Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spiro-fused bis-hexa-peri-hexabenzocoronene†
Yunbin
Hu
 ab,
Di
Wang
a,
Martin
Baumgarten
ab,
Di
Wang
a,
Martin
Baumgarten
 a,
Dieter
Schollmeyer
c,
Klaus
Müllen
*a and
Akimitsu
Narita
a,
Dieter
Schollmeyer
c,
Klaus
Müllen
*a and
Akimitsu
Narita
 *a
*a
aMax-Planck-Institut für Polymerforschung, Ackermannweg 10, 55128 Mainz, Germany. E-mail: muellen@mpip-mainz.mpg.de; narita@mpip-mainz.mpg.de
bDepartment of Organic and Polymer Chemistry, College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, P. R. China
cInstitut für Organische Chemie, Johannes Gutenberg-Universität Mainz, Duesbergweg 10-14, 55099 Mainz, Germany
First published on 26th October 2018
Abstract
A spiro-fused hexa-peri-hexabenzocoronene dimer was synthesized, and its physicochemical properties were studied by UV-Vis absorption and emission spectroscopy as well as cyclic voltammetry. Chemical oxidation of SB-HBC afforded its radical cation and dication derivatives, which could be reversibly reduced to the neutral state.
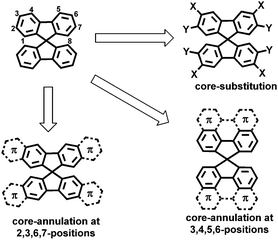
Spiro-fused polycyclic aromatic compounds, in which orthogonal π-systems are linked by a common sp3-hybridized atom, have been widely investigated due to their three-dimensional (3D) geometries, high thermal stability, good processability and unique spiro-conjugation effects.1,2 Spirobifluorene (SBF) represents the most studied class of spiro-conjugated molecules, which has been recognized as a promising building block for functional materials in organic light-emitting diodes, phototransistors and solar cells.3–11 The structural modification of SBF has been achieved by core-substitution at the 3,6- and/or 2,7-positions aiming at tuning the optical and electronic properties (Fig. 1).12–19 However, much less effort has been devoted to extend the π-system of SBF by annulation with aromatic units (Fig. 1),20–23 although this can lead to rigid and enlarged spiro π-systems with increased intermolecular π–π overlap in the solid state.24 In particular, the core-annulation at 3, 4, 5, and 6 positions of SBF that enables the construction of spiro-fused large polycyclic aromatic hydrocarbons (PAHs) has remained elusive (Fig. 1).25
Hexa-peri-hexabenzocoronene (HBC) is an intriguing PAH with a large π-system which gives rise to a pronounced self-assembly and high intrinsic charge-carrier mobility.25,26 There are numerous planar HBC derivatives in the literature, but there has been only a limited number of reports on the incorporation of HBC into 3D scaffolds,27–29 despite the intriguing properties demonstrated for smaller PAHs arranged in 3D structures, such as intrinsic chirality,30,31 amorphous aggregation1 and unique packing modes.32,33 Herein, we report an efficient synthesis of a novel 3,4,5,6-annulated SBF derivative, bearing two spiro-linked HBC units (spiro-bis-HBC, SB-HBC, Scheme 1). Its robust orthogonally arranged structure was confirmed by X-ray crystallography. The photophysical and electrochemical properties of SB-HBC were investigated in comparison with the parent HBC, revealing largely enhanced absorptivity and two-electron oxidation. Moreover, chemical oxidation and reduction were shown to be reversible.
