Mechanochemical carbon–carbon bond formation that proceeds via a cocrystal intermediate†
Abstract
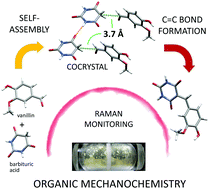
We report the first cocrystal as an intermediate in a solid-state organic reaction wherein molecules of barbituric acid and vanillin assume a favorable orientation for the subsequent Knoevenagel condensation.


 Please wait while we load your content...
Please wait while we load your content...