Highlights from the 53rd EUCHEM conference on stereochemistry, Bürgenstock, Switzerland, May 2018
Anat
Milo
*a and
Mónica H.
Pérez Temprano
*b
aDepartment of Chemistry, Ben-Gurion University of the Negev, Beer Sheva, Israel. E-mail: anatmilo@bgu.ac.il
bInstitute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain. E-mail: mperez@iciq.es
First published on 28th August 2018
Abstract
When we first heard of the Bürgenstock conference it was described as a guarded meeting in a remote location, undisturbed by modern diversions, with mysterious customs and a secret handshake. All of these rumors turned out to be completely true. We arrived at an undisclosed location and three young men, who knew our names upon sight, greeted us and gave us an agenda in which we discovered the identity of the speakers, moderators and other participants. We then had a few moments to bask in their glory before being treated to a delicious dose of chemistry. During the banquet, before the first lecture, this year's president, Prof. Ilan Marek, gave the opening address from a balcony reserved only for these meetings, rumors hold that it stands vacant all year in anticipation. He mentioned several of the traditions of the meeting, which we are not allowed to share of course, and described the joy of putting together this year's exciting program with the help of an exceptional organizing committee that included Prof. Cristina Nevado, Prof. Christian Bochet, Dr Fabrice Gallou and Dr Alain De Mesmaeker. He also introduced this year's guest of honor, Prof. Yitzhak Apeloig, and wished the best luck to the current Vice President and future President, Prof. Véronique Gouverneur, not only for preparing the 54th Bürgenstock Conference, but also with her duty this year, maintaining the good weather for the whole week. Although the official title of the conference is the EUCHEM conference on stereochemistry, the topics covered span a broad range of cutting edge chemical transformations and insights, which can appeal to anyone working in chemistry and its interfaces with other disciplines. We will briefly describe each of the talks following one of our favorite citable quotes from these inspiring speakers.
“If chemistry is more than stamp collecting, one would like to understand the ligand dependence of product distribution” Helmut Schwarz
The opening talk was moderated by Prof. Véronique Gouverneur, who introduced the newly elected Foreign Associate of the U.S. National Academy of Sciences, Prof. Helmut Schwarz (Technische Universität Berlin). He gave a masterclass entitled “The methane challenge: a cold approach to a hot problem” where he illustrated not only the challenges, but also the opportunities associated with one of the “Holy Grails in Chemistry”, the activation and functionalization of CH4. Prof. Schwarz showed his efforts of more than 30 years to provide mechanistic insights on the complex landscape of methane activation at the molecular level by merging gas-phase and computational studies.1 He disclosed the possible mechanisms involved in the C–H bond cleavage of methane when using cluster oxides and metal carbides, ranging from hydrogen atom transfer (HAT)2 to proton-coupled electron transfer (PCET),3 and introduced concepts such as oriented external electric fields, spin-crossing and doping effects. At the end of his lecture, Prof. Schwarz gave his perspective on this research and the progress made by quoting Armentrout and Beauchamp's seminal account from 1989 “… it remains a source of frustration to know so much about the reactions of transition-metal ions with hydrocarbons and yet have so many questions remain unanswered”.4
“My personal preference is not to work on reactions we cannot do enantioselectively” James Morken
The main theme of Monday morning's scientific session, moderated by Prof. Mark Gandelman, was stereochemistry. The first lecture titled “Stereoselective catalysis enabled by metal-induced metallate rearrangements” was delivered by Prof. James Morken (Boston College), a world-leader in organoboron chemistry. Prof. Morken started his talk by introducing the main goal of his group, the development of experimentally simple, efficient, enantioselective and sustainable transformations. In this context, he first explained the potential of catalytic enantioselective diboration processes toward transforming alkenes into chiral products.5 The rest of the talk was focused on recent advances the Morken group had achieved in the development of “conjunctive cross-coupling” reactions.6 He coined this term, mentioning he welcomes catchier suggestions, to explain the combination of three simple starting materials, two nucleophilic reagents and an organic electrophile, for the synthesis of versatile chiral organoboronic esters using Pd or Ni catalysts. Although the mechanistic details are still inconclusive, preliminary studies suggest that these reactions, which involve boron “ate” species, proceed through a metal-induced 1,2-metallate rearrangement. Prof. Morken commented that perhaps he should put his name on the chalk board for the questions and discussion session since there are still a lot of unanswered questions regarding nature of the reactive species or the scope of these transformations, and indeed, a vivid discussion ensued.
