Opposite mechanoluminescence behavior of two isomers with different linkage positions†
Abstract
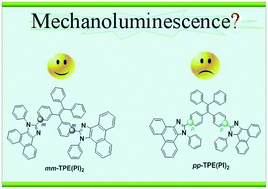
Two isomers of mm-TPE(PI)2 and pp-TPE(PI)2, constructed by the two same aromatic blocks of tetraphenylethene and phenanthro[9,10-d]imidazole, exhibit totally different mechanoluminescence, as a result of the ignorable different linkage positions on the tetraphenylethene moiety. Detailed analysis and theoretical calculations demonstrate the structure–packing–property relationship of organic mechanoluminescent luminogens, with the emphasis on the important role of molecular packing in the solid state.


 Please wait while we load your content...
Please wait while we load your content...