Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single-compartment hydrogen peroxide fuel cells with poly(3,4-ethylenedioxythiophene) cathodes
Eva
Miglbauer
ab,
Paweł Jerzy
Wójcik
c and
Eric Daniel
Głowacki
 *ad
*ad
aLaboratory of Organic Electronics, ITN Campus Norrköping, Linköping University, Norrköping, Sweden
bFaculty of Technical Chemistry, Chemical and Process Engineering and Biotechnology, Graz University of Technology, Graz, Austria
cRedox.me AB, Norrköping, Sweden
dWallenberg Centre for Molecular Medicine, Linköping University, Linköping, Sweden. E-mail: eric.glowacki@liu.se
First published on 3rd October 2018
Abstract
Single-compartment hydrogen peroxide fuel cells have recently emerged as a promising energy conversion platform since H2O2 is a high energy-density liquid that functions as both fuel and oxidizer. Finding suitable electrocatalysts is challenging since most metallic electrodes also catalyze the disproportionation reaction of H2O2 into H2O and O2, representing a significant loss mechanism in peroxide fuel cells. Herein we demonstrate that the conducting polymer poly(3,4-ethylenedioxythiophene), PEDOT, is a versatile electrocatalyst for peroxide fuel cells without generating losses due to disproportionation. We find that PEDOT is a cathodic catalyst for reduction of peroxide to water, performing at a level on par with the best reported inorganic catalysts. Using PEDOT as the cathode and nickel as the anode material, open circuit potentials in the range of 0.5–0.6 V are possible, with power densities of 0.20–0.30 mW cm−2. We provide evidence to understand mechanistically how PEDOT functions as a catalyst for hydrogen peroxide reduction to water. The result of our efforts is a scalable hydrogen peroxide fuel cell cathode, which serves to demonstrate also the capabilities of organic semiconducting materials as electrocatalysts.
Fuel cells represent an important family of energy storage and conversion devices, with several mature technologies and well-established applications. The most prominent example is the hydrogen fuel cell, which consumes hydrogen gas as a fuel and utilizes atmospheric oxygen as an oxidizer, producing water as a byproduct. Despite its elegance, the hydrogen fuel cell has two disadvantages – the problem of storage of H2 as a gaseous fuel and the necessity of optimizing the proton-conducting separator membrane between the cathode and anode chambers to isolate the fuel from the oxidizer.1 Hydrogen peroxide fuel cells are a newer concept which retains the advantage of having a fuel cell with water as a byproduct, but with the significant feature of aqueous hydrogen peroxide as a liquid fuel and the single-compartment nature where H2O2 is both fuel and oxidizer and thus no separator is necessary.2–4 These fuel cells are part of a broader growing field of energy conversion and storage with H2O2, driven largely by improvements in photocatalytic platforms for conversion of solar energy into peroxide.5–8 In a hydrogen peroxide fuel cell, energy is produced because of the exergonic reactions of oxidation of hydrogen peroxide to produce oxygen and the reduction of hydrogen peroxide to water. If each of these half-reactions proceed on an anode and cathode, respectively, the energy contained in hydrogen peroxide can be electrochemically converted to electrical energy. Hydrogen peroxide reduction, at neutral to acidic pH values, proceeds on the cathode viaeqn (1). Oxidation at the anode proceeds viaeqn (2). The net reaction (eqn (3)) of producing water and oxygen from peroxide gives a net potential gain of 1.09 V. This is close to two well-known fuel cell systems: hydrogen fuel cells have a theoretical open circuit potential of 1.23 V and methanol fuel cells 1.21 V.
