Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbide clusterfullerene DyYTiC@C80 featuring three different metals in the endohedral cluster and its single-ion magnetism†
Ariane
Brandenburg
 ,
Denis S.
Krylov
,
Alexander
Beger
,
Anja U. B.
Wolter
,
Bernd
Büchner
and
Alexey A.
Popov
,
Denis S.
Krylov
,
Alexander
Beger
,
Anja U. B.
Wolter
,
Bernd
Büchner
and
Alexey A.
Popov
 *
*
Leibniz Institute for Solid State and Materials Research (IFW), D-01069 Dresden, Germany. E-mail: a.popov@ifw-dresden.de
First published on 26th July 2018
Abstract
Carbide clusterfullerene DyYTiC@C80-Ih with three different metal atoms in the endohedral cluster is obtained by arc-discharge synthesis with methane as reactive gas and is successfully isolated by HPLC. The compound shows single-molecule magnetism (SMM) with magnetic hysteresis below 8 K. The SMM properties of DyYTiC@C80 are compared to those of DySc2N@C80 and the influence of the central atom in the endohedral cluster is analyzed.
A possibility of combining different types of metal atoms within one endohedral metallofullerene (EMF) molecule gives new insight into the chemical and physical properties of EMFs.1 For instance, combination of metals with different ionic radii can substantially change the yield or structural, chemical, or electrochemical properties of EMFs.2 A combination of metals with different contrast properties can be beneficial for biomedical applications,3 whereas “substitution” of lanthanides with diamagnetic metals has a dramatic influence on the magnetic properties of EMFs.4
The vast majority of mixed-metal EMFs are nitride clusterfullerenes, M3N@C2n, where M is usually a trivalent metal such as Sc, Y, or lanthanides. Sc-based mixed-metal nitride clusterfullerenes have been especially popular, and Sc–Y,2n Sc–Ti,5 Sc–V,2l and numerous Sc–lanthanide binary systems have been synthesized.2a,d,e,g,i,j,6 The list of studied non-Sc mixed-metal nitride clusterfullerenes is also rather long and includes Ti–Y,7 Ce–Y,2d,e Ce–Lu,2h,8 Gd–Ho,3b Gd–Lu,3 Ho–Y,4c Ho–Lu,3a,4c and Lu–Y9 binary systems. Mixed-metal EMFs with the same fullerene cage but different cluster compositions may have very similar retention times, which complicates their chromatographic separation. For ternary systems the complexity is increasing dramatically, and only one EMF with a ternary-metal cluster, ScYErN@C80, has been reported so far.10
M2TiC@C2n is a special type of trimetal carbide clusterfullerene,2f,11 which exists only as a mixed-metal EMF. The composition of the carbide cluster is fixed by Ti, which forms a double bond with the central carbon atom; M is thus a trivalent metal such as Sc, Y, or a lanthanide.2f,11 The variation of the cluster composition is not possible, i.e. formation of carbide clusterfullerenes with Ti3C, MTi2C, or M3C clusters has not been detected, which dramatically simplifies the isolation. If methane is used as a reactive gas in the arc-discharge synthesis, lanthanide-based M2TiC@C80-Ih can be obtained with a high degree of selectivity.11a The carbide cluster in M2TiC@C80 is isoelectronic and isostructural to the nitride cluster in M2ScN@C80, and both types of clusters have very similar charge distribution.11b Similar to Dy2ScN@C80,12 Dy2TiC@C80 has been found to be a single molecule magnet (SMM),11a but considerably softer than the former. In this work we show that this Ti-carbide template enables facile access to mixed-metal EMFs with three different metals in the cluster, synthesize DyYTiC@C80 and analyse its magnetic properties in comparison to DySc2N@C80, which was studied in detail earlier.13
EMF-containing soots were obtained by arc-discharge syntheses with graphite electrodes filled with the mixture of Dy, Y, Ti, and graphite powder (molar ratio 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:
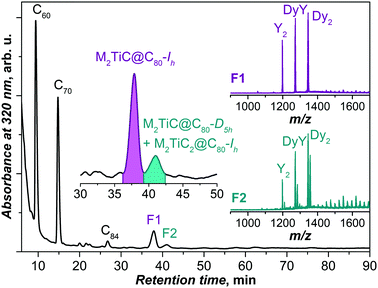
1:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.5). The atmosphere in the reactor contained a mixture of He (237 mbar) and CH4 (13 mbar).2f,11a After pre-extraction with acetone, the soot was Soxhlet-extracted with CS2 for 20 hours. A typical chromatogram of the fullerene extract obtained in these synthesis conditions is shown in Fig. 1. Based on mass-spectrometric analysis (laser-desorption ionization, LDI), the compounds with retention times less than 30 min are assigned to empty fullerenes (C60, C70, C84etc.). In the range of endohedral metallofullerenes (t > 30 min), two main chromatographic peaks are observed, denoted as F1 and F2 (Fig. 1). The dominant EMF fraction F1 (36–39 min) is found to be a mixture of three M2TiC@C80 EMFs (M2 = Dy2, Y2, and DyY). Mass-spectral analysis of the fraction F2 (39–42 min) shows the presence of M2TiC@C80 and M2TiC2@C80 (M2 = Dy2, Y2, and DyY).
12.5). The atmosphere in the reactor contained a mixture of He (237 mbar) and CH4 (13 mbar).2f,11a After pre-extraction with acetone, the soot was Soxhlet-extracted with CS2 for 20 hours. A typical chromatogram of the fullerene extract obtained in these synthesis conditions is shown in Fig. 1. Based on mass-spectrometric analysis (laser-desorption ionization, LDI), the compounds with retention times less than 30 min are assigned to empty fullerenes (C60, C70, C84etc.). In the range of endohedral metallofullerenes (t > 30 min), two main chromatographic peaks are observed, denoted as F1 and F2 (Fig. 1). The dominant EMF fraction F1 (36–39 min) is found to be a mixture of three M2TiC@C80 EMFs (M2 = Dy2, Y2, and DyY). Mass-spectral analysis of the fraction F2 (39–42 min) shows the presence of M2TiC@C80 and M2TiC2@C80 (M2 = Dy2, Y2, and DyY).
Based on the previous studies of the synthesis of lanthanide-titan carbide clusterfullerenes,2f,11a the fullerenes in fraction F1 can be identified as M2TiC@C80 with the Ih-symmetric cage isomer, whereas those in fraction F2 are identified as D5h cage isomers of M2TiC@C80 and Ih isomers of M2TiC2@C80.
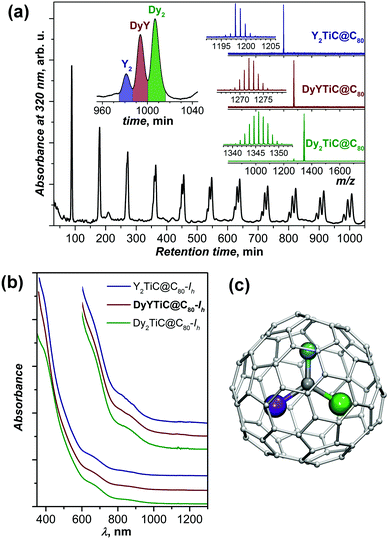
Fraction F1 was further subjected to recycling HPLC, which afforded the separation of Y2TiC@C80-Ih, DyYTiC@C80-Ih, and Dy2TiC@C80-Ih after 11 cycles (Fig. 2a). UV-vis-NIR absorption spectra of all three EMFs are virtually identical (Fig. 2b), which proves that they are isostructural. The spectra exhibit the characteristic absorption pattern observed earlier for M2TiC@C80 with the Ih cage isomer (the metal M has no significant influence on the spectra).11a,b Hence the molecular structure of the newly isolated DyYTiC@C80 can be unequivocally assigned to the C80-Ih cage (Fig. 2c).
The relative yield of M2TiC@C80-Ih (M2 = Y2, DyY, and Dy2), i.e. the ratio between Y2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DyY
DyY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dy2 in fraction F1, is estimated using mass-spectrometry (Fig. 1, 1
Dy2 in fraction F1, is estimated using mass-spectrometry (Fig. 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4
2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6) and from the peak areas after recycling HPLC (Fig. 2a, 1
3.6) and from the peak areas after recycling HPLC (Fig. 2a, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8
2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4). Two methods give consistent results, which deviate from the Y2
4.4). Two methods give consistent results, which deviate from the Y2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DyY
DyY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dy2 ratio of 1
Dy2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 expected for a purely statistical distribution given the Y
1 expected for a purely statistical distribution given the Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dy ratio in the starting material is 1
Dy ratio in the starting material is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A significant deviation indicates that Dy is more preferable for the formation of Ti-carbide clusterfullerenes than Y despite the slightly smaller ionic radius of the latter (0.90 Å for Y3+ and 0.91 Å for Dy3+ according to ref. 14). This finding also agrees with our previous observation that in the binary Dy–Ti and Y–Ti systems the yield of Dy2TiC@C80-Ih is higher than that of Y2TiC@C80-Ih.11a
1. A significant deviation indicates that Dy is more preferable for the formation of Ti-carbide clusterfullerenes than Y despite the slightly smaller ionic radius of the latter (0.90 Å for Y3+ and 0.91 Å for Dy3+ according to ref. 14). This finding also agrees with our previous observation that in the binary Dy–Ti and Y–Ti systems the yield of Dy2TiC@C80-Ih is higher than that of Y2TiC@C80-Ih.11a
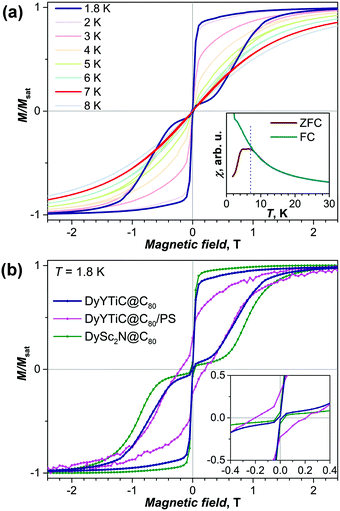
The central carbon in the endohedral carbide cluster bears a large negative charge similar to that in nitride clusterfullerenes, which results in a strong quasi-uniaxial ligand field and hence in a large magnetic anisotropy of the Dy ion(s) bonded to that carbon. Dy-based clusterfullerenes thus often behave as single molecule magnets.4a,11a,13b,15Fig. 3a shows magnetization curves of DyYTiC@C80 measured between 1.8 and 8 K. The low-temperature curves show the butterfly-shaped magnetic hysteresis characteristic for single ion magnets exhibiting quantum tunneling of magnetization (QTM) near zero field.16 As intermolecular interactions are known to be one of the major perturbations causing the QTM in single-ion magnets, magnetization measurements were also performed for DyYTiC@C80 diluted in polystyrene (PS). Recently we showed that dilution in PS substantially reduces the QTM step in DySc2N@C80.13a The decrease of the drop of the magnetization at zero-field caused by QTM is also observed in this work for PS-diluted DyYTiC@C80 (Fig. 3b). However, dilution in PS also leads to a strong diamagnetic background, which affects the shape of the magnetization curve. Besides, dilution increases the relaxation rate in a finite magnetic field.
Magnetic hysteresis is observed for DyYTiC@C80 up to 7 K, and is closed at higher temperatures. In agreement with these findings, the magnetic susceptibility measured during the temperature increase of a zero-field cooled sample (χZFC) and the magnetic susceptibility measured during cooling down the sample in a field of 0.2 T (χFC) diverge below 8 K (Fig. 3a, inset). Interestingly, χZFC shows not a sharp peak such as observed in DySc2N@C80 at 7.0 K,13a but a broad peak with a plateau between 4.7 and 6.9 K. We thus determine the blocking temperature of magnetization of DyYTiC@C80 as TB = 6.9 K. The overall magnetization behavior of DyYTiC@C80 and DySc2N@C80 is very similar. Both compounds exhibit butterfly hysteresis in the same temperature range with TB values close to 7 K. However, a comparison of the magnetic hysteresis curves (Fig. 3b) shows that the hysteresis in DySc2N@C80 is broader, which points to a slower relaxation of the magnetization in the nitride clusterfullerene.
Relaxation times of the magnetization for DyYTiC@C80 were measured by first magnetizing the sample to saturation at 5 T, then sweeping the field fast to 0.2 T, and then recording a decay of the magnetization while the system was slowly restoring its equilibrium state. Decay curves were measured at several temperatures between 1.8 and 4 K and then fitted with a stretched exponential. Above 4 K the relaxation time of DyYTiC@C80 is shorter than 100 s, and the determination of the relaxation time by DC SQUID magnetometry is less reliable because of the finite field sweep rates and the time necessary for the stabilization of the magnetic field before recording the decay curve. A detailed discussion of the procedure can be found in ref. 13a. The low yield of the compound precluded an accumulation of the amounts necessary for measurements of shorter relaxation times by AC magnetometry.
The temperature dependence of the relaxation time of the magnetization in DyYTiC@C80 is plotted in Fig. 4. As QTM is quenched by a finite magnetic field of 0.2 T, a very slow decay of the magnetization with the relaxation time of 2.3 × 104 s is observed at 1.8 K. An increase of the temperature accelerates the relaxation, and the temperature dependence takes a linear form in Arrhenius coordinates, which is usually associated with the Orbach relaxation mechanism:
τ−1 = τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/T) exp(−Ueff/T) | (1) |
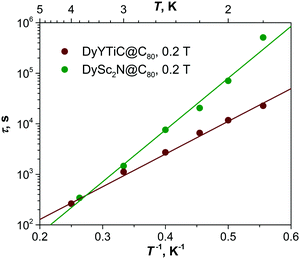 | ||
| Fig. 4 Relaxation times of magnetization of DyYTiC@C80 and DySc2N@C80 measured in the field of 0.2 T (dots) and their fits with eqn (1) (straight lines). | ||
It is quite remarkable that whereas single-ion magnets DyYTiC@C80 and DySc2N@C80 are not that different in their SMM properties, at least at low temperatures accessible for the current measurements, their dinuclear counterparts, Dy2TiC@C80 and Dy2ScN@C80, exhibit a stronger variation of the magnetic properties. The relatively narrow magnetic hysteresis in Dy2TiC@C80 is closing already near 3 K,11a whereas the blocking temperature of magnetization in Dy2ScN@C80 is as high as 8 K.12 The central atoms in the cluster play a two-fold role in SMM properties. First, it is the source of the large single-ion magnetic anisotropy.15b Second, it is a bridge between two Dy ions and hence plays a certain role in their exchange interactions. Replacement of a nitride ion by a carbide in the trimetal cluster can therefore affect both factors. Our study of DyYTiC@C80 and its comparison to DySc2N@C80 shows that the variation of the single-ion anisotropy appears to be of lesser importance for the low-temperature SMM behavior of the EMFs than the exchange coupling.
To conclude, in this work we synthesized the first carbide clusterfullerene with three different metals in the cluster, DyYTiC@C80-Ih. The Ti-carbide template limits the possible range of compositions of the mixed-metal clusters, and EMFs with three different metals can be obtained relatively straightforwardly. This opens a way for combining metals with different functionalities within one molecule. We also showed that DyYTiC@C80-Ih is a single molecule magnet with QTM near zero field and a blocking temperature of magnetization at 7 K.
The authors acknowledge funding by the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (grant no. 648295) and by Deutsche Forschungsgemeinschaft (grant PO 1602/5-1)
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989 CrossRef PubMed; (b) M. N. Chaur, F. Melin, A. L. Ortiz and L. Echegoyen, Angew. Chem., Int. Ed., 2009, 48, 7514 CrossRef PubMed; (c) X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723 RSC.
- (a) A. L. Svitova, A. A. Popov and L. Dunsch, Inorg. Chem., 2013, 52, 3368 CrossRef PubMed; (b) S. F. Yang, A. A. Popov, M. Kalbac and L. Dunsch, Chem.– Eur. J., 2008, 14, 2084 CrossRef PubMed; (c) S. F. Yang, M. Kalbac, A. Popov and L. Dunsch, ChemPhysChem, 2006, 7, 1990 CrossRef PubMed; (d) Y. Zhang, A. A. Popov and L. Dunsch, Nanoscale, 2014, 6, 1038 RSC; (e) Y. Zhang, S. Schiemenz, A. A. Popov and L. Dunsch, J. Phys. Chem. Lett., 2013, 4, 2404 CrossRef; (f) K. Junghans, K. B. Ghiassi, N. A. Samoylova, Q. Deng, M. Rosenkranz, M. M. Olmstead, A. L. Balch and A. A. Popov, Chem.– Eur. J., 2016, 22, 13098 CrossRef PubMed; (g) X. L. Wang, T. M. Zuo, M. M. Olmstead, J. C. Duchamp, T. E. Glass, F. Cromer, A. L. Balch and H. C. Dorn, J. Am. Chem. Soc., 2006, 128, 8884 CrossRef PubMed; (h) S. Stevenson, H. R. Thompson, K. D. Arvola, K. B. Ghiassi, M. M. Olmstead and A. L. Balch, Chem.– Eur. J., 2015, 21, 10362 CrossRef PubMed; (i) S. Stevenson, C. Chancellor, H. M. Lee, M. M. Olmstead and A. L. Balch, Inorg. Chem., 2008, 47, 1420 CrossRef PubMed; (j) Y. Zhang, K. B. Ghiassi, Q. Deng, N. A. Samoylova, M. M. Olmstead, A. L. Balch and A. A. Popov, Angew. Chem., Int. Ed., 2015, 52, 495 Search PubMed; (k) N. Chen, E. Y. Zhang, K. Tan, C. R. Wang and X. Lu, Org. Lett., 2007, 9, 2011 CrossRef PubMed; (l) T. Wei, S. Wang, X. Lu, Y. Tan, J. Huang, F. Liu, Q. Li, S. Xie and S. Yang, J. Am. Chem. Soc., 2016, 138, 207 CrossRef PubMed; (m) S. Wang, J. Huang, C. Gao, F. Jin, Q. Li, S. Xie and S. Yang, Chem.– Eur. J., 2016, 22, 8309 CrossRef PubMed; (n) N. Chen, L. Z. Fan, K. Tan, Y. Q. Wu, C. Y. Shu, X. Lu and C. R. Wang, J. Phys. Chem. C, 2007, 111, 11823 CrossRef.
- (a) E. B. Iezzi, J. C. Duchamp, K. R. Fletcher, T. E. Glass and H. C. Dorn, Nano Lett., 2002, 2, 1187 CrossRef; (b) Z. Zhang, Y. Liu, P. Han, S. Zhuang, T. Wang, S. Luo and B. Xu, ChemPhysChem, 2015, 16, 295 CrossRef PubMed.
- (a) R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, K. Krämer, S.-X. Liu, S. Decurtins, A. Popov, S. Yang, L. Dunsch and T. Greber, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 060406 CrossRef; (b) A. L. Svitova, Y. Krupskaya, N. Samoylova, R. Kraus, J. Geck, L. Dunsch and A. A. Popov, Dalton Trans., 2014, 43, 7387 RSC; (c) Y. Zhang, D. Krylov, S. Schiemenz, M. Rosenkranz, R. Westerstrom, J. Dreiser, T. Greber, B. Buchner and A. A. Popov, Nanoscale, 2014, 6, 11431 RSC.
- S. Yang, C. Chen, A. Popov, W. Zhang, F. Liu and L. Dunsch, Chem. Commun., 2009, 6391 RSC.
- (a) S. Stevenson, C. B. Rose, J. S. Maslenikova, J. R. Villarreal, M. A. Mackey, B. Q. Mercado, K. Chen, M. M. Olmstead and A. L. Balch, Inorg. Chem., 2012, 51, 13096 CrossRef PubMed; (b) S. Yang, A. A. Popov, C. Chen and L. Dunsch, J. Phys. Chem. C, 2009, 113, 7616 CrossRef; (c) Y. Zhang, D. Krylov, M. Rosenkranz, S. Schiemenz and A. A. Popov, Chem. Sci., 2015, 6, 2328 RSC; (d) Y. Zhang, A. A. Popov, S. Schiemenz and L. Dunsch, Chem.– Eur. J., 2012, 18, 9691 CrossRef PubMed; (e) R. M. Macfarlane, D. S. Bethune, S. Stevenson and H. C. Dorn, Chem. Phys. Lett., 2001, 343, 229 CrossRef.
- C. Chen, F. Liu, S. Li, N. Wang, A. A. Popov, M. Jiao, T. Wei, Q. Li, L. Dunsch and S. Yang, Inorg. Chem., 2012, 51, 3039 CrossRef PubMed.
- L. Zhang, A. A. Popov, S. Yang, S. Klod, P. Rapta and L. Dunsch, Phys. Chem. Chem. Phys., 2010, 12, 7840 RSC.
- S. Yang, A. A. Popov and L. Dunsch, Angew. Chem., Int. Ed., 2008, 47, 8196 CrossRef PubMed.
- N. Chen, E. Y. Zhang and C. R. Wang, J. Phys. Chem. B, 2006, 110, 13322 CrossRef PubMed.
- (a) K. Junghans, C. Schlesier, A. Kostanyan, N. A. Samoylova, Q. Deng, M. Rosenkranz, S. Schiemenz, R. Westerström, T. Greber, B. Büchner and A. A. Popov, Angew. Chem., Int. Ed., 2015, 54, 13411 CrossRef PubMed; (b) A. L. Svitova, K. Ghiassi, C. Schlesier, K. Junghans, Y. Zhang, M. Olmstead, A. Balch, L. Dunsch and A. A. Popov, Nat. Commun., 2014, 5, 3568 CrossRef PubMed; (c) F. Liu, F. Jin, S. Wang, A. A. Popov and S. Yang, Inorg. Chim. Acta, 2017, 468, 203 CrossRef.
- D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Büchner and A. A. Popov, Chem. Commun., 2017, 53, 7901 RSC.
- (a) D. Krylov, F. Liu, A. Brandenburg, L. Spree, V. Bon, S. Kaskel, A. Wolter, B. Buchner, S. Avdoshenko and A. A. Popov, Phys. Chem. Chem. Phys., 2018, 20, 11656 RSC; (b) R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, H. Brune, S. Rusponi, F. Nolting, A. Popov, S. Yang, L. Dunsch and T. Greber, J. Am. Chem. Soc., 2012, 134, 9840 CrossRef PubMed.
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- (a) F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Büchner and A. A. Popov, Nat. Commun., 2017, 8, 16098 CrossRef PubMed; (b) C.-H. Chen, D. S. Krylov, S. M. Avdoshenko, F. Liu, L. Spree, R. Yadav, A. Alvertis, L. Hozoi, K. Nenkov, A. Kostanyan, T. Greber, A. U. B. Wolter and A. A. Popov, Chem. Sci., 2017, 8, 6451 RSC; (c) R. Westerström, A.-C. Uldry, R. Stania, J. Dreiser, C. Piamonteze, M. Muntwiler, F. Matsui, S. Rusponi, H. Brune, S. Yang, A. Popov, B. Büchner, B. Delley and T. Greber, Phys. Rev. Lett., 2015, 114, 087201 CrossRef PubMed.
- (a) S. G. McAdams, A.-M. Ariciu, A. K. Kostopoulos, J. P. S. Walsh and F. Tuna, Coord. Chem. Rev., 2017, 346, 216 CrossRef; (b) J. Dreiser, J. Phys.: Condens. Matter, 2015, 27, 183203 CrossRef PubMed; (c) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef PubMed; (d) J.-L. Liu, Y.-C. Chen and M.-L. Tong, Chem. Soc. Rev., 2018, 47, 2431 RSC.
- (a) M. K. Singh and G. Rajaraman, Chem. Commun., 2016, 52, 14047 RSC; (b) V. Vieru, L. Ungur and L. F. Chibotaru, J. Phys. Chem. Lett., 2013, 4, 3565 CrossRef; (c) F. Cimpoesu, N. Dragoe, H. Ramanantoanina, W. Urland and C. Daul, Phys. Chem. Chem. Phys., 2014, 16, 11337 RSC.
- (a) D. L. Mills, Phys. Rev., 1966, 146, 336 CrossRef; (b) A. Lunghi, F. Totti, R. Sessoli and S. Sanvito, Nat. Commun., 2017, 8, 14620 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, mass-spectra, and details of relaxation measurements. See DOI: 10.1039/c8cc04736g |
| This journal is © The Royal Society of Chemistry 2018 |