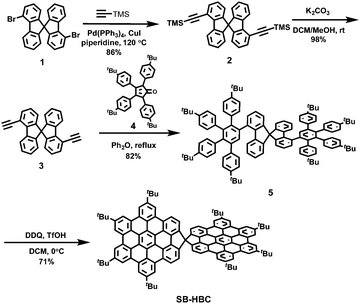
The synthesis of SB-HBC is depicted in Scheme 1. Starting from 4,4′-dibromo-9,9′-spirobifluorene (1),34 a two-fold palladium-catalyzed Sonogashira coupling of 1 with trimethylsilylacetylene was performed in the presence of tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) and copper(I) iodide (CuI) in piperidine, affording 4,4′-bis(trimethylsilylethynyl)-9,9′-spirobifluorene 2 in 86% yield. The relatively high reaction temperature (120 °C) was necessary to overcome the steric hindrance at the bay positions of 1. Then, the trimethylsilyl groups were removed upon treatment of 2 with potassium carbonate (K2CO3) in dichloromethane (DCM) and methanol (MeOH), furnishing 4,4′-diethynyl-9,9′-spirobifluorene (3) in 98% yield. Bisdienophile 3 was then subjected to a two-fold Diels–Alder reaction with cyclopentadienone 4, providing the spirobifluorene-based polyphenylene precursor 5 in 82% yield. The final oxidative cyclodehydrogenation of 5 resulted in the formation of spiro-fused HBC dimer SB-HBC as a yellow solid in 71% yield. The chemical structure of SB-HBC was unambiguously disclosed by mass and NMR spectroscopies as well as X-ray crystallography. It is noteworthy that well-resolved 1H-NMR spectrum of SB-HBC was recorded, where all the aromatic protons could be clearly assigned by 1H–1H correlation spectroscopy (COSY) and nuclear overhauser enhancement spectroscopy (NOESY) measurements (see ESI† for further details).
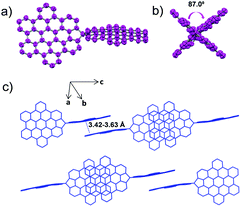
Single crystals of SB-HBC were grown by slowly diffusing pentane vapor into its tetrahydrofuran solution. As shown in Fig. 2, the SB-HBC molecule revealed a nearly orthogonal spiro-shaped structure with the dihedral angle of 87.0° between the two fused five-membered rings. This is close to the reported torsion angle of SBF (ca. 89.0°)35 and also in good agreement with the value of 88.7° calculated for the gas phase by density functional theory (DFT) at the B3LYP/6-31G(d,p) level (Fig. S2, ESI†). The HBC subunits in SB-HBC are slightly bent, with the largest distance of 0.48 Å between the outermost carbons and the plane of the central benzene ring. This is attributed to the concurrent effects of the steric interactions among tert-butyl groups and the strains induced by the five-membered rings. It is noteworthy that adjacent SB-HBC molecules are stacked by π–π overlap of the HBC subunits along the c-axis, forming one-dimensional arrays (Fig. 2c). The intermolecular π–π stacking distances are in the range of 3.42 to 3.63 Å. This molecular stacking mode is similar to that of previously reported spirobiphenalenyl neutral radicals, which show long-range one-dimensional charge-transporting properties.36,37
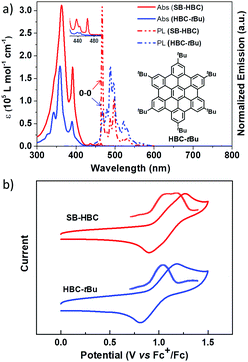
The optoelectronic behavior of SB-HBC was initially investigated by UV-Vis absorption spectroscopy in comparison with 2,5,8,11,14,17-hexa-tert-butyl-HBC (HBC-tBu).38,39 As displayed in Fig. 3a, SB-HBC exhibits a similar but less structured absorption pattern. The significantly enhanced absorption behavior of SB-HBC relative to HBC-tBu can be clearly disclosed from the nearly two-fold enhancement of the maximum molar extinction coefficient (εmax(SB-HBC) at 363 nm: ∼3.1 × 105 L mol−1 cm−1 and εmax(HBC-tBu) at 359 nm: ∼1.8 × 105 L mol−1 cm−1). The slightly red-shifted absorption maximum of SB-HBC implied the spiro-conjugation effect between two spiro-fused HBC moieties, which has been previously observed in other spirocyclic fused aromatics.24 Further lower-energy absorption bands for SB-HBC were observed at 432 nm (ε: ∼6.0 × 103 L mol−1 cm−1) with a shoulder at 442 nm and at 464 nm (ε: ∼6.3 × 103 L mol−1 cm−1), which are more pronounced compared with the corresponding peaks from HBC-tBu (inset Fig. 3a). Excitation spectrum of SB-HBC is well consistent with the absorption with distinct long-wavelength excitation bands at 438, 446 and 465 nm (Fig. S6, ESI†). Notably, compared to HBC-tBu, SB-HBC showed an extremely sharp emission band at 467 nm assignable