| Cathode: H2O2 + 2H+ + 2e− → 2H2O (1.78 V vs. NHE) | (1) |
| Anode: H2O2 → O2 + 2H+ + 2e− (0.682 V vs. NHE) | (2) |
| Total: 2H2O2 → 2H2O + O2 (1.09 V) | (3) |
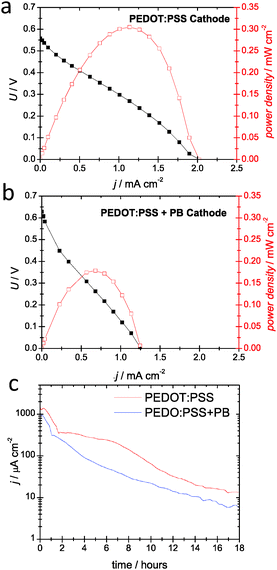
To measure in detail the fuel cell performance, we conducted step chronopotentiometry scans (Fig. 2). Different current densities were maintained for 1 minute and the final potential registered. In addition to PEDOT:PSS, we tested a PEDOT:PSS composite with Prussian blue (PB) nano/microcrystals, synthesized according to the literature.20 PB is a well-known catalyst for reduction of peroxide to water, and is one of the to-date best materials as cathodes for peroxide fuel cells. PEDOT:PSS cathodes (Fig. 2a) afforded a VOC of 0.56 V and JSC of 2.0 mA cm−2, and maximum power density of 0.31 mW cm−2. The addition of PB increases the VOC to 0.64 V, very close to the previously reported value for PB on carbon in a peroxide fuel cell with a nickel anode (Fig. 2b). Current density does not improve however, remaining at 1.25 mA cm−2. Taken together, these data show that PEDOT:PSS is an efficient catalyst in its own right, as it gives an open circuit potential very close to that of PB. Step chronopotentiometry experiments were repeated for three cycles (each cycle = 12 minutes), and demonstrated constant power density for both types of samples. We conducted a chronoamperometric measurement of holding the cell at short circuit with a starting concentration of 0.1 M H2O2. Both types of PEDOT cathodes were found to discharge at similar rates, declining to about a tenth of the starting current density after 5 hours (Fig. 2c). Having established that PEDOT is a suitable cathodic catalyst for single-compartment peroxide fuel cells, we tested if it induces the nonelectrochemical disproportionation of H2O2 into water and oxygen. This is a major loss mechanism in peroxide fuel cells and an obstacle for deploying many metallic catalysts. Peroxide decomposition was quantified by measuring aliquots from test solution using the horseradish peroxidase/tetramethyl benzidine assay.6,21 We found that a 0.1 M H2O2 in 0.05 M HCl decomposes at a rate of 0.52 mM h−1. Nickel accelerated peroxide decomposition to 2.6 mM h−1. The presence of PEDOT formulations was found not only to not induce decomposition, but to actually stabilize the peroxide solution. PEDOT:PSS slowed decomposition to 0.34 mM h−1.
In order to shed light on the mechanisms at play with PEDOT interacting with H2O2 in the context of familiar processes like oxygen-induced doping and oxygen reduction on PEDOT, we performed a series cyclic voltammetry experiments with and without H2O2 present (Fig. 3). In ambient conditions in 0.05 M HCl electrolyte, in the range of −0.4 to 0.8 V versus Ag/AgCl, PEDOT electrodes do not show any electrochemical activity save a relatively large capacitive current, characteristic of PEDOT:PSS.22 Purging with Ar prior to the experiment removes oxygen, which is known to contribute to p-doping of the PEDOT. This results is the appearance of quasi-reversible redox peaks between 0.2–0.4 V, attributable to the oxidation and re-reduction of PEDOT(0). This indicates that argon purging produces some quantity of neutral PEDOT by removing oxygen. Oxygen-purging of the electrolyte eliminates these redox peaks, and produces a smaller set of faradaic peaks resulting from the oxygen reduction reaction and accompanying reoxidation of oxygen reduction products. The oxygen reduction reaction on PEDOT was recently described, as was the effect of oxygen-induced doping increasing significantly the conductivity of PEDOT.23 The addition of H2O2 to a concentration of 0.1 M results in a drastic change of the cyclic voltammograms. Ambient PEDOT electrodes demonstrate a cathodic current with an onset around 0.4 V versus Ag/AgCl, signaling the reduction of H2O2 to water. Oxygen purging results in a three-fold increase in this cathodic current, which can be attributed to the effect of oxygen increasing the conductivity of PEDOT.23 Purging with Ar, however, results in a five-fold increase in the H2O2 reduction current relative to ambient conditions. This final result, taken together with the Ar-dedoping effect described above and shown in Fig. 3a, provides strong evidence that neutral PEDOT is the key species enabling the peroxide reduction reaction. These findings support the hypothesized reaction cycle proposed in Fig. 1b. Conducting polymers have a rich redox behavior and are stable in aqueous conditions, making them interesting 3D networks where electrons, ions, and reactants can meet. This approach led us to try using the archetypical conducting polymer PEDOT to see if it can participate in the redox cycle necessary to reduce hydrogen peroxide to water, critical to make cathodes for hydrogen peroxide fuel cells. These fuel cells are an interesting alternative technology to established hydrogen fuel cells, and their further development hinges on improving the performance of both cathodic and anodic catalysts. PEDOT fulfills its role as a cathodic catalyst on a level competitive with some of the best inorganic catalysts, with the benefit of not promoting peroxide disproportionation. Performance must be improved by optimizing PEDOT formulations, or by turning to other, next-generation conducting polymers. Tuning conducting polymers to become anodic catalysts for peroxide oxidation would lead to a complete “plastic” single-compartment hydrogen peroxide fuel cell. Once performance is optimized, long-term stability of conducting polymer electrodes must be carefully verified. The known stability of conducting polymer formulations in oxidizing, acidic environments speaks to them being a superior choice over metallic alternatives. Our demonstration serves as a promising first step in this direction.
 | ||
| Fig. 3 Cyclic voltammograms (5 mV s−1) of PEDOT:PSS – GOPS under Ar purged, ambient and oxygen purged conditions: (a) 0.05 M HCl without peroxide (WO); (b) 0.05 M HCl with 0.1 M peroxide (W). | ||
This work was supported by Vinnova in the framework of Treesearch.se and by the Knut and Alice Wallenberg Foundation. E. M. is grateful for support from the Erasmus+ scholarship program of the European Commission and useful discussions with G. Trimmel at TU Graz.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Srinivasan, Fuel Cells From Fundamentals to Applications, Springer, New York, 2006 Search PubMed.
- S. Fukuzumi, Biochim. Biophys. Acta, Bioenerg., 2016, 1857, 604–611 CrossRef CAS PubMed.
- S. Fukuzumi and Y. Yamada, ChemElectroChem, 2016, 3, 1978–1989 CrossRef CAS.
- S. Fukuzumi, Joule, 2017, 1, 689–738 CrossRef CAS.
- K. Mase, M. Yoneda, Y. Yamada and S. Fukuzumi, Nat. Commun., 2016, 7, 11470 CrossRef CAS PubMed.
- M. Jakešová, D. H. Apaydin, M. Sytnyk, K. Oppelt, W. Heiss, N. S. Sariciftci and E. D. Głowacki, Adv. Funct. Mater., 2016, 26, 5248–5254 CrossRef.
- Y. Sun, I. Sinev, W. Ju, A. Bergmann, S. Dresp, S. Kühl, C. Spöri, H. Schmies, H. Wang, D. Bernsmeier, B. Paul, R. Schmack, R. Kraehnert, B. Roldan Cuenya and P. Strasser, ACS Catal., 2018, 8, 2844–2856 CrossRef CAS.
- Y. Yamada, Y. Fukunishi, S. Yamazaki and S. Fukuzumi, Chem. Commun., 2010, 46, 7334–7336 RSC.
- R. S. Disselkamp, Energy Fuels, 2008, 22, 2771–2774 CrossRef CAS.
- R. S. Disselkamp, Int. J. Hydrogen Energy, 2010, 35, 1049–1053 CrossRef CAS.
- S. Yamazaki, Z. Siroma, H. Senoh, T. Ioroi, N. Fujiwara and K. Yasuda, J. Power Sources, 2008, 178, 20–25 CrossRef CAS.
- S. Fukuzumi, Y. Yamada and K. D. Karlin, Electrochim. Acta, 2012, 82, 493–511 CrossRef CAS PubMed.
- S. A. Mousavi Shaegh, N.-T. Nguyen, S. M. Mousavi Ehteshami and S. H. Chan, Energy Environ. Sci., 2012, 5, 8225–8228 RSC.
- Y. Yamada, M. Yoneda and S. Fukuzumi, Inorg. Chem., 2014, 53, 1272–1274 CrossRef CAS PubMed.
- M. Warczak, M. Gryszel, M. Jakešová, V. Đerek and E. D. Głowacki, Chem. Commun., 2018, 54, 1960–1963 RSC.
- M. Gryszel, M. Sytnyk, M. Jakesova, G. Romanazzi, R. Gabrielsson, W. Heiss and E. D. Głowacki, ACS Appl. Mater. Interfaces, 2018, 10, 13253–13257 CrossRef CAS PubMed.
- M. K. Węcławski, M. Jakešová, M. Charyton, N. Demitri, B. Koszarna, K. Oppelt, S. Sariciftci, D. T. Gryko and E. D. Głowacki, J. Mater. Chem. A, 2017, 5, 20780–20788 RSC.
- A. Elschner, S. Kirchmeyer, W. Lovenich, U. Merker and K. Reuter, PEDOT Principles and Applications of an Intrinsically Conductive Polymer, CRC Press, 2011 Search PubMed.
- P. Stadler, D. Farka, H. Coskun, E. D. Głowacki, C. Yumusak, L. M. Uiberlacker, S. Hild, L. N. Leonat, M. C. Scharber, P. Klapetek, R. Menon and N. S. Sariciftci, J. Mater. Chem. C, 2016, 4, 6982–6987 RSC.
- M. Vázquez-González, R. M. Torrente-Rodríguez, A. Kozell, A. Cecconello, S. Campuzano, J. M. Pingarron and I. Willner, Nano Lett., 2017, 17, 4958–4963 CrossRef PubMed.
- P. D. Josephy, T. Eling and R. P. Mason, J. Biol. Chem., 1982, 257, 3669–3675 CAS.
- C. M. Proctor, J. Rivnay and G. G. Malliaras, J. Polym. Sci., Part B: Polym. Phys., 2016, 1433–1436 CrossRef CAS.
- E. Mitraka, M. J. Jafari, M. Vagin, X. Liu, M. Fahlman, T. Ederth, M. Berggren, M. P. Jonsson and X. Crispin, J. Mater. Chem. A, 2017, 5, 4404–4412 RSC.
| This journal is © The Royal Society of Chemistry 2018 |