 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Synthesis of figure-of-eight helical bisBODIPY macrocycles and their chiroptical properties
Makoto
Saikawa
,
Takashi
Nakamura
,
Junji
Uchida
,
Masaki
Yamamura
and
Tatsuya
Nabeshima
*
Graduate School of Pure and Applied Sciences and Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8571, Japan. E-mail: nabesima@chem.tsukuba.ac.jp
First published on 3rd September 2018
Abstract
Correction for ‘Synthesis of figure-of-eight helical bisBODIPY macrocycles and their chiroptical properties’ by Makoto Saikawa et al., Chem. Commun., 2016, 52, 10727–10730.
The authors regret that there were some errors in the original article. The authors reported that the mononuclear boron complex [H31B] and dinuclear boron complex [1B2] were synthesized by the reaction of the ligand H61 with phenylboronic acid. However, it was found that the phenylboronic acid that they used was contaminated with boric acid (w/w 33–44%). The use of pure phenylboronic acid (TCI Co., Ltd, purity > 97%) gave the corresponding phenylboronic ester instead of [H31B] or [1B2], which were characterized using 1H NMR and MALDI TOF-MS. Therefore, boric acid was found to be the actual boron source in the synthesis.
The authors have confirmed that [H31B] and [1B2] are obtained using boric acid or trimethyl borate as the boron source. The following parts of the original article should be corrected.
On pages 10727–10728, the paragraph beginning “The boron complexes…” should be replaced by the following:
“The boron complexes of the macrocyclic ligand H61 were synthesized by the reaction with boric acid. Interestingly, the mononuclear boron complex [H31B] and the dinuclear boron complex [1B2] were selectively synthesized depending on the amount of boric acid and the reaction temperature (Scheme 1). The use of a 2.4 molar amount of boric acid against H61 in the reaction at 50 °C for 22.5 h resulted in the formation of [H31B] in 82% yield, while the reaction with an 8.4 molar amount of boric acid in refluxing chloroform for 66 h yielded [1B2] in quantitative yield. The successful isolation of the mononuclear boron complex [H31B] implied that the second complexation reaction is slower than the first one, presumably due to the greater steric hindrance during the second complexation. The reaction of trimethyl borate with H61 also gave [H31B] and [1B2] under similar reaction conditions (see ESI).”
The original Scheme 1 should be replaced by the corrected version presented below.
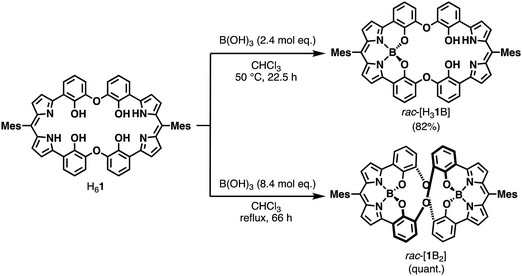 | ||
| Scheme 1 Selective syntheses of the mononuclear boron complex rac-[H31B] and dinuclear boron complex rac-[1B2]. | ||
In the supporting information, Schemes S1 and S2, and the synthetic procedures for [H31B] and [1B2] should be corrected. The characterization data are the same as in the original version. The corrected supporting information is provided separately.
The authors apologize for the inconvenience this may have caused. They acknowledge Dr Ryota Matsuoka and Mr Takumu Noda for their assistance in the production of the correction.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2018 |