to the 0–0 vibronic transition (Fig. 2a), which is attributed to a reduced symmetry of SB-HBC.40 Furthermore, the fluorescence quantum yield of SB-HBC was determined to be 0.063, which is higher than the reported values of HBC-tBu (ΦF = 0.030) and other alkyl substituted HBC derivatives.41 In contrast to the doubly degenerated HOMO and LUMO of unsubstituted HBC, DFT calculations demonstrated the non-degenerate frontier molecular orbitals (HOMO and LUMO) of SB-HBC, with the electron density shared over the spiro bridge (Fig. 4). On the basis of the time-dependent DFT (TD-DFT) method, HBC shows a symmetry-forbidden low-energy transition at 429 nm (oscillator strength (f) = 0.000) (Fig. S4 and Table S1, ESI†), which was previously assigned to the α-band.42 In contrast, the low-energy transition at 432 nm of SB-HBC is partially symmetry allowed with f = 0.023 (Fig. S5 and Table S2, ESI†), which is in line with the experimental observations. When studied by cyclic voltammetry, SB-HBC presented reversible two-electron oxidation in the range of 0–1.5 V as evidenced by differential pulse voltammetry (DPV) curves in contrast to the one-electron oxidation process of HBC-tBu (Fig. 3b). The onset oxidation potentials of SB-HBC and HBC-tBu were close to each other (SB-HBC: 0.95 V, HBC-tBu: 0.92 V), which implied their comparable electron-donating abilities.
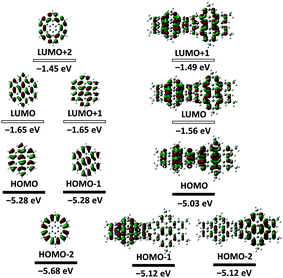 | ||
| Fig. 4 Energies and shapes of frontier orbitals of HBC and model SB-HBC at the B3LYP/6-31G level of theory. | ||
We also undertook chemical oxidation of SB-HBC using antimony chloride (SbCl5) as the oxidant, which were monitored by UV-Vis-NIR absorption spectroscopy and electron paramagnetic resonance (EPR) measurements. Upon incremental addition of a solution of SbCl5 in DCM, the light yellow solution of SB-HBC turned red with the appearance of a sharp absorption band at 548 nm and a broad band from 600 to 1000 nm centered at 815 nm (red lines in Fig. 5a). We assign these new absorption bands to the radical cation of SB-HBC (SB-HBC˙+), also based on the observation of an intensive EPR signal (g = 2.0033) (inset of Fig. 5a and Fig. S8, S9, ESI†). Excess amounts of SbCl5 were necessary for this oxidation process (Fig. S7, ESI†). Nevertheless, loss of degeneracy in the HOMO of SB-HBC, as predicted by the DFT calculation (Fig. 4), precludes the formation of bis(radical cation) species.
Further oxidation of the red SB-HBC˙+ with an increasing amount of SbCl5 afforded a green solution with a broad absorption band centered at 756 nm (green lines in Fig. 5a). These spectral and color changes coincided with the disappearance of the EPR signal (inset of Fig. 5a), suggesting the formation of diamagnetic SB-HBC dication (SB-HBC2+). This is in agreement with the loss of degeneracy of the HOMO mentioned above and in contrast to oxidation of coronene which can form a paramagnetic diradical dication due to the two-fold degenerate HOMOs.43
It is noteworthy that the green SB-HBC2+ could be reversibly reduced to the neutral state by adding tin(II) chloride (SnCl2). A stepwise color change was observed from green to red and then to light yellow, which was accompanied by simultaneous changes of the absorption and EPR spectra, demonstrating the recovery of SB-HBC˙+ and then of the neutral SB-HBC (Fig. 5b). The reversible redox property of SB-HBC indicated the good stability of its oxidized species.
In summary, we have demonstrated the efficient synthesis of a spiro-fused bis-HBC (SB-HBC), representing a novel PAH with a robust spiro-linkage. The orthogonally arranged π-system of SB-HBC was proven by X-ray crystallography, with the adjacent molecules stacking by the marked one-dimensional π–π overlapping pattern. The chemical oxidation of SB-HBC into the stable oxidized species and reversible reduction process are demonstrated. Our result sheds light on π-extended SBF with modulated optical and electronic properties, and can become a starting point for the investigation of other spiro-fused nanographene structures.
We acknowledge the financial support from the Max Planck Society and the European Commission through the FET-Proactive Project “MoQuaS” (No. 610449) and Graphene Flagship (No. CNECT-ICT-604391). Open Access funding provided by the Max Planck Society.
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011–1065 CrossRef CAS PubMed.
- C. Poriel and J. Rault-Berthelot, J. Mater. Chem. C, 2017, 5, 3869–3897 RSC.
- C. Quinton, S. Thiery, O. Jeannin, D. Tondelier, B. Geffroy, E. Jacques, J. Rault-Berthelot and C. Poriel, ACS Appl. Mater. Interfaces, 2017, 9, 6194–6206 CrossRef CAS PubMed.
- Z. Jiang, H. Yao, Z. Zhang, C. Yang, Z. Liu, Y. Tao, J. Qin and D. Ma, Org. Lett., 2009, 11, 2607–2610 CrossRef CAS PubMed.
- S. Thiery, D. Tondelier, C. Dedairieux, B. Geffroy, O. Jeannin, R. Metivier, J. Rault-Berthelot and C. Poriel, J. Phys. Chem. C, 2015, 119, 5790–5805 CrossRef CAS.
- Y.-L. Liao, W.-Y. Hung, T.-H. Hou, C.-Y. Lin and K.-T. Wong, Chem. Mater., 2007, 19, 6350–6357 CrossRef CAS.
- T. P. I. Saragi, R. Pudzich, T. Fuhrmann and J. Salbeck, Appl. Phys. Lett., 2004, 84, 2334–2336 CrossRef CAS.
- T. P. Osedach, S. M. Geyer, J. C. Ho, A. C. Arango, M. G. Bawendi and V. Bulović, Appl. Phys. Lett., 2009, 94, 043307 CrossRef.
- T. P. I. Saragi, R. Pudzich, T. Fuhrmann-Lieker and J. Salbeck, Opt. Mater., 2007, 29, 879–884 CrossRef CAS.
- X.-F. Wu, W.-F. Fu, Z. Xu, M. Shi, F. Liu, H.-Z. Chen, J.-H. Wan and T. P. Russell, Adv. Funct. Mater., 2015, 25, 5954–5966 CrossRef CAS.
- W. H. Nguyen, C. D. Bailie, E. L. Unger and M. D. McGehee, J. Am. Chem. Soc., 2014, 136, 10996–11001 CrossRef CAS PubMed.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissörtel, J. Salbeck, H. Spreitzer and M. Grätzel, Nature, 1998, 395, 583–585 CrossRef CAS.
- J.-H. Fournier, T. Maris and J. D. Wuest, J. Org. Chem., 2004, 69, 1762–1775 CrossRef CAS PubMed.
- K. C. Song, R. Singh, J. Lee, D. H. Sin, H. Lee and K. Cho, J. Mater. Chem. C, 2016, 4, 10610–10615 RSC.
- N. J. Jeon, H. G. Lee, Y. C. Kim, J. Seo, J. H. Noh, J. Lee and S. I. Seok, J. Am. Chem. Soc., 2014, 136, 7837–7840 CrossRef CAS PubMed.
- L. E. Polander, P. Pahner, M. Schwarze, M. Saalfrank, C. Koerner and K. Leo, APL Mater., 2014, 2, 081503 CrossRef.
- L. Pop, F. Dumitru, N. D. Hadade, Y. M. Legrand, A. van der Lee, M. Barboiu and I. Grosu, Org. Lett., 2015, 17, 3494–3497 CrossRef CAS PubMed.
- T. Nakagawa, S. Y. Ku, K. T. Wong and C. Adachi, Chem. Commun., 2012, 48, 9580–9582 RSC.
- C. Poriel, F. Barrière, D. Thirion and J. Rault-Berthelot, Chem. – Eur. J., 2009, 15, 13304–13307 CrossRef CAS PubMed.
- M. Romain, D. Tondelier, J.-C. Vanel, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, Angew. Chem., Int. Ed., 2013, 52, 14147–14151 CrossRef CAS PubMed.