“The sulfoxide is activated faster than you can say Ilan Marek” Nuno Maulide
The second talk before lunch titled “The simplicity of rearrangements: sulfur and other heteroatoms” was delivered by Prof. Nuno Maulide (University of Vienna) who introduced variations on the theme of umpolung, or polarity reversal, not at a carbonyl carbon, but rather α to it.7 Prof. Maulide started with the activation of amides with N-oxides to generate an enolonium equivalent.8 He then described the perceptive study his group carried out, which allowed them to harness this discrete electrophile to develop a unique route to 1,4-dicarbonyl compounds.9 A twist in the plot came with the idea of exploiting abundant chiral sulfur reagents, notably sulfoxides, to impose chirality transfer between sulfur and carbon stereocenters through carefully orchestrated [3,3] rearrangements.10 As a coda to this topic, he presented an exquisite mechanistic study that enabled the interception of a cationic intermediate leading to a C–C cross-coupling product from which sulfur is expelled as a traceless intermediate.11 Prof. Maulide then allured the audience to attend ESOC 2019 by presenting wonderful pictures of the host city, Vienna.12 The final minutes of the talk were dedicated to processes for selectively opening allylic lactones as an alternative route to useful coupling products.
“My student isolated a radical by silica gel column – it was amazing!” Shigehiro Yamaguchi
The moderator for the afternoon session was Prof. Jérôme Waser and the first talk “Main-group strategy toward NIR and photostable fluorescent dyes for bioimaging” was given by Prof. Yamaguchi (Nagoya University). He started by describing the concept of a mixed lab in the Institute of Transformative Bio-Molecules (ITbM) to which he now belongs, where there are no walls separating between chemistry and biology. In this environment, his group are involved in creating new fluorophore scaffolds to enable one of the super-resolution microscopies, stimulated emission depletion (STED) microscopy. Prof. Yamaguchi identified the main limitation for this technique currently as the poor photo-stability of organic dyes. To address this issue, his group cleverly use main-group elements to produce new scaffolds, for example boron-stabilized stable radicals.13 By doing so they are able to attain a red shift using main group chemistry without the need for extended π systems. Indeed, their phosphine-oxide-substituted rhodamines showed great utility in various types of imaging techniques, such as long term, deep and single molecular imaging.14 Prof. Yamaguchi's group also develop dyes based on phosphole oxide that are stable against photo-bleaching and allow for 3d imaging by running hundreds of multiple scans.15 He mentioned that these new phosphorus containing dyes would have great practical potential as they could be widely utilized in various life-science research fields.
In the afternoon, 5 minute talks were given by JSPS fellows as appetizers for the poster session by Job Boekhoven (Technische Universität München), Charles Diesendruck (Technion – Israel Institute of Technology), Sunkyu Han (KAIST), Mónica Pérez-Temprano (ICIQ) and Marcos Suero (ICIQ).
“When you came in here you thought that we were the superior species, now… you think, perhaps a cabbage can do better!” Andreas Marx
The last talk on Monday by Andreas Marx (Universität Konstanz) titled “Deciphering the information layer beyond the genome sequence” was focused on post translational protein and post transcriptional RNA modifications. He started with an overview of genome sequencing and ubiquitinations, mentioning that biologists invent the best names, for example they called a protein binding motif the RING domain, which stands for really interesting new gene. Prof. Marx described his group's efforts in the targeted generation of all seven homogeneous ubiquitin chains in large quantities.16 One of the systems his group works with is DNA polymerase and he noted that they are especially interested in evolved polymerases, which are useful for modern applications in molecular diagnostics, for example, polymerase chain reactions (PCR).17 In this context, Prof. Marx presented their explorations testing the potential of protein-modified nucleotides as a tool for diagnosing mutations by the naked eye using glycoprotein horseradish peroxidase (HPR) proteins.18 This colorimetric method for the detection of nucleic acids provides a high fidelity readout, since only primers that bind to a specific target sequence will be modified by HRP due to the specificity of DNA polymerases.
