C(sp2)–H Trifluoromethylation of enamides using TMSCF3: access to trifluoromethylated isoindolinones, isoquinolinones, 2-pyridinones and other heterocycles†
Abstract
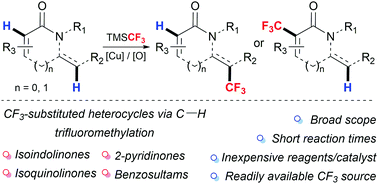
A method for the direct C(sp2)–H trifluoromethylation of enamides, including biologically relevant isoindolinones, isoquinolinones and 2-pyridinones using TMSCF3 under oxidative conditions is presented. The protocol is convenient, operationally simple and exhibits high tolerance across a multitude of relevant handles and functional groups.
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...