 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ball-free mechanochemistry: in situ real-time monitoring of pharmaceutical co-crystal formation by resonant acoustic mixing†
Adam A. L.
Michalchuk
 *abc,
Karl S.
Hope
*abc,
Karl S.
Hope
 ad,
Stuart R.
Kennedy
ad,
Stuart R.
Kennedy
 a,
Maria V.
Blanco
a,
Maria V.
Blanco
 e,
Elena V.
Boldyreva
e,
Elena V.
Boldyreva
 *cf and
Colin R.
Pulham
*cf and
Colin R.
Pulham
 *ab
*ab
aEaStCHEM School of Chemistry and Centre for Science at Extreme Conditions, University of Edinburgh, Edinburgh, UK. E-mail: c.r.pulham@ed.ac.uk; adam.michalchuk@ed.ac.uk
bEPSRC Centre for Continuous Manufacturing and Crystallisation (CMAC), UK
cREC-008 Novosibirsk State University, Novosibirsk, Russian Federation
dISIS Neutron and Muon Source, Harwell Science & Innovation Campus, Didcot, UK
eEuropean Synchrotron Radiation Facility (ESRF), Grenoble, France
fInstitute of Solid State Chemistry and Mechanochemistry SB RAS, Novosibirsk, Russian Federation. E-mail: eboldyreva@yahoo.com
First published on 29th March 2018
Abstract
Resonant acoustic mixing (RAM) is a new technology designed for intensive mixing of powders that offers the capability to process powders with minimal damage to particles. This feature is particularly important for mixing impact-sensitive materials such as explosives and propellants. While the RAM technique has been extensively employed for the mixing of powders and viscous polymers, comparatively little is known about its use for mechanosynthesis. We present here the first in situ study of RAM-induced co-crystallisation monitored using synchrotron X-ray powder diffraction. The phase profile of the reaction between nicotinamide and carbamazepine in the presence of a small amount of water was monitored at two different relative accelerations of the mixer. In marked contrast to ball-milling techniques, the lack of milling bodies in the RAM experiment does not hinder co-crystallisation of the two starting materials, which occurred readily and was independent of the frequency of oscillation. The reaction could be optimised by enhancing the number of reactive contacts through mixing and comminution. These observations provide new insight into the role of various experimental parameters in conventional mechanochemistry using liquid-assisted grinding techniques.
Mechanochemical techniques (e.g. planetary and vibratory ball milling, grinding, etc.) are a useful method for multi-component, one-pot syntheses of a wide range of compounds and materials.1 The mechanosynthesis of multi-component organic materials is believed to proceed via fluid-like intermediate phases. These phases may be formed through a eutectic melt,2 atmospheric moisture,3 sublimation of one component,4 or melting due to global temperature rise. The desolvation of solvated reactants has also been suggested to facilitate mechanochemical reactions.5 While a single general mechanism for liquid-assisted grinding (LAG) is not known, the liquid is believed to enhance molecular mobility, likely through dissolution.6 Despite the rather limited understanding of the mechanism of LAG, it has proved to be a promising technique for the “solvent-free” production in high yield of novel organic and inorganic compounds7 and materials,8,9 and has been demonstrated to provide control over selection of polymorphic form.10
A key feature of traditional mechanochemical experiments is the addition of milling bodies: hard, unreactive masses such as steel or ceramic balls, which introduce mechanical (and often thermal) energy through impact, shear, or a combination thereof. The addition of milling bodies is central to nearly all mechanochemical literature, with explicit discussion about the effects of the number and masses of the milling ball, together with the ratios of balls to the amount of sample.11,12 To date, the focus has remained on the amount of energy imparted by these balls on the powder sample at particular milling frequencies, rather than the physical effects of these balls and the associated energy input.8,11–13
The substantial mechanical energy introduced by the addition of milling bodies can cause considerable damage to particles, and may lead to generation of large numbers of defects into the crystalline lattice or complete amorphisation.14 This is particularly problematic if the powders being treated are highly sensitive to mechanical stimulus, e.g. energetic materials such as explosives and propellants, or when highly crystalline products are required. Furthermore, issues such as aggregation of particulates, “snow balling”, and compaction can all be encountered during conventional ball milling.15,16
In Resonant Acoustic Mixing (RAM), the sample vessel is placed on a plate connected to a bed of springs, and forced to oscillate at the fixed mechanical resonance frequency of the device. This motion is transferred to the powder, introducing intense, local mixing zones. The RAM technique, Fig. 1, has therefore been proposed as a method to perform mechanochemical processes under significantly more gentle conditions than those experienced during ball milling.17 For example, the impact-sensitive energetic co-crystal, 2CL-20·HMX, has been successfully prepared using this technique.18 A limited number of other co-crystals have also been produced using the RAM technique.17,19,20 The RAM technique offers particular benefits for mechanochemistry on account of its ability to rapidly and thoroughly mix materials.21
 | ||
| Fig. 1 Schematic representation of the resonant acoustic mixer (left), and photograph of experimental setup at ESRF (right). | ||
The monitoring of mechanochemical processes in real time has proved to be highly beneficial in order to identify and understand the correlation between instrument parameters,11,22 temperature23 and reaction rates, together with identification of short-lived intermediate phases24 and changes in reaction conditions.15 Real-time and in situ monitoring of mechanochemical processes is therefore vital for gaining an understanding of and hence controlling mechanochemical processes, and can provide different interpretations of reaction mechanisms when compared to ex situ analysis.15 This is particularly true of novel mechano-reactors that offer new control parameters and may induce novel reactivities.25 We report here the first in situ real-time monitoring of a RAM process using synchrotron X-ray radiation and a Resodyn LabRAM instrument, and the first example of in situ monitoring of mechanochemistry in the absence of milling bodies.