“We use small molecules to generate function that is normally reserved for larger molecular systems” Helma Wennemers
The Tuesday morning session was moderated by Prof. Virginie Vidal and the first talk, presented by Prof. Helma Wennemers (ETH Zurich) on “Peptides in asymmetric catalysis and more”, was kicked off with a chiral nanometric Kagome weave.19 This unique material, inspired by traditional Japanese basket making, is based solely on organic units and possesses increased mechanical strength compared to non-woven threads. The repeating units could be described as a planar tiling of stars of David, which is also part of the Israeli flag, so Prof. Wennemers dedicated this part of the talk to the conference president. After this fascinating appetizer, she presented the impressive advances achieved by her group in the field of small molecule catalysis. The first focus was on aldol reactions catalyzed by cinchona alkaloid urea derivatives using fluoromalonic acid half thioesters as activated fluoroacetate equivalents.20 This biomimetic approach is inspired by polyketide synthases and can provide medicinally relevant enantiomerically enriched fluorinated polyketides and statins. Finally, Prof. Wennemers presented a thorough mechanistic study focused on determining the structural origins of selectivity using tailored peptidic catalysts.21 This work highlights the importance of the trans/cis amide bond ratio in determining enantio- and diastereo-selectivity and enabled a highly selective gram scale reaction at an extremely low organocatalyst loading of 0.05 mol%.
“If you really want to get confused, come to biology” Ashraf Brik
The second Tuesday morning lecture was delivered by Prof. Ashraf Brik (Technion – Israel Institute of Technology) who spoke about “Organic chemistry applied to synthetic proteins: the case of ubiquitination and deubiquitination”. Prof. Brik started his talk with memories from his previous participation in the Bürgenstock conference, in 2011, as a JSP Fellow who gave a five minute talk. Now, in 2018, he was back as one of the plenary lectures to disclose his journey of one decade in organic chemistry for protein synthesis. In particular, he focused on the development of chemical strategies to access reversible post-translational protein modifications by ubiquitin. The importance of the ubiquitin-mediated protein degradation was recognized in 2004, when the Nobel Prize in Chemistry was awarded to Ciechanover, Hershko and Rose for their groundbreaking discoveries in this field. In the first part of his talk, Brik showed the challenges, limitations and opportunities in studying ubiquitination.22 Next, he described how his group resourcefully identified the potential of palladium chemistry in protein synthesis,23 strategies for installing ubiquitin chains linked to protein substrates24 or key aspects on the mono-ubiquitination of histone H2B by the SAGA coactivator.25
Tuesday afternoon was dedicated to excursions in the area, spending time with old and new friends, intense scientific discussions over Feldschlösschen beer, and as tradition has it, yodelling. Everyone knew that there was a special concert after dinner but the players and score were kept secret. We only received the following flyer with some mysterious notes, a cryptic message “When Classic meets Jazz…” and odd looking chemical structures. Once we entered the hall a grand piano was awaiting us and a feeling of excitement soaked the Mythen Saal. Prof. Nuno Maulide then presented a wonderful lecture on the tight relationship between art and science accompanied by masterfully played piano pieces to punctuate his points. He played short pieces by Schubert, Chopin, Debussy and Bach while talking about similarities between being a scientist and an artist. For example, to illustrate how fashion can change he played Bach's Jesu, meine Freude and showed that the corale melody by Johann Crüger that was famous in Bach's time is relatively obscure today, while Bach's variations remain well known today. The similarity wasn’t lost on the audience when Prof. Maulide showed a relatively obscure paper from 1968 by one Richard Heck.26
After the Classic came Jazz. Prof. Amnon Stanger (Technion) started off by explaining that he never formally learnt to play the piano, but then dazzled the audience with his jazz interpretation of a famous children's song, The Beatles’ Yesterday and Pink Floyd's Atom Heart Mother suite. For the latter, one could easily imagine the famous sound of a motorcycle passing by after the first section called “father's shout”. Finally, Dr Renana Gershoni-Poranne (ETH), Prof. Stanger's former PhD student, who is not only a talented computational chemist, but also a professionally trained soprano, joined him for Cat Steven's Morning has Broken, Elton John's My Song, John Denver's Leaving on a Jet Plane and Leonard Cohen's Hallelujah.
