Open Access Article
Open Access ArticleTemplate-promoted self-replication in dynamic combinatorial libraries made from a simple building block†
B.
Bartolec
,
M.
Altay
and
S.
Otto
 *
*
Centre for Systems Chemistry, Stratingh Institute, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: s.otto@rug.nl
First published on 5th November 2018
Abstract
We report dynamic combinatorial libraries made from a simple building block that is on the verge of enabling self-assembly driven self-replication. Adding a template provides a sufficient additional push yielding self-replication. Self-assembly and self-replication can emerge with building blocks that are considerably smaller than those reported thus far.
Dynamic combinatorial libraries (DCLs)1 are attracting increasing attention as a powerful tool in the emerging field of systems chemistry.1b,2 DCLs allow the discovery and development of systems that exploit non-covalent interactions. In a DCL the library members interconvert continuously by exchanging building blocks with each other, leading to an (equilibrium) pool of interchanging molecules. Non-covalent interactions tend to stabilize those library members that are most efficient at forming them, causing the library composition to shift in favour of these compounds. Such non-covalent interactions can occur within or between library members, giving rise to foldamers3 or self-assembling systems,4 respectively. Dynamic combinatorial self-assembly processes can give rise to self-replication,5,6 producing self-synthesizing materials4b,7 in an autocatalytic fashion. Upon addition of a template DCLs may also produce molecules or nanoparticles that selectively bind to this template. Molecular recognition between the template and library species leads to the amplification of the library members which bind to the template. This effect may be utilized for the discovery of ligands for biomacromolecules,8 surface-functionalised nanoparticles,9 synthetic receptors10 and even catalysts.11 On rare occasions templates can also induce assembly processes and trigger the emergence of a self-replicator from a dynamic combinatorial library.12
The criteria for the design of building blocks to generate DCLs that efficiently exploit non-covalent interactions are still relatively obscure, in particular when it comes to self-assembly and replication phenomena. Past work on self-assembly driven self-replication has mostly featured relatively elaborate peptide building blocks.4b,6a,b,13 We now show that a building block containing only a single amino acid has already a tendency to produce a self-replicator and that this process can be promoted by addition of a template molecule.
The design of new building blocks for DCLs aimed at self-assembly involves a balancing act between two requirements: firstly, building blocks need to endow library members with the possibility to form attractive intermolecular interactions and secondly, building block and library members should be soluble and therefore have attractive interactions with the solvent. When targeting self-assembly in water the latter typically requires the presence of charged or ethylene oxide groups. We designed building block 1 (Scheme 1) as a structurally simple (minimal) building block that features a carboxylate for water solubility, two hydrophobic aromatic rings for hydrophobic interactions and an amide group as a potential hydrogen bonding site. It also contains two thiol groups which can be oxidized to form a DCL of macrocyclic disulfides when exposed to oxygen from the air.14 At neutral to mildly basic pH residual thiolate mediates the disulfide exchange reaction that enables the equilibration of the different library members.
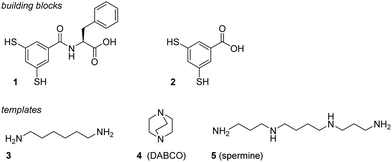
We set up a DCL made by dissolving 1 at a 3.0 mM concentration in aqueous borate buffer (50 mM, pH 8.5) and monitored the product distribution by UPLC-MS. A diverse set of oligomers is formed, ranging from cyclic trimer to cyclic 16mer.‡![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 This DCL is considerably more diverse than that formed from control compound 2 which is an analogue of 1 but lacking the phenylalanine residue (Fig. 1c). The latter DCL consisted predominantly of cyclic trimer and tetramer.
15 This DCL is considerably more diverse than that formed from control compound 2 which is an analogue of 1 but lacking the phenylalanine residue (Fig. 1c). The latter DCL consisted predominantly of cyclic trimer and tetramer.
 | ||
| Fig. 1 UPLC-MS analysis of 50 days old DCLs made from building block 1 in aqueous borate buffer (50 mM, pH 8.5), stirred at 1200 rpm at room temperature: (a) [1] = 3.0 mM; (b) [1] = 1.0 mM. The inserts emphasise the higher percentage of larger oligomers at higher concentrations. (c) UPLC-MS analysis of a DCL made from control compound 2 under the same conditions ([2] = 1.0 mM).‡ | ||
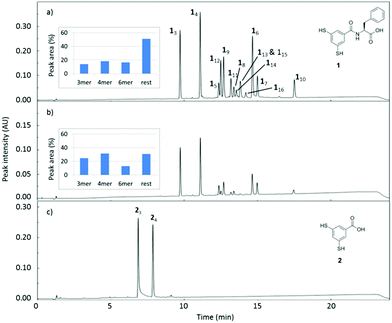
Inspection of TEM micrographs revealed ill-defined supramolecular aggregates in DCLs made from building block 1 (Fig. S1, ESI†), while no aggregates were observed in DCLs made from building block 2. Thus, it appears that the introduction of a single phenylalanine residue in 1 is sufficient to allow for the formation of self-assembled structures. Such assembly may also explain the formation of larger macrocycles.15 In the assemblies there is a high local concentration of material that enables the formation of relatively high molecular weight macrocycles through disulfide exchange. In DCLs of non-assembling library members, such as those made from 2, the formation of such large oligomers tends to be entropically disfavoured, hence only the smallest non-strained rings are formed (trimer and tetramer).§ Note that in DCLs made from 1 at lower concentrations (Fig. 1b) the larger macrocycles are less populated, suggesting that the assembled structures are at equilibrium with non-assembled structures in the concentration range that was probed. This observation, combined with the ill-defined nature of the aggregates (Fig. S1, ESI†) suggests that the design of building block 1 is only just good enough to lead to self-assembled library members. As assembly most likely comes about through a trade-off between attractive interactions (hydrogen bonding and hydrophobically enhanced π-stacking interactions) and electrostatic repulsion between the carboxylate groups, we reasoned that it might be possible to promote assembly by adding a template that could shield charge repulsion. Thus, amines 3–5 were tested for their ability to direct self-assembly. The linear primary amines 3 and 5 induced a template effect, selectively promoting the formation of the cyclic hexamer, while virtually no effect was observed with secondary amine 4 (Fig. 2).
 | ||
| Fig. 2 Change of the product distribution with time for DCLs made from building block 1 (1.0 mM in 50 mM borate buffer, pH 8.5, stirred at 1200 rpm, room temperature) in the presence of different templates (1.0 mM): (a) no template; (b) 3; (c) DABCO, 4; (d) spermine, 5.‡ | ||
We speculate that hydrogen bonding between primary amine groups of the template molecules and carboxylate groups present in library members causes the observed template effect, although we cannot exclude that the close proximity between the two amines in 4 or steric hindrance provided by the ethylene bridges would preclude efficient interaction with the fibres.
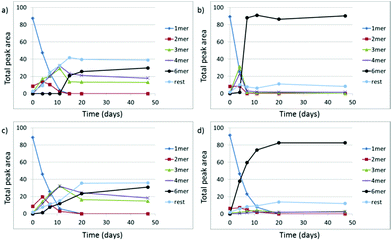
The extent to which the formation of 16 is autocatalytic was probed by seeding experiments. A small amount of 16 was added to a library composed of different oligomers, containing only a low amount of the suspected replicator. The rate of growth of 16 was monitored and clearly faster than the corresponding rate in the absence of seed, confirming that 16 is a self-replicator (Fig. 3). This is in agreement with the sigmoidal growth curve of 16 in the absence of seed (Fig. 2a). Note that 16 is the only macrocycle to show such sigmoidal growth. The latter two observations suggest that 16 can also self-replicate in the absence of a template.
 | ||
| Fig. 3 Seeding experiment for assessing the ability of 16 to self-replicate. A library of building block 1 (10% volume) containing approximately 90% of hexamer (Fig. 2b, day 20) was added to an 80% oxidized DCL made from building block 1 (1.0 mM, stirred at 1200 rpm, room temperature). | ||
Self-replication of 16 is driven by self-assembly of this macrocycle into fibrous structures that were visualised by TEM (Fig. 4). In a library prepared without template, isolated fibres were observed suggesting a simple one-dimensional self-assembly mode (Fig. 4a). In the presence of the amine templates 3 or 5 laterally associated fibres were observed (Fig. 4b and c), suggesting that the templates, by partially compensating the negative charge of the fibres, reduce charge repulsion allowing fibres to assemble into larger aggregates.
 | ||
| Fig. 4 Representative TEM micrographs of 15 days old DCLs made from building block 1 (1.0 mM in 50 mM borate buffer, pH 8.5, stirred at 1200 rpm, room temperature) (a) in the absence of template; (b) in the presence of 20 mol% 3; (c) in the presence of 20 mol% 5. Corresponding library compositions are shown in Fig. 3 and Fig. 2a, b, d, respectively. | ||
Further confirmation that building block 1 yields DCLs that are on the border of allowing access to self-assembling molecules was obtained by experiments in which we mixed this building block with analogue 2. The resulting DCL consisted of small trimeric and tetrameric macrocycles of mixed composition, irrespective of the addition of template (Fig. S3, ESI†), suggesting that no significant self-assembly takes place in these mixed DCLs.
In conclusion, we have identified a minimal building block design which can produce DCLs with just enough potential for intermolecular non-covalent interactions to facilitate self-assembly. Unassisted by templates this building block produces a diverse mixture of macrocycles. In the presence of suitable primary amine templates a self-replicating cyclic hexamer is formed selectively, driven by assembly into fibres. These results show that self-replication can occur with surprisingly simple building blocks, much less complex than the relatively long peptides used in most studies thus far,4b,6a,b,13,16 showing the generality and potential of self-assembly driven self-replication.
We are grateful for support from the European Research Council (ERC advanced grant no. 741774 – ToDL), the NWO (grant no. VICI - 724.012.002) and the Dutch Ministry of Education, Culture and Science (Gravitation program 024.001.035). Reference spectroscopic and reaction data can be found at DOI: 10.1039/c8cc06253f.
Conflicts of interest
There are no conflicts to declare.References
- (a) D. Komaromy, P. Nowak and S. Otto, in Dynamic Covalent Chemistry: Principles, Reactions, and Applications, ed. W. Zhang and Y. Jin, Wiley, Hoboken, 1997, ch. 2, pp. 31–120 Search PubMed; (b) O. S. Miljanic, Chem, 2017, 2, 502–524 CrossRef CAS; (c) Y. H. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634–6654 RSC; (d) J. M. Lehn, Angew. Chem., Int. Ed., 2013, 52, 2836–2850 CrossRef CAS PubMed; (e) F. B. L. Cougnon and J. K. M. Sanders, Acc. Chem. Res., 2012, 45, 2211–2221 CrossRef CAS PubMed; (f) P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS PubMed.
- (a) G. Ashkenasy, T. M. Hermans, S. Otto and A. F. Taylor, Chem. Soc. Rev., 2017, 46, 2543–2554 RSC; (b) E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed; (c) K. Ruiz-Mirazo, C. Briones and A. de la Escosura, Chem. Rev., 2014, 114, 285–366 CrossRef CAS PubMed; (d) J. R. Nitschke, Nature, 2009, 462, 736–738 CrossRef CAS PubMed; (e) R. F. Ludlow and S. Otto, Chem. Soc. Rev., 2008, 37, 101–108 RSC.
- (a) L. Roy and M. A. Case, J. Am. Chem. Soc., 2010, 132, 8894–8896 CrossRef CAS PubMed; (b) Y. Krishnan-Ghosh and S. Balasubramanian, Angew. Chem., Int. Ed., 2003, 42, 2171–2173 CrossRef CAS PubMed; (c) K. Oh, K. S. Jeong and J. S. Moore, Nature, 2001, 414, 889–893 CrossRef CAS PubMed; (d) M. A. Case and G. L. McLendon, J. Am. Chem. Soc., 2000, 122, 8089–8090 CrossRef CAS.
- (a) A. Pal, M. Malakoutikhah, G. Leonetti, M. Tezcan, M. Colomb-Delsuc, V. D. Nguyen, J. van der Gucht and S. Otto, Angew. Chem., Int. Ed., 2015, 54, 7852–7856 CrossRef CAS PubMed; (b) J. M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A. Stuart, J. J. P. Peyralans and S. Otto, Science, 2010, 327, 1502–1506 CrossRef CAS PubMed; (c) R. Nguyen, L. Allouche, E. Buhler and N. Giuseppone, Angew. Chem., Int. Ed., 2009, 48, 1093–1096 CrossRef CAS PubMed.
- For reviews on self-replication, see: (a) H. Duim and S. Otto, Beilstein J. Org. Chem., 2017, 13, 1189–1203 CrossRef CAS PubMed; (b) T. Kosikova and D. Philp, Chem. Soc. Rev., 2017, 46, 7274–7305 RSC; (c) G. Clixby and L. Twyman, Org. Biomol. Chem., 2016, 14, 4170–4184 RSC; (d) A. J. Bissette and S. P. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800–12826 CrossRef CAS PubMed; (e) V. Patzke and G. von Kiedrowski, ARKIVOC, 2007, 5, 293–310 Search PubMed.
- For recent examples on self-replicators from dynamic combinatorial libraries, see: (a) M. Altay, Y. Altay and S. Otto, Angew. Chem., Int. Ed., 2018, 57, 10564–10568 CrossRef CAS PubMed; (b) Y. Altay, M. Tezcan and S. Otto, J. Am. Chem. Soc., 2017, 139, 13612–13615 CrossRef CAS PubMed; (c) J. W. Sadownik, T. Kosikova and D. Philp, J. Am. Chem. Soc., 2017, 139, 17565–17573 CrossRef CAS PubMed; (d) T. Kosikova and D. Philp, J. Am. Chem. Soc., 2017, 139, 12579–12590 CrossRef CAS PubMed.
- N. Giuseppone, Acc. Chem. Res., 2012, 45, 2178–2188 CrossRef CAS PubMed.
- For recent examples, see: (a) J. Soubhye, M. Gelbcke, P. Van Antwerpen, F. Dufrasne, M. Y. Boufadi, J. Neve, P. G. Furtmuller, C. Obinger, K. Z. Boudjeltia and F. Meyer, ACS Med. Chem. Lett., 2017, 8, 206–210 CrossRef CAS PubMed; (b) L. Monjas, L. Swier, I. Setyawati, D. J. Slotboom and A. K. H. Hirsch, ChemMedChem, 2017, 12, 1693–1696 CrossRef CAS PubMed; (c) J. Fu, H. X. Fu, M. Dieu, I. Halloum, L. Kremer, Y. F. Xia, W. D. Pan and S. P. Vincent, Chem. Commun., 2017, 53, 10632–10635 RSC.
- (a) W. Edwards, N. Marro, G. Turner and E. R. Kay, Chem. Sci., 2018, 9, 125–133 RSC; (b) P. Nowak, V. Saggiomo, F. Salehian, M. Colomb-Delsuc, Y. Han and S. Otto, Angew. Chem., Int. Ed., 2015, 54, 4192–4197 CrossRef CAS PubMed; (c) F. della Sala and E. R. Kay, Angew. Chem., Int. Ed., 2015, 54, 4187–4191 CrossRef CAS; (d) Y. Han, P. Nowak, M. Colomb-Delsuc, M. P. Leal and S. Otto, Langmuir, 2015, 31, 12658–12663 CrossRef CAS PubMed.
- For recent examples, see: (a) F. Ulatowski and J. Jurczak, Pure Appl. Chem., 2017, 89, 801–807 CAS; (b) B. C. Peacor, C. M. Ramsay and M. L. Waters, Chem. Sci., 2017, 8, 1422–1428 RSC; (c) J. Atcher, J. Sola and I. Alfonso, Org. Biomol. Chem., 2017, 15, 213–219 RSC; (d) J. Li, P. Nowak and S. Otto, Angew. Chem., Int. Ed., 2015, 54, 833–837 CrossRef CAS PubMed.
- (a) H. Fanlo-Virgos, A. N. R. Alba, S. Hamieh, M. Colomb-Delsuc and S. Otto, Angew. Chem., Int. Ed., 2014, 53, 11346–11350 CrossRef CAS PubMed; (b) L. Vial, J. K. M. Sanders and S. Otto, New J. Chem., 2005, 29, 1001–1003 RSC; (c) B. Brisig, J. K. M. Sanders and S. Otto, Angew. Chem., Int. Ed., 2003, 42, 1270–1273 CrossRef CAS PubMed.
- (a) D. Komaromy, M. Tezcan, G. Schaeffer, I. Maric and S. Otto, Angew. Chem., Int. Ed., 2017, 56, 14658–14662 CrossRef CAS PubMed; (b) P. Nowak, M. Colomb-Delsuc, S. Otto and J. Li, J. Am. Chem. Soc., 2015, 137, 10965 CrossRef CAS PubMed.
- (a) M. Malakoutikhah, J. J. P. Peyralans, M. Colomb-Delsuc, H. Fanlo-Virgos, M. C. A. Stuart and S. Otto, J. Am. Chem. Soc., 2013, 135, 18406–18417 CrossRef CAS PubMed; (b) B. Rubinov, N. Wagner, M. Matmor, O. Regev, N. Ashkenasy and G. Ashkenasy, ACS Nano, 2012, 6, 7893–7901 CrossRef CAS PubMed; (c) B. Rubinov, N. Wagner, H. Rapaport and G. Ashkenasy, Angew. Chem., Int. Ed., 2009, 48, 6683–6686 CrossRef CAS PubMed.
- (a) S. P. Black, J. K. M. Sanders and A. R. Stefankiewicz, Chem. Soc. Rev., 2014, 43, 1861–1872 RSC; (b) S. Otto, R. L. E. Furlan and J. K. M. Sanders, J. Am. Chem. Soc., 2000, 122, 12063–12064 CrossRef CAS.
- D. Komaromy, M. C. A. Stuart, G. M. Santiago, M. Tezcan, V. V. Krasnikov and S. Otto, J. Am. Chem. Soc., 2017, 139, 6234–6241 CrossRef CAS PubMed.
- (a) J. Nanda, B. Rubinov, D. Ivnitski, R. Mukherjee, E. Shtelman, Y. Motro, Y. Miller, N. Wagner, R. Cohen-Luria and G. Ashkenasy, Nat. Commun., 2017, 8, 434 CrossRef PubMed; (b) Z. Dadon, N. Wagner, S. Alasibi, M. Samiappan, R. Mukherjee and G. Ashkenasy, Chem. – Eur. J., 2015, 21, 648–654 CrossRef CAS PubMed; (c) D. H. Lee, J. R. Granja, J. A. Martinez, K. Severin and M. R. Ghadiri, Nature, 1996, 382, 525–528 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Building block synthesis, supplementary figures and UPLC-MS data. See DOI: 10.1039/c8cc06253f |
| ‡ Peak areas can be taken to represent concentrations (expressed in units of building blocks) as control experiments showed that the total peak area observed for DCLs with substantially different compositions is approximately constant, indicating that the building block unit has a molar absorptivity that is essentially independent of the size of the macrocycle in which it resides. |
| § We cannot exclude that also non-covalent interactions within the macrocycles lead to the stabilization of larger rings. The fact that UPLC peak area does not fall off gradually with increasing ring size suggests that specific ring sizes may benefit from such intramolecular stabilisation. |
| This journal is © The Royal Society of Chemistry 2018 |