The reaction between form III of carbamazepine (CBZ; ca. 0.4 mmol) and form I nicotinamide (NIC; ca. 0.4 mmol) to form the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal CBZ·NIC was investigated according to Fig. 2. This system has been the subject of previous investigations as a model pharmaceutical co-crystal system,26 and its mechanosynthesis was the first example of a co-crystal produced using the RAM technique.19 However, to date, no investigations have been reported for the rates and reaction profiles of RAM-induced co-crystallisation or the effects of RAM conditions on these processes. CBZ is known to exist in five anhydrous crystal forms, as well as a dihydrate (CBZDH), which is formed by slurrying CBZ in water. Form III is the most thermodynamically stable form under ambient conditions.27 Likewise, NIC is known to exist in two solid forms, with form I being most stable.28 Previous ex situ ball-milling studies of CBZDH and NIC have demonstrated that CBZ·NIC is the final product and that this remains stable even at high relative humidities.29 This indicates that the co-crystal is more thermodynamically stable than CBZDH under normal conditions.
1 co-crystal CBZ·NIC was investigated according to Fig. 2. This system has been the subject of previous investigations as a model pharmaceutical co-crystal system,26 and its mechanosynthesis was the first example of a co-crystal produced using the RAM technique.19 However, to date, no investigations have been reported for the rates and reaction profiles of RAM-induced co-crystallisation or the effects of RAM conditions on these processes. CBZ is known to exist in five anhydrous crystal forms, as well as a dihydrate (CBZDH), which is formed by slurrying CBZ in water. Form III is the most thermodynamically stable form under ambient conditions.27 Likewise, NIC is known to exist in two solid forms, with form I being most stable.28 Previous ex situ ball-milling studies of CBZDH and NIC have demonstrated that CBZ·NIC is the final product and that this remains stable even at high relative humidities.29 This indicates that the co-crystal is more thermodynamically stable than CBZDH under normal conditions.
 | ||
| Fig. 2 Reaction scheme for the synthesis of CBZ·NIC co-crystal from CBZ III and NIC. Atoms shown: C (grey), O (red), N (blue), H (white). | ||
In the absence of a small addition of liquid, no reaction occurred between CBZ and NIC under any of the RAM conditions studied here. However, on addition of a single drop of water (ca. 20 μL, 1 mmol), the reaction products were observed. Unlike in a conventional ball mill, the LabRAM instrument maintains a constant frequency of approximately 61 Hz that corresponds to the mechanical resonance frequency of the device. Instead, the amplitude of the oscillation is varied, thus altering the relative acceleration experienced by the powder particles, termed the ‘G-force’.
The co-crystallisation was monitored at two acceleration settings of 50 G and 100 G. Both possible products (CBZDH and CBZ·NIC) display characteristic Bragg peaks in the region above a d-spacing of 7 Å, and so a qualitative indication of the relative rates of the co-crystallisation process could be assessed by monitoring the normalised area of these Bragg peaks. Fig. 3 shows that when the mixture was subjected to 50 G, an initial induction period was observed. This indicates that the two starting materials did not react prior to the beginning of the RAM mixing process. Within 10 seconds, the first indications of product peaks at d-spacings of ca. 9.78 and ca. 7.20 Å were observed. These correspond to the (100) and (040) planes of CBZDH, respectively. Formation of CBZDH occurred at a nearly continuous rate before reaching a final plateau, Fig. 3(II). The continuous and consistent growth of CBZDH before this point, however, suggests that formation of reactive contacts occurred readily, despite the absence of any milling bodies. Some indications of CBZ·NIC were observed, Fig. 3(I), with a characteristic Bragg peak appearing at a d-spacing of ca. 13.10 Å, corresponding to the (011) plane. This peak grew slowly in intensity before reaching a maximum. On mixing at 50 G, the major product peaks do not correspond to the thermodynamically most favourable phase (CBZ·NIC), but instead indicate the formation of CBZDH as the kinetically controlled product. Rietveld refinement, Fig. 3(III), of a subset of the in situ XRPD profiles suggests that >10% conversion to CBZDH was reached after only 90 seconds of mixing and that this phase fraction continued to grow steadily, (see ESI†) up to a limiting value of ca. 40%. Noting that water is present in excess, this conversion is likely limited by hydration of CBZ surfaces, impeding the hydration of deeper particle layers. Rietveld refinement also indicates that no more than 10 mol% of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CBZ·NIC co-crystal was formed over the period of the experiment.
1 CBZ·NIC co-crystal was formed over the period of the experiment.
The reaction profile was substantially different when a stoichiometric mixture of CBZ and NIC was subjected to mixing at 100 G – see Fig. 4(I) and (II) (full profiles are shown in ESI†). Within the first 30 s of mixing, the reaction proceeded rapidly, leaving only a small amount of the reactants. It should be noted that some Bragg peaks appeared and disappeared somewhat stochastically throughout the first ca. 560 s, suggesting that reactant phases had not been completely eliminated from the mixture, but simply remain undetected. This is most likely due to inhomogeneities in the mixing over the course of the reaction. In marked contrast to the reaction conducted at 50 G, Rietveld refinement of the XRPD profiles show that the dominant phase at 100 G was indeed the thermodynamically favoured co-crystal CBZ·NIC, with a final phase fraction of ca. 70 mol%. Some CBZDH was initially formed, followed by partial consumption to a final value of 15–20 mol%, Fig. 4(II). This is consistent with previous reports of the co-crystal being formed on RAM treatment with water, although the presence of CBZDH was not observed in those studies.19 The persistence of a small quantity of CBZDH, despite the apparent complete consumption of CBZ, can be attributed to the observation that there was residual powder (CBZ) that had aggregated at the corners of the reaction vessel and which had therefore not been efficiently dispersed into the reaction zone. This reflects the less than ideal geometry of the mixing vessel, which had been optimised for transmission of X-rays rather than for the most efficient mixing.
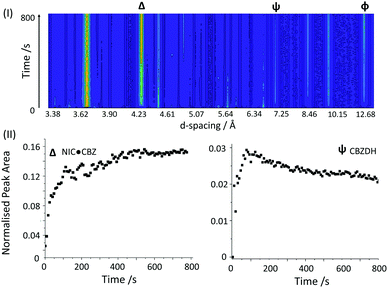 | ||
Fig. 4 RAM treatment of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture CBZ and NIC at 100 G. (I) Time-resolved XRPD profile of RAM treatment. (II) Normalised integrated peak areas of product peaks at d-spacings of ca. (Δ) 4.30 Å, (Ψ) 7.20 Å. (ϕ) 13.10 Å is given in ESI.† 1 mixture CBZ and NIC at 100 G. (I) Time-resolved XRPD profile of RAM treatment. (II) Normalised integrated peak areas of product peaks at d-spacings of ca. (Δ) 4.30 Å, (Ψ) 7.20 Å. (ϕ) 13.10 Å is given in ESI.† | ||
The XRPD background associated with the 100 G data were notably higher than at 50 G, with Rietveld refinements possible for only a few patterns. Rietveld refinement of the final mixture showed that the major phase at the end of the reaction was CBZ·NIC. Unfortunately, the most intense Bragg peaks, the (041) and (121) planes at a d-spacing of ca. 4.30 Å, overlap partially with a minor peak of CBZ. Hence we monitored both the (011) reflection at a d-spacing of 13.10 Å, as well as the reflections associated with the (041) and (121) planes, Fig. 4(II) and ESI.† For both reflections, a very rapid increase in the intensity of the Bragg peak was observed in the first 100 s of RAM treatment. This is followed by very slow, but continued growth until a final plateau is reached.
