H. Lee, Asian J. Pharm. Sci., 2014, 9, 163 CrossRef.
- H. G. Brittain, J. Pharm. Sci., 2011, 4, 1260 CrossRef PubMed.
- G. Kumar and R. Gupta, Chem. Soc. Rev., 2013, 42, 9403 RSC.
- C. B. Aakeröy, N. R. Champness and C. Janiak, CrystEngComm, 2010, 12, 22 RSC.
- (a) C.-P. Li and M. Du, Chem. Commun., 2011, 47, 5958 RSC; (b) M. Rancan and L. Armelao, Chem. Commun., 2015, 51, 12947 RSC; (c) P. A. Gale, M. E. Light and R. Quesada, Chem. Commun., 2005, 2232 Search PubMed.
- (a) K. Rissanen, Chem. Soc. Rev., 2017, 46, 2638 RSC; (b) L. Mandelcorn, Chem. Rev., 1959, 59, 827 CrossRef.
- C. Reichardt, Solvent and Solvent Effects in Organic Chemistry, Wiley-VCH, Weinheim, 2003 Search PubMed.
- (a) G. U. Bublitz and S. G. Boxer, J. Am. Chem. Soc., 1998, 120, 3988 CrossRef; (b) E. B. Tada, P. L. Silva and O. A. El Seoud, Phys. Chem. Chem. Phys., 2003, 5, 5378 RSC.
- (a) G. Machado, R. I. Stock and C. Reichardt, Chem. Rev., 2014, 114, 10429–10475 CrossRef PubMed; (b) C. Reichardt, Pure Appl. Chem., 2008, 80, 1415 CrossRef.
- W. S. Hammack, D. N. Hendrickson and H. G. Drickamer, J. Phys. Chem., 1989, 93, 3483 CrossRef.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef.
- S. Kurjatschij, W. Seichter and E. Weber, New J. Chem., 2010, 34, 1465 RSC.
- (a) E.-Y. Cho, J.-M. Gu, I.-H. Choi, W.-S. Kim, Y.-K. Hwang, S. Huh, S.-J. Kim and Y. Kim, Cryst. Growth Des., 2014, 14, 5026–5033 CrossRef; (b) K. Ichimura, A. Funabiki and K. Aoli, Chem. Lett., 2010, 39, 586 CrossRef.
- J. Baran, A. J. Barnes, M. Drozd, J. Janczak, H. Ratajczak and M. Sledz, J. Mol. Struct., 2001, 598, 117 CrossRef.
- CAVER3.0 was used to determine the narrowest point along the channel, termed the bottleneck, and the internal diameter is quoted as twice the bottleneck radius: E. Chovancová, A. Pavelka, P. Beneŝ, O. Strnad, J. Brezovsky, B. Kozlikova, A. Gora, V. Sustr, M. Klvana, P. Medek, L. Biedermannova, J. Sochor and J. Damborsky, PLoS Comput. Biol., 2012, 8, 10 Search PubMed.
- PLATON SQUEEZE was used to calculate the disordered solvent contribution to the structure; see A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9 CrossRef PubMed.
- M. Egli, V. Tereshko, G. N. Mushudov, R. Sunushvilli, X. Liu and F. D. Lewis, J. Am. Chem. Soc., 2003, 125, 10842 CrossRef PubMed.
- S. Horowitz and R. C. Trievel, J. Biol. Chem., 2012, 287, 41576–41582 CrossRef PubMed.
- N. Goutev and H. Matsuura, J. Phys. Chem. A, 2001, 105, 4741–4748 CrossRef.
- (a) G. K. Ghosh and P. K. Bharadwaj, Inorg. Chem., 2012, 43, 5180 CrossRef PubMed; (b) N. S. Oxtoby, A. J. Blake, N. R. Champness and C. Wilson, Chem. – Eur. J., 2005, 11, 4643 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallography data available. CCDC 1827182–1827193. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ce00480c |
| This journal is © The Royal Society of Chemistry 2018 |