- Y. Wu, J. Zhang, Z. Fei and Z. Bo, J. Am. Chem. Soc., 2008, 130, 7192–7193 CrossRef CAS PubMed.
- N. Harada, H. Ono, T. Nishiwaki and H. Uda, J. Chem. Soc., Chem. Commun., 1991, 1753–1755 RSC.
- J. Ramakrishna and P. Venkatakrishnan, Chem. – Asian J., 2017, 12, 181–189 CrossRef CAS PubMed.
- G. Gao, N. Liang, H. Geng, W. Jiang, H. Fu, J. Feng, J. Hou, X. Feng and Z. Wang, J. Am. Chem. Soc., 2017, 139, 15914–15920 CrossRef CAS PubMed.
- T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed.
- A. Narita, X.-Y. Wang and X. Feng, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed; K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC.
- J. N. Smith, J. M. Hook and N. T. Lucas, J. Am. Chem. Soc., 2018, 140, 1131–1141 CrossRef CAS PubMed.
- H.-J. Yen, H. Tsai, M. Zhou, E. F. Holby, S. Choudhury, A. Chen, L. Adamska, S. Tretiak, T. Sanchez, S. Iyer, H. Zhang, L. Zhu, H. Lin, L. Dai, G. Wu and H.-L. Wang, Adv. Mater., 2016, 28, 10250–10256 CrossRef CAS PubMed.
- C. Zhang, Y. Liu, X.-Q. Xiong, L.-H. Peng, L. Gan, C.-F. Chen and H.-B. Xu, Org. Lett., 2012, 14, 5912–5915 CrossRef CAS PubMed.
- T. Fujikawa, D. V. Preda, Y. Segawa, K. Itami and L. T. Scott, Org. Lett., 2016, 18, 3992–3995 CrossRef CAS PubMed.
- K. Kato, Y. Segawa, L. T. Scott and K. Itami, Angew. Chem., Int. Ed., 2018, 57, 1337–1341 CrossRef CAS PubMed.
- C. L. Hilton, C. R. Jamison, H. K. Zane and B. T. King, J. Org. Chem., 2009, 74, 405–407 CrossRef CAS PubMed.
- T. M. Long and T. M. Swager, Adv. Mater., 2001, 13, 601–604 CrossRef CAS.
- C. Fan, Y. Chen, P. Gan, C. Yang, C. Zhong, J. Qin and D. Ma, Org. Lett., 2010, 12, 5648–5651 CrossRef CAS PubMed.
- R. E. Douthwaite, A. Taylor and A. C. Whitwood, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2005, 61, O328–O331 CrossRef PubMed.
- S. K. Pal, M. E. Itkis, F. S. Tham, R. W. Reed, R. T. Oakley, B. Donnadieu and R. C. Haddon, J. Am. Chem. Soc., 2007, 129, 7163–7174 CrossRef CAS PubMed.
- S. K. Pal, P. Bag, M. E. Itkis, F. S. Tham and R. C. Haddon, J. Am. Chem. Soc., 2014, 136, 14738–14741 CrossRef CAS PubMed.
- P. T. Herwig, V. Enkelmann, O. Schmelz and K. Müllen, Chem. – Eur. J., 2000, 6, 1834–1839 CrossRef CAS.
- R. Rathore and C. L. Burns, J. Org. Chem., 2003, 68, 4071–4074 CrossRef CAS PubMed.
- C.-Y. Tsai, Q. Zhang, Y.-Z. Wang, J. Shyong, H.-L. Chen and D.-J. Liaw, Polym. Chem., 2017, 8, 3327–3332 RSC.
- R. Yamaguchi, S. Ito, B. S. Lee, S. Hiroto, D. Kim and H. Shinokubo, Chem. – Asian J., 2013, 8, 178–190 CrossRef CAS PubMed.
- M. Kastler, J. Schmidt, W. Pisula, D. Sebastiani and K. Müllen, J. Am. Chem. Soc., 2006, 128, 9526–9534 CrossRef CAS PubMed.
- P. J. Krusic and E. Wasserman, J. Am. Chem. Soc., 1991, 113, 2322–2323 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, syntheses, spectra, properties, characterization data and structures, including Fig. S1–S23 and Tables S1 and S2. CCDC 1848515. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc07405d |
| This journal is © The Royal Society of Chemistry 2018 |