“My group's goal is taming Nickel, the spirited horse of the periodic table” Rubén Martín
The Wednesday morning session was moderated by Prof. Belén Martín-Matute, and the first lecture was presented by Prof. Rubén Martín (ICIQ – Institute of Chemical Research of Catalonia) titled “Turning simplicity into complexity with Ni catalysis: from comprehension to prediction”. During his talk, Prof. Martín demonstrated his group's efforts in harnessing nickel catalysis for the development of carboxylation protocols using carbon dioxide as a renewable C1 feedstock. Prof. Martin initially focused on Ni-catalyzed carboxylation of organic (pseudo)halides with CO2 through “abnormal” cross-electrophile coupling reactions.27 By a detailed analysis of the mechanism and product distribution, his group ingeniously took a parasitic reaction, the β-hydride elimination by nickel catalysts, and turned it into a strategic advantage. Through a tunable and controllable chain-walking route they were able to promote a site-selective remote sp3 C–H carboxylation of aliphatic hydrocarbons.28 In the final part of the lecture, Martín showed exciting results of catalytic carboxylation of unsaturated hydrocarbons under mild conditions utilizing H2O as the hydride source29 and involving multiple catalytic CO2 insertions into 1,3-dienes.30 The following discussion was focused on the requirements from ligands employed in these transformations and the chain-walking strategy for remote functionalization.
“I’m sorry, I have 20 more structures, but the slide was too small” Ang Li
The second lecture on Wednesday morning was a tour de force of natural product synthesis titled “Total synthesis of polycyclic natural products” and presented by Prof. Ang Li (Shanghai Institute of Organic Chemistry). The Li group view the challenge of natural product total synthesis as a driving force in the development of new strategies and methods in organic chemistry. Prof. Li first presented his group's efforts in the synthesis of Daphniphyllum alkaloids. These alkaloids can be extracted from evergreen plants native to Asia, whose roots and leaves are a source of Chinese herbal medicine. Structurally, the alkaloids usually possess a bridged and fused hexa- or penta-cyclic scaffold riddled with continuous stereogenic centers. The Li group employed a divergent synthesis strategy by identifying a common 6,n,5-bridged tricycle structural motif which allowed them to access multiple members of this alkaloid family.31 Prof. Li finished his talk with a unified and bioinspired Prins-type oxidative cyclization strategy that his group developed to achieve the total syntheses of several structurally diverse natural products from the hapalindole indole terpenoid family.32
“When you write it that way you have carefully hidden all the important information” Odile Eisenstein
The moderator for the Wednesday afternoon and evening sessions was Prof. Franziska Schoenebeck and the first talk, presented by Prof. Odile Eisenstein, was entitled “Analyzing reaction pathways: is there an alternative to energy?” She started off the talk with the provocative questions: can there be an alternative to energy calculations towards understanding and predicting reactivity and is there a hidden message in the NMR spectrum that can explain reactivity and selectivity in chemical transformations. The goal of Prof. Eisenstein's talk was to convey the value of considering the information coming from solid-state NMR spectra not for the sake of prediction, but for the sake of understanding a phenomenon. She demonstrated that, in the context of organometallic complexes for olefin metathesis33 and polymerization,34 it is difficult to predict the reactivity of a catalyst based on the chemical shift of the metal bound carbon species. Furthermore, careful analysis of the chemical shifts revealed that there isn’t a simple correlation between the chemical shift and the charge at carbon of these complexes. However, Prof. Eisenstein demonstrated that the anisotropy of the chemical shift revealed by solid state NMR measurements, known as the chemical shift tensor, could be interpreted in terms of the molecular orbital pattern at the active atom. This pattern served to reveal reactivity tendencies of organometallic complexes.
In the afternoon, 5 minute talks were given as appetizers for the poster session by Roey Amir (Tel-Aviv University), Gabriel Lemcoff (Ben-Gurion University), Amandine Kolleth (Syngenta), Thomas Snaddon (Indiana University) and Christof Sparr (University of Basel).
“Once it turns black you know it's working and that the reaction is finished” Philippe Renaud
After dinner, the evening lecture by Prof. Philippe Renaud (University of Bern) was titled “Extending the scope of radical chain reactions: how far can we go?”. The session began by introducing the challenges associated with radical chemistry and the design of efficient chain reactions. Prof. Renaud described the use of organoboranes as very efficient radical sources. In this regard, his group has explored B-alkylcatecholboranes, easily prepared from olefins via hydroboration with catecholborane derivatives, as a radical source in alkenylation, acylation, cyanation or alkynylation procedures.35 Renaud highlighted the powerful combination of organoboranes and 4-tert-butylcatechol (TBC), a stabilizer and an inhibitor of alkene polymerization, as reducing agents in radical chain reactions.36 The employment of BEt3 and catechol was also applied in a chain-reaction repair process in thiol–ene coupling processes.37 Of particular interest was the elegant strategy described by Renaud for the BEt3-mediated radical deuteration of organic molecules in a selective manner using D2O as deuterium source.38 These results were framed in the context of the recent developments of deuterated drugs, including the recent approval of deutetrabenazine by the Food and Drug Administration (FDA) and open the door to the development of enantioselective radical reactions.