It is interesting to observe such a substantial difference in reaction rate when comparing the experiments conducted at two different accelerations. In ball-milling experiments an increased milling frequency generally results in an increasing reaction rate.22 In RAM, increasing acceleration affects only the magnitude of the oscillation, with no influence on the frequency of this motion. Previous work on the RAM technique has suggested that higher accelerations are often associated with more thorough and more rapid mixing of the sample.21,30 Higher accelerations have also been demonstrated to induce particle comminution25 and dissociation of particle aggregates.30 These factors all contribute to enhanced mixing, and formation of a larger number of reactive heterogeneous interfaces. While we note that higher mixing can also be associated with heating, earlier measurements suggest these temperature rises to be no higher than ca. 40 °C at both 50 and 100 G.17 Based on thermodynamic arguments, NIC and CBZ would be expected to form CBZ·NIC – this is the product formed in solution. However, in contrast to crystallisation from solution, the reaction zones in mechanochemical processes involving solid are limited to particle–particle interfaces. The composition of the interface is therefore crucial in determining the nature of the associated reaction, with heterogeneous interfaces required for multi-phase reactions to occur. At low accelerations, formation of interfaces between NIC and CBZ particles are likely inhibited by agglomeration, insufficient mixing, and by the presence of reaction products coating reactant particles. Thus CBZ particles are more likely to react with liquid water to form CBZDH. Conversely, higher accelerations lead to the disruption of agglomerates, comminution of larger particles, and thus overall more intimate mixing. As a result, a greater number of fresh reactive interfaces are generated and the reaction rate increases.
At lower accelerations in the RAM, CBZDH does not continue to react with NIC; it instead presumably coats unreacted CBZ, which hinders further reaction. This is in stark contrast to ball milling29 and the co-crystallisation under higher accelerations in the RAM. Indeed, on ball milling, the intensity of impacts does not allow solvate intermediates to be isolated, and highlights the “softness” of the RAM technique. The faster and more complete co-crystallisation under high G-force RAM conditions can be explained by comminution of particles and agglomerates. However, this mechanism does not explain the continued reaction of CBZDH with unreacted NIC. Previous measurements have suggested that the global temperatures obtained in the RAM at 50 and 100 G accelerations vary only slightly, and do not exceed ca. 40 °C.17 Dehydration is therefore not a bulk effect, with CBZDH remaining at the end of the 100 G reaction. To induce comminution, higher accelerations must be associated with increased particle–particle contact energies. These higher energies must therefore lead to surface effects, presumably involving dehydration of CBZDH. Hence the subsequent reaction of CBZDH with residual NIC.
The RAM technique provides a powerful alternative to conventional ball milling technologies, particularly for liquid-assisted mechanochemistry. Co-crystallisation by RAM is not restricted to the present system; in situ real-time XRPD offers great potential to probe a variety of RAM-induced processes. The rate of a mechanochemical co-crystallisation induced by RAM has been followed for the first time, and was found to increase with increasing acceleration. Higher accelerations lead to more intimate mixing (comminution, particle–particle mixing, surface regeneration, etc.) and thus a higher number of reactive interfaces. Moreover, it is possible to alter the pathways of the RAM-induced co-crystallisation using different accelerations. These reactions proceed despite the absence of milling bodies. It is therefore apparent that milling bodies are not always essential in order to obtain the high reaction rates that make mechanochemistry so appealing to the academic and industrial communities. Provided thorough mixing can be achieved, and new reactive interfaces can be formed, mechanochemical reactions can occur.
The authors thank ESRF ID31 (CH4805) for beam time. Thanks to an Edinburgh Global Research Scholarship (AM), EPSRC CMAC EP/I033459/1 (AM), Russian Ministry of Science & Education (AM), an ISIS Facility Development Studentship (KH), WSTC/0047 (SK/CP) and the Russian Academy of Sciences Project 0301-2018-0007 (EB) for funding. Final thanks to Prof. VV Boldyrev for many fruitful discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) L. Batzdorf, N. Zientek, D. Rump, F. Fischer, M. Maiwald and F. Emmerling, J. Mol. Struct., 2017, 1133, 18 CrossRef CAS
; (b) D. Douroumis, S. A. Ross and A. Nokhodchi, Adv. Drug Delivery Rev., 2017, 117, 178 CrossRef CAS PubMed
.
-
(a) O. Dolotko, J. W. Wiench, K. W. Dennis, V. K. Pecharsky and V. P. Balema, New J. Chem., 2010, 34, 25 RSC
; (b) R. Trotzki, M. M. Hoffmann and B. Ondruschka, Green Chem., 2008, 10, 873 RSC
.
- I. A. Tumanov, A. A. L. Michalchuk, A. A. Politov, E. V. Boldyreva and V. V. Boldyrev, CrystEngComm, 2017, 19, 2830 RSC
.
- M. A. Mikhailenko, T. P. Shakhtshneider and V. V. Boldyrev, J. Mater. Sci., 2004, 39, 5435 CrossRef CAS
.
- E. A. Losev and E. V. Boldyreva, CrystEngComm, 2014, 16, 3857 RSC
.
- T. Friscic and W. Jones, Cryst. Growth Des., 2009, 9, 1621 CAS
.
-
(a) V. Porte, M. Thioloy, T. Pigoux, T. X. Metro, J. Martinez and F. Lamaty, Eur. J. Org. Chem., 2016, 3505 CrossRef CAS
; (b) D. Tan and T. Friščić, Eur. J. Org. Chem., 2018, 18 CrossRef CAS
.
- J. L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13 CrossRef CAS PubMed
.
- E. V. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719 RSC
.
-
(a) F. Fischer, A. Heidrich, S. Greiser, S. Benemann, K. Rademann and F. Emmerling, Cryst. Growth Des., 2016, 16, 1701 CrossRef CAS
; (b) D. Cinčić, I. Brekalo and B. Kaitner, Cryst. Growth Des., 2012, 12, 44 CrossRef
.
- F. Fischer, N. Fendel, S. Greiser, K. Rademann and F. Emmerling, Org. Process Res. Dev., 2017, 21, 655 CrossRef CAS
.
- H. Kulla, F. Fischer, S. Benemann, K. Rademann and F. Emmerling, CrystEngComm, 2017, 19, 3902 RSC
.
- I. A. Tumanov, A. F. Achkasov, E. V. Boldyreva and V. V. Boldyrev, CrystEngComm, 2011, 13, 2213 RSC
.
- M. Descamps and J. F. Willart, Adv. Drug Delivery Rev., 2016, 100, 51 CrossRef CAS PubMed
.
- A. A. L. Michalchuk, I. A. Tumanov, S. Konar, S. A. J. Kimber, C. R. Pulham and E. V. Boldyreva, Adv. Sci., 2017, 4, 1700132 CrossRef PubMed
.
-
(a) E. V. Boldyreva, Curr. Pharm. Des., 2016, 22, 1 CrossRef
; (b) B. P. Hutchings, D. E. Crawford, L. Gao, P. Hu and S. L. James, Angew. Chem., Int. Ed., 2017, 1 Search PubMed
.
-
K. S. Hope, H. J. Lloyd, D. Ward, A. A. L. Michalchuk and C. R. Pulham, NTREM, Pardubice, Czech Republic, 2015 Search PubMed
.
- S. R. Anderson, D. J. Am Ende, J. S. Salan and P. Samuels, Propellants, Explos., Pyrotech., 2014, 39, 637 CrossRef CAS
.
- D. J. Am Ende, S. R. Anderson and J. S. Salan, Org. Process Res. Dev., 2014, 18, 331 CrossRef CAS
.
- K. Nagapudi, E. Y. Umanzor and C. Masui, Int. J. Pharm., 2017, 521, 337 CrossRef CAS PubMed
.
-
(a) J. G. Osorio and F. J. Muzzio, Powder Technol., 2015, 278, 46 CrossRef CAS
; (b) R. Tanaka, N. Takahashi, Y. Nakamura, Y. Hattori, K. Ashizawa and M. Otsuka, Anal. Sci., 2017, 33, 41 CrossRef CAS PubMed
.
- P. A. Julien, I. Malvestiti and T. Friščić, Beilstein J. Org. Chem., 2017, 13, 2160 CrossRef CAS PubMed
.
-
(a) K. Užarević, V. Štrukil, C. Mottillo, P. A. Julien, A. Puškarić, T. Friščić and I. Halasz, Cryst. Growth Des., 2016, 16, 2342 CrossRef
; (b) F. Fischer, K. J. Wenzel, K. Rademann and F. Emmerling, Phys. Chem. Chem. Phys., 2016, 18, 23320 RSC
.
- H. Kulla, S. Greiser, S. Benemann, K. Rademann and F. Emmerling, Cryst. Growth Des., 2017, 17, 1190 CAS
.
- I. A. Tumanov, A. