Open Access Article
Open Access ArticleOptically-regulated thermal energy storage in diverse organic phase-change materials†
Grace G. D.
Han
 a,
Joshua H.
Deru
b,
Eugene N.
Cho
a,
Joshua H.
Deru
b,
Eugene N.
Cho
 a and
Jeffrey C.
Grossman
a and
Jeffrey C.
Grossman
 *a
*a
aDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: jcg@mit.edu
bDepartment of Materials, University of Oxford, 16 Parks Road, Oxford, OX1 3PH, UK
First published on 21st August 2018
Abstract
Thermal energy storage and release in aliphatic phase-change materials are actively controlled by adding azobenzene-based photo-switches. UV activation of the additives induces supercooling of the composites, allowing for longer thermal storage at lower temperatures. The mechanism of this process is studied by comparing phase change behavior across diverse materials.
Low-grade thermal energy storage in organic phase-change materials (PCMs) via solid–liquid phase transition shows potential efficacy in unique applications including thermo-regulating fabrics,1 temperature-adaptable buildings,2 and thermal protection of electronic devices,3 biomedical products,4 and food5 due to the low melting points (between 0 and 250 °C),6 low cost,7 and diverse form factors8,9 that organic PCMs present. Latent heat storage provides generally higher storage capacity and efficiency than sensible heat storage and also enables heat release at a targeted temperature determined by the phase transition temperature.10 One drawback of latent heat storage in PCM is the sole dependence of PCM crystallization on the ambient temperature and consequent lack of control in determining when the stored energy is released.11 Nano-confinement of organic PCMs in diverse porous materials,12 particularly carbon-based materials such as carbon aerogels13 and graphene oxides,14 has been reported as a successful method which increases thermal conductivity of PCM composites and shifts crystallization points (TC) of PCMs. However, the nano-confined PCMs still exhibit fixed TC, determined by the composition of PCMs and nano-structured host materials, thus their phase passively responds to the changing ambient temperature.
Recently, we developed an active and dynamic method to prevent crystallization as the PCM cools to temperatures below the original phase transition temperature by incorporating photo-switching dopants.15 Although photo-switches were previously investigated as dopants in liquid crystals16–18 to optically change their phases, the application in thermal energy storage as integrated to traditional latent heat storage materials was newly discovered. In that proof-of-concept work, we demonstrated that the liquid phase of an organic PCM, tridecanoic acid, can be preserved at temperatures lower than its pure-phase crystallization point due to the increased interaction with photo-switching azobenzene dopants upon UV activation. When cooled below TC the new energy barrier for the liquid-to-solid transition introduced by the presence of switched azobenzene dopants19 can be overcome by simple visible light illumination, which switches the dopants back to their starting configuration and triggers the heat release from the liquid PCM composite. This hybrid photo-switch/PCM system demonstrated prolonged thermal storage well below the original TC, enabled by the activated photo-switches, without compromising storage capacity due to the presence of metastable cis dopants that store additional thermal energy.15 The hybrid system show stability over 100 cycles (50 hours of fast cycling operation) and thermal storage time over 10 hours under a supercooled condition, while pristine PCMs crystallize within minutes when cooled below TC.
Herein, we explore the optical regulation of thermal energy storage in diverse aliphatic PCMs and demonstrate the different degrees of lowering TC of each PCM depending on their chain lengths and functional groups. The structures of the PCMs determine the degree of intermolecular interaction and the operation temperature of heat storage, and our results shed light on parameters and conditions that can be controlled to change the nucleation and crystallization of this class of thermal storage materials.
The strategy to make the TC of organic PCMs optically triggerable is described in Fig. 1a. Upon heat absorption, the PCMs first melt (Tm ranging from 10 to 70 °C) while the azobenzene dopants with higher melting point of 73 °C remain aggregated and dispersed in the liquid PCM. UV illumination on the suspension activates trans-Azo dopants to isomerize into the cis, which presents higher polarity and steric bulk compared to the trans form. The interaction between cis-Azo and the liquid PCM molecules effectively stabilizes the liquid phase of the composite down to lower temperatures than the intrinsic TC of the initial composite (T2) with trans-Azo. The new TC of the UV-activated composite (T1) and the difference between T1 and T2, defined as ΔTC, become important metrics for the system. ΔTC, in particular, represents the degree of light-induced supercooling obtained in each composite or the range of temperature over which the liquid-phase heat storage material is stabilized without losing heat through solidification. The stored heat can be released by optical triggering with visible light which causes the cis-to-trans reverse isomerization, aggregation of trans-Azo which forms nucleation seeds, and the rapid crystallization of the PCM composite.
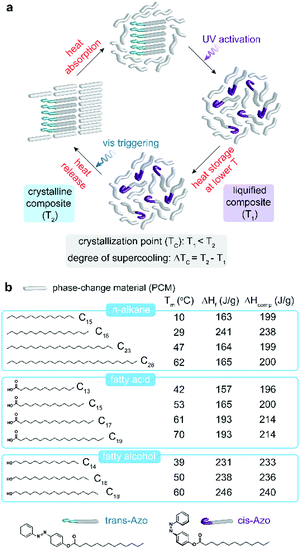 | ||
| Fig. 1 (a) Schematic illustration of heat storage and release cycle in the composite of PCMs and photo-switching dopants. (b) Chemical structures of PCMs investigated in this work along with their melting point (Tm), their intrinsic heat of fusion (ΔHf), and heat storage capacity of respective composites containing 50 wt% azobenzene dopants (ΔHcomp). We note that the ΔHcomp values are estimated, based on the calculations (Supporting Note 1, ESI†). Chemical structures of photo-switching dopants in two isomeric conformations before and after UV activation. | ||
The structural variation of aliphatic organic PCMs (Fig. 1b) provides a wide range of melting points (Tm), which defines temperatures of heat storage, and heat of fusion (ΔHf) that generally increases as the chain becomes longer, despite some exceptions.20 Three groups of aliphatic PCMs including n-alkane,21 fatty acid,22 and fatty alcohol,23 were investigated in this study to expand the scope of PCMs and to compare the impact of relative polarity of PCMs on the efficiency of heat storage and on the intermolecular interactions that operate the heat storage cycle. We used one type of azobenzene dopant with a C13 chain, unlike the previous work15 that investigated the variation of dopant structures, in order to now focus on the role of PCM types and their interaction with the azobenzene unit upon isomerization. The expected heat storage capacities in the composites are listed next to the heat of fusion of pristine PCMs based on assuming complete cis-to-trans conversion of dopants and complete crystallization of composites (Supporting Note 1, ESI†).
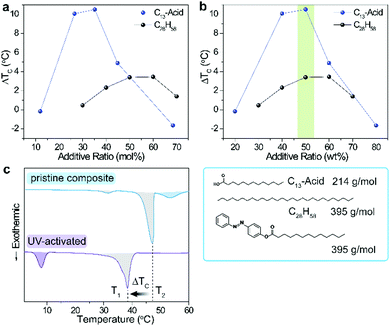
With the aim of maximizing the degree of supercooling (ΔTC), we first varied the ratio of dopants in the PCMs (Fig. 2a) which resulted in volcano-shaped plots (ΔTCvs. additive ratio) for two distinct PCMs with significantly different polarity, chain length, and melting point. Highly polar C13-acid exhibits a max. ΔTC of 10.5 °C at 35 mol% additive ratio as a result of the maximized supercooling induced by the polar cis-Azo dopants with steric bulk. Upon further increasing the additive ratio, the incomplete UV charging of trans-Azo and the remaining nucleation seeds reduce the degree of supercooling.15 Non-polar C28 alkane shows a similar plot with max. ΔTC of 3.5 °C at higher (50–60 mol%) additive ratios.
These offset plots for two dissimilar PCM systems show overlapping features when ΔTC is plotted as a function of mass (as opposed to mol) % of dopants in the composite (Fig. 2b). For both composites, the max. ΔTC is found at around 50 wt%, which infers that mass or volume ratio of dopants determines max. ΔTC independent of the type of PCM. The molecular weight of C28H28 is identical to that of the azobenzene dopant, while the molecular mass of C13-acid is about half (54%) that of the azobenzene dopant. Therefore, the degree of supercooling caused by each dopant isomerization in the C13-acid is much higher than in the C28H28 as shown in Fig. 2a. Upon identifying the optimal additive ratio (50 wt%), we applied the identical condition to other PCM systems for further investigation and to achieve max. ΔTC. We also note that the C13-acid composite with the highest additive ratio (80 wt%) has a small negative ΔTC (−1.7 °C), which is caused by the presence of excess dopant that has a higher TC (61 °C) than that of the PCM (38 °C). As shown in Fig. S1 (ESI†), both C13-acid and trans-Azo are significantly supercooled in the composite even before UV activation, displaying a TC of 26 °C and 48 °C, respectively. Upon UV irradiation, only 20% trans-to-cis isomerization occurs, as analyzed by the integration of differential scanning calorimetry (DSC) exothermic peaks, and the non-uniform dopant mixtures solidify over a broader temperature range of 49–55 °C (Fig. S1, ESI†). In this particular case, ΔTC is defined as the difference between TC of excess dopants, which leads to the abnormal and insignificant value.
Fig. 2c shows representative DSC plots taken on a composite (C15-acid, 50 wt% dopant) before and after UV activation. For the uncharged composites, trans-Azo solidifies at ∼55 °C, followed by crystallization of the PCM at around its intrinsic TC (T2). A small exothermic peak ∼32 °C represents a minor polymorph of the C15-acid.24 At any temperature between T1 and T2, the heat release from the supercooled liquid PCM composite can be controllably triggered by visible light illumination that isomerizes dopants and creates nucleation seeds of trans-Azo.
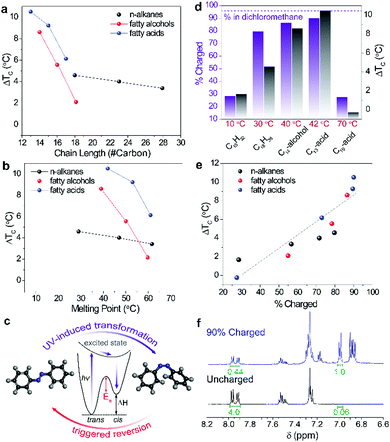
Fig. 3a shows the average ΔTC measured for each composite containing 50 wt% photo-switches. Notably, ΔTC generally decreases as the PCM possesses longer chains and higher melting points (Fig. 3b), except for C15H32 which showed a negligible ΔTC. Tm of the PCM defines the heat storage temperature, and the lower ΔTC measured at operation temperatures above 50 °C can be explained by the presence of an activation energy for azobenzene reverse isomerization (Fig. 3c). The activation energy for azobenzene derivatives that are mono-functionalized with ester or amide groups is known to be ΔH‡ of 88–92 kJ mol−1 and ΔS‡ of −41 to 56 J mol−1,25,26 and the thermal triggering of cis-to-trans isomerization occurs at a temperature range of 70–120 °C (Fig. S2, ESI†) in the solid state, which effectively deactivates the photo-switch. Therefore, at higher temperatures, the ratio of cis isomer decreases, leaving the discharged trans-Azo acting as nucleation seeds in the composite, which in turn results in a lower value for ΔTC. Once the temperature is as high as 70 °C, there is a very low number of cis-Azo molecules in the system, making the difference between the composite before and after UV activation negligible. C15H32, despite the low Tm of ∼10 °C, showed negligible supercooling, due to the poor miscibility of azobenzene in the short non-polar linear alkane.
The ratio between cis and trans isomers after UV activation in each composite can be studied by analysing 1H NMR of the composite, following the UV charging for an identical time period (Fig. 3d). The percent charged indicates the yield of UV-activated trans-to-cis conversion. The max charging achieved in dilute solutions (e.g. dichloromethane) was 96%, shown for comparison in the figure (dotted line). The bar graphs illustrate the degree of dopant charging in selected composites of varying PCMs where the liquid PCM molecules solvate dopants. The charging amount ranges from 28% to 90% depending on the heat storage temperature as indicated under each graph. We observed that ΔTC of each composite generally scales with the charging amount which is separately measured by 1H NMR. Fig. 3e confirms that charging amount is indeed one of the most significant factors that influence the degree of supercooling, ΔTC. In the case of C18H38, the charging was very efficient, achieving 80% conversion of azobenzene dopants from trans to cis, while ΔTC is lower than the predicted value according to the general trend shown in Fig. 3e. We observed that non-polar PCMs are generally less supercooled than polar counterparts, as a result of the weaker interaction with cis-Azo (Fig. S3, ESI†). The measurement of charging amount is performed by integrating the relative 1H NMR peaks that correspond to the aromatic protons on trans and cis dopants, as shown in Fig. 3f.
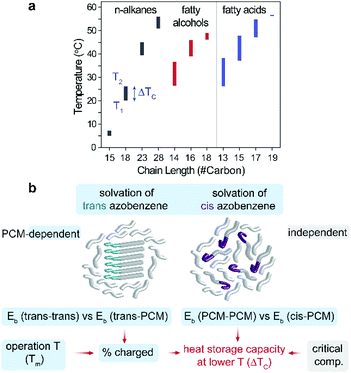
Fig. 4a summarizes the measured values, T1 and T2, from the charged and uncharged composites containing 50 wt% of azobenzene. This type of chart can provide guidance for the selection of suitable PCM composites for a given application that necessitates a certain condition for heat storage such as storage time. Based on the range of chemistries considered and the comparison between PCM systems with diverse physical properties, we are able to decouple several factors that influence ΔTC during different stages of the thermal storage cycle (Fig. 4b). Before UV activation, the interaction between molten PCM and trans-Azo molecules defines how effectively the additives are solvated by the PCM. If the binding energy (Eb) among trans-Azo additives is strong and the solvation is relatively weak, the additives remain aggregated and are less prone to photo-switching upon UV illumination. Therefore, the selection of PCM and its polarity heavily affects the UV charging amount (% charged) which our results show to be one of the most significant factors that determine ΔTC (Fig. 3e). Once trans-Azo is UV activated, the interaction between PCM and cis-Azo plays a dominant role in the supercooling process. In this case, if Eb among PCM molecules is strong relative to Eb between PCM and cis-Azo, the crystallization of the PCM is likely to occur as the temperature drops. The selection of a PCM that interacts strongly with the cis-Azo can thus enhance stabilization of the liquid PCM composite at lower temperatures, increasing ΔTC. The operation temperature which is dependent on the PCM selection (Fig. 3b) also determines the charging amount of additives. Lastly, a PCM-independent factor is the critical composition (additive % in the composite), which should be considered when designing the heat storage systems.
We gratefully acknowledge the support from Tata Center at MIT Energy Initiative (Award No. 021447–00033). J. H. D. thanks the support from MIT-Oxford Exchange Summer program.
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. Sarier and E. Onder, Thermochim. Acta, 2007, 452, 149–160 CrossRef.
- A. M. Khudhair and M. M. Farid, Energy Convers. Manage., 2004, 45, 263–275 CrossRef.
- F. L. Tan and C. P. Tso, Appl. Therm. Eng., 2004, 24, 159–169 CrossRef.
- E. Oró, A. de Gracia, A. Castell, M. M. Farid and L. F. Cabeza, Appl. Energy, 2012, 99, 513–533 CrossRef.
- R. K. Sharma, P. Ganesan, V. V. Tyagi, H. S. C. Metselaar and S. C. Sandaran, Energy Convers. Manage., 2015, 95, 193–228 CrossRef.
- J. Pereira da Cunha and P. Eames, Appl. Energy, 2016, 177, 227–238 CrossRef.
- S. A. Memon, Renewable Sustainable Energy Rev., 2014, 31, 870–906 CrossRef.
- Y.-J. Jin, B. Shin-Il Kim, W.-E. Lee, C.-L. Lee, H. Kim, K.-H. Song, S.-Y. Jang and G. Kwak, NPG Asia Mater., 2014, 6, e137 CrossRef.
- T. Khadiran, M. Z. Hussein, Z. Zainal and R. Rusli, Sol. Energy Mater. Sol. Cells, 2015, 143, 78–98 CrossRef.
- F. Agyenim, N. Hewitt, P. Eames and M. Smyth, Renewable Sustainable Energy Rev., 2010, 14, 615–628 CrossRef.
- K. Kant, A. Shukla, A. Sharma and P. H. Biwole, J. Energy Storage, 2018, 15, 274–282 CrossRef.
- W. Aftab, X. Huang, W. Wu, Z. Liang, A. Mahmood and R. Zou, Energy Environ. Sci., 2018, 11, 1392–1424 RSC.
- X. Huang, W. Xia and R. Zou, J. Mater. Chem. A, 2014, 2, 19963–19968 RSC.
- S. Zhang, Q. Tao, Z. Wang and Z. Zhang, J. Mater. Chem., 2012, 22, 20166–20169 RSC.
- G. G. D. Han, H. Li and J. C. Grossman, Nat. Commun., 2017, 8, 1446 CrossRef PubMed.
- C. Denekamp and B. L. Feringa, Adv. Mater., 1998, 10, 1080–1082 CrossRef.
- T. Ikeda and O. Tsutsumi, Science, 1995, 268, 1873–1875 CrossRef PubMed.
- T. Ikeda, T. Sasaki and K. Ichimura, Nature, 1993, 361, 428–430 CrossRef.
- A. M. Kolpak and J. C. Grossman, Nano Lett., 2011, 11, 3156–3162 CrossRef PubMed.
- M. Kenisarin and K. Mahkamov, Renewable Sustainable Energy Rev., 2007, 11, 1913–1965 CrossRef.
- T. Kousksou, A. Jamil, T. E. Rhafiki and Y. Zeraouli, Sol. Energy Mater. Sol. Cells, 2010, 94, 2158–2165 CrossRef.
- Y. Yuan, N. Zhang, W. Tao, X. Cao and Y. He, Renewable Sustainable Energy Rev., 2014, 29, 482–498 CrossRef.
- S. Ali Memon, T. Yiu Lo, X. Shi, S. Barbhuiya and H. Cui, Appl. Therm. Eng., 2013, 59, 336–347 CrossRef.
- A. D. Bond, New J. Chem., 2004, 28, 104–114 RSC.
- G. D. Han, S. S. Park, Y. Liu, D. Zhitomirsky, E. Cho, M. Dinca and J. C. Grossman, J. Mater. Chem. A, 2016, 4, 16157–16165 RSC.
- T. J. Kucharski, N. Ferralis, A. M. Kolpak, J. O. Zheng, D. G. Nocera and J. C. Grossman, Nat. Chem., 2014, 6, 441 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc05919e |
| This journal is © The Royal Society of Chemistry 2018 |