“SF6is also the worst greenhouse gas if you believe in global warming” Jonathan Nitschke
The final day of the Bürgenstock conference, moderated by Prof. Bert Meijer, started with Prof. Jonathan Nitschke, who took the audience into the world of supramolecular capsules with a lecture entitled “Transformative cages and luminous chains: functional materials via subcomponent self-assembly”. Nitschke described the advances by his group in the construction of metal–organic cages induced by the self-assembly of simple building blocks, normally aldehydes and amines, using metal-ion templates.39 Prof. Nitschke's group serve as conductors in the orchestrated binding of these components into metallo-supramolecular structures of different geometries and volumes, from prisms to cyclic catenanes, paying special attention to M4L6 metal–organic hosts.40 The resulting complex architectures can accommodate a wide variety of guests in their central cavities, ranging from pyrophoric reagents such as white phosphorus (P4) to drugs and peptides.41 Among the potential utilities of these functional materials, Nitschke mentioned their use for the selective extraction and separation of valuable chemicals, as light-driven molecular machines, or to design new chemical separations mimicking biological signal transduction pathways.42 The following discussion focused on the principles governing the formation of these complex structures and the possibility to use them to rationally design novel cages.
“Please yank yourself from the molecular world for a moment” Omar Yaghi
The closing lecture of the 53rd Bürgenstock conference was delivered by one of the 2018 laureates of the prestigious Wolf Prize, Prof. Omar M. Yaghi (UC Berkeley), who explained the story behind “The atom, the molecule, and the covalent organic framework”. As alluded to by the title, Prof. Yaghi commenced this talk with concepts proposed by the seminal work of Lewis in 1916 in order to illuminate the chemistry of frameworks and the associated challenges.43 As Nobel laureate Roald Hoffmann pointed out in 1993,44 the field of synthetic organic chemistry with structures that extend into 2-dimensions and 3-dimensions were non-existing at that time. The challenge has been in developing crystallization and linkage chemistry for such synthetic targets. This situation changed in 2005 when the first 2D covalent organic frameworks (COFs),45 followed by the synthesis of the first 3D COFs in 2007 were created by Yaghi and colleagues,46 thereby extending the field of organic chemistry beyond discrete molecules (0D) and polymers (1D) (Fig. 1). COFs are the natural extension from atoms to molecules. He introduced the term reticular chemistry, the linking of molecular building blocks by strong bonds to make crystalline extended structures. These COFs are only based on light elements, such as boron, carbon, nitrogen, oxygen or silicon, linked by strong covalent bonds and attain perfectly controlled pore dimensions.47 To put in perspective the difficulties associated with the early design, synthesis and particularly crystallization of COFs, Yaghi commented that, “he does not remember how many students tried to do COFs, but all of them have fantastic jobs”. Since this pioneering work, the group commanded by Prof. Yaghi has also created innovative strategies for molecular weaving as exemplified by the first molecularly woven material, COF-505.48 COFs have a wide variety of applications in electronics, gas storage/separation processes and catalysis.49
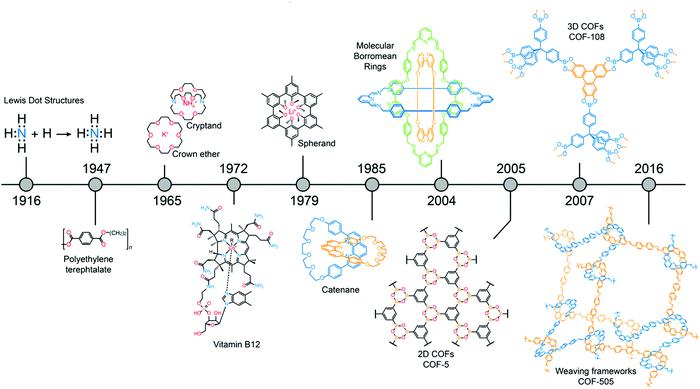 | ||
| Fig. 1 Chronology of advances from Lewis’ original concept of the covalent bond to organic molecules and to linking molecules into frameworks. | ||
The conference ended with the announcement of the new vice president, Prof. Janine Cossy, and two new members of the organizing committee, Prof. Thomas Ward and Prof. Jerome Waser. Our report will end with a comment from the president of the 53rd Bürgenstock conference: “the participants, warm interactions, numerous questions and long evening at the bar… made this meeting a success!” We will add that the tone set by Prof. Marek led to a kind and inclusive atmosphere and we are certain that the connections forged at this wonderful meeting will bear fruit for years to come.
References
- (a) D. Schröder, S. Shaik and H. Schwarz, Acc. Chem. Res., 2000, 33, 139 CrossRef; (b) H. Schwarz, Angew. Chem., Int. Ed., 2011, 50, 10096 CrossRef PubMed; (c) H. Schwarz, Angew. Chem., Int. Ed., 2015, 54, 10090 CrossRef PubMed; (d) H. Schwarz, S. Shaik and J. Li, J. Am. Chem. Soc., 2017, 139, 17201 CrossRef PubMed.
- N. Dietl, M. Schlangen and H. Schwarz, Angew. Chem., Int. Ed., 2012, 51, 5544 CrossRef PubMed.
- J. Li, S. Zhou, J. Zhang, M. Schlangen, T. Weiske, D. Usharani, S. Shaik and H. Schwarz, J. Am. Chem. Soc., 2016, 138, 7973 CrossRef PubMed.
- P. B. Armentrout and J. L. Beauchamp, Acc. Chem. Res., 1989, 22, 315 CrossRef.
- (a) S. N. Mlynarski, C. H. Schuster and J. P. Morken, Nature, 2014, 505, 386 CrossRef PubMed; (b) L. Yan, Y. Meng, F. Haeffner, R. M. Leon, M. P. Crockett and J. P. Morken, J. Am. Chem. Soc., 2017, 140, 3663 CrossRef PubMed.
- (a) L. Zhang, G. J. Lovinger, E. K. Edelstein, A. A. Szymaniak, M. P. Chierchia and J. P. Morken, Science, 2016, 351, 70 CrossRef PubMed; (b) G. J. Lovinger, M. D. Aparece and J. P. Morken, J. Am. Chem. Soc., 2017, 139, 3153 CrossRef PubMed; (c) E. K. Edelstein, S. Namirembe and J. P. Morken, J. Am. Chem. Soc., 2017, 139, 5027 CrossRef PubMed; (d) M. Chierchia, C. Law and J. P. Morgen, Angew. Chem., Int. Ed., 2017, 56, 11870 CrossRef PubMed; (e) G. J. Lovinger and J. P. Morken, J. Am. Chem. Soc., 2017, 139, 17293 CrossRef PubMed.
- D. Kaiser, A. de la Torre, S. Shaaban and N. Maulide, Angew. Chem., Int. Ed., 2017, 129, 6015–6019 CrossRef.
- A. de la Torre, D. Kaiser and N. Maulide, J. Am. Chem. Soc., 2017, 139, 6578–6581 CrossRef PubMed.
- D. Kaiser, C. J. Teskey, P. Adler and N. Maulide, J. Am. Chem. Soc., 2017, 139, 16040–16043 CrossRef PubMed.
- D. Kaldre, B. Maryasin, D. Kaiser, O. Gajsek, L. González and N. Maulide, Angew. Chem., Int. Ed., 2017, 129, 2248–2252 CrossRef.
- B. Maryasin, D. Kaldre, R. Galaverna, I. Klose, S. Ruider, M. Drescher, H. Kählig, L. González, M. N. Eberlin, I. D. Jurberg and N. Maulide, Chem. Sci., 2018, 9, 4124–4131 RSC.
- http://esoc2019.conf.tuwien.ac.at/welcome/ .
- T. Kushida, S. Shirai, N. Ando, T. Okamoto, H. Ishii, H. Matsui, M. Yamagishi, T. Uemura, J. Tsurumi, S. Watanabe, J. Takeya and S. Yamaguchi, J. Am. Chem. Soc., 2017, 139, 14336–14339 CrossRef PubMed.
- M. Grzybowski, M. Taki, K. Senda, Y. Sato, T. Ariyoshi, Y. Okada, R. Kawakami, T. Imamura and S. Yamaguchi, Angew. Chem., Int. Ed., 2018, 57, 10137–10141 CrossRef PubMed.
- C. Wang, M. Taki, Y. Sato, A. Fukazawa, T. Higashiyama and S. Yamaguchi, J. Am. Chem. Soc., 2018, 139, 10374–10381 CrossRef PubMed.
- X. Zhao, J. Lutz, E. Höllmüller, M. Scheffner, A. Marx and F. Stengel, Angew. Chem., Int. Ed., 2017, 56, 15764–15768 CrossRef PubMed.
- A. Hottin and A. Marx, Acc. Chem. Res., 2016, 49, 418–427 CrossRef PubMed.
- M. Welter, D. Verga and A. Marx, Angew. Chem., Int. Ed., 2016, 128, 10286–10290 CrossRef.
- U. Lewandowska, W. Zajaczkowski, S. Corra, J. Tanabe, R. Borrmann, E. M. Benetti, S. Stappert, K. Watanabe, N. A. K. Ochs, R. Schaeublin, C. Li, E. Yashima, W. Pisula, K. Müllen and H. Wennemers, Nat. Chem., 2017, 9, 1068 CrossRef PubMed.
- J. Saadi and H. Wennemers, Nat. Chem., 2016, 8, 276–280 CrossRef PubMed.
- T. Schnitzer and H. Wennemers, J. Am. Chem. Soc., 2017, 139, 15356–15362 CrossRef PubMed.
- (a) L. Spasser and A. Brik, Angew. Chem., Int. Ed., 2012, 51, 6840 CrossRef PubMed; (b) S. Bondalapati, M. Jbara and A. Brik, Nat. Chem., 2016, 8, 407 CrossRef PubMed; (c) P. Gopinath, S. Ohayon, M. Nawatha and A. Brik, Chem. Soc. Rev., 2016, 45, 4171 RSC.
- M. Jbara, S. K. Maity and A. Brik, Angew. Chem., Int. Ed., 2017, 56, 10644 CrossRef PubMed.
- (a) M. Haj-Yahya, B. Fauvet, Y. Herman-Bachinsky, M. Hejjaoui, S. N. Bavikar, S. V. Karthikeyan, A. Ciechanover, H. A. Lashuel and A. Brik, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17726 CrossRef PubMed; (b) H. P. Hemantha, S. N. Bavikar, Y. Herman-Bachinsky, N. Haj-Yahya, S. Bondalapati, A. Ciechanover and A. Brik, J. Am. Chem. Soc., 2014, 136, 2665 CrossRef PubMed.
- (a) M. T. Morgan, M. Haj-Yahya, A. E. Ringel, P. Bandi, A. Brik and C. Wolberger, Science, 2016, 351, 725 CrossRef PubMed; (b) M. Jbara, S. K. Maity, M. Morgan, C. Wolberger and A. Brik, Angew. Chem., Int. Ed., 2016, 55, 4972 CrossRef PubMed.
- R. F. Heck, J. Am. Chem. Soc., 1968, 90, 5526–5531 CrossRef.
- (a) M. Börjesson, T. Moragas, D. Gallego and R. Martin, ACS Catal., 2016, 6, 6739 CrossRef PubMed; (b) A. Tortajada, F. Juliá-Hernández, M. Börjesson, T. Moragas and R. Martin, Angew. Chem., Int. Ed., 2018 DOI:10.1002/anie.201803186.
- (a) F. Juliá-Hernández, T. Moragas, J. Cornella and R. Martin, Nature, 2017, 545, 84 CrossRef PubMed; (b) H. Sommer, F. Juliá-Hernández, R. Martín and I. Marek, ACS Cent. Sci., 2018, 4, 153 CrossRef PubMed.
- M. Gaydou, T. Moragas, F. Juliá-Hernández and R. Martin, J. Am. Chem. Soc., 2017, 139, 12161 CrossRef PubMed.
- A. Tortajada, R. Ninokata and R. Martin, J. Am. Chem. Soc., 2018, 140, 2050 CrossRef PubMed.
- (a) W. Zhang, M. Ding, J. Li, Z. Guo, M. Lu, Y. Chen, L. Liu, Y.-H. Shen and A. Li, J. Am. Chem. Soc., 2018, 140, 4227–4231 CrossRef PubMed; (b) Z. Lu, Y. Li, J. Deng and A. Li, Nat. Chem., 2013, 5, 679–684 CrossRef PubMed.
- Z. Lu, M. Yang, P. Chen, X. Xiong and A. Li, Angew. Chem., Int. Ed., 2014, 53, 13840–13844 CrossRef PubMed.
- (a) S. Halbert, C. Copéret, C. Raynaud and O. Eisenstein, J. Am. Chem. Soc., 2016, 138, 2261–2272 CrossRef PubMed; (b) C. P. Gordon, K. Yamamoto, W.-C. Liao, F. Allouche, R. A. Andersen, C. Copéret, C. Raynaud and O. Eisenstein, ACS Cent. Sci., 2017, 5, 759–768 CrossRef PubMed.
- C. P. Gordon, S. Shirase, K. Yamamoto, R. A. Andersen, O. Eisenstein and C. Copéret, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E5867–E5876 CrossRef PubMed.
- A.-P. Schaffner, V. Darmency and P. Renaud, Angew. Chem., Int. Ed., 2006, 45, 5847 CrossRef PubMed.
- G. Villa, G. Povie and P. Renaud, J. Am. Chem. Soc., 2011, 133, 5913 CrossRef PubMed.
- G. Povie, A. T. Tran, D. Bonnaffé, J. Habegger, Z. Y. Hu, C. Le Narvor and P. Renaud, Angew. Chem., Int. Ed., 2014, 53, 3894 CrossRef PubMed.
- V. Soulard, G. Villa, D. P. Vollmar and P. Renaud, J. Am. Chem. Soc., 2018, 140, 155 CrossRef PubMed.
- (a) S. Zarra, D. M. Wood, D. A. Roberts and J. R. Nitschke, Chem. Soc. Rev., 2015, 44, 419 RSC; (b) D. A. Roberts, B. S. Pilgrim and J. R. Nitschke, Chem. Soc. Rev., 2017, 47, 626 RSC.
- (a) C. S. Wood, T. K. Ronson, A. M. Belenguer, J. J. Holstein and J. R. Nitschke, Nat. Chem., 2015, 7, 354 CrossRef PubMed; (b) C. Browne, W. J. Ramsay, T. K. Ronson, J. Medley-Hallam and J. R. Nitschke, Angew. Chem., Int. Ed., 2015, 54, 11122 CrossRef PubMed; (c) M. Kieffer, B. S. Pilgrim, T. K. Ronson, D. A. Roberts, M. Aleksanyan and J. R. Nitschke, J. Am. Chem. Soc., 2016, 138, 6813 CrossRef PubMed.
- (a) P. Mal., D. Schultz, K. Beyeh, K. Rissanen and J. R. Nitschke, Angew. Chem., Int. Ed., 2008, 47, 8297 CrossRef PubMed; (b) P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697 CrossRef PubMed; (c) J. Mosquera, B. Szyszko, S. K. Y. Ho and J. R. Nitschke, Nat. Commun., 2017, 8, 14882 CrossRef PubMed.
- (a) I. A. Riddell, M. M. J. Smulders, J. K. Clegg, Y. R. Hristova, B. Breiner, J. D. Thoburn and J. R. Nitschke, Nat. Chem., 2012, 4, 751 CrossRef PubMed; (b) B. S. Pilgrim, D. A. Roberts, T. G. Lohr, T. K. Ronson and J. R. Nitschke, Nat. Chem., 2017, 8, 1276 CrossRef; (c) A. J. Musser, P. P. Neelakandan, J. M. Richter, H. Mori, R. H. Friend and J. R. Nitschke, J. Am. Chem. Soc., 2017, 139, 12050 CrossRef PubMed; (d) D. Zhang, T. K. Ronson, J. Mosquera, A. Martinez and J. R. Nitschke, Angew. Chem., Int. Ed., 2018, 57, 3717 CrossRef PubMed.
- G. N. Lewis, J. Am. Chem. Soc., 1916, 38, 762 CrossRef.
- R. Hoffmann, Sci. Am., 1993, 268, 66 CrossRef.
- A. P. Côte, A. I. Benin, N. W. Ockwing, A. J. Matzger, M. O’Keeffe and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef PubMed.
- H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268 CrossRef PubMed.
- (a) P. J. Waller, F. Gándara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053 CrossRef PubMed; (b) C. S. Diercks and O. M. Yaghi, Science, 2017, 355, 923 CrossRef PubMed.
- Y. Liu, Y. Ma, Y. Zhao, X. Sun, F. Gándara, H. Furukawa, Z. Liu, H. Zhu, C. Zhu, K. Suenaga, P. Oleynikov, A. S. Alshammari, X. Zhang, O. Terasaki and O. M. Yaghi, Science, 2016, 351, 36 Search PubMed.
- S. Lin, C. S. Diercks, Y.-B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Science, 2015, 349, 1208 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |