Monitoring ADP and ATP in vivo using a fluorescent Ga(iii)-probe complex†
Abstract
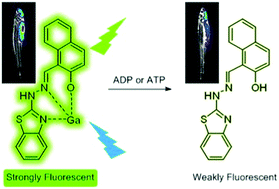
A naphthol-based sensor (L) was designed and synthesized for the specific recognition of Ga3+ using fluorescence enhancement. An in situ generated L–Ga3+ ensemble detected ADP and ATP more selectively through a fluorescence “switch off” response, which was confirmed both in cells and in adult zebrafish.


 Please wait while we load your content...
Please wait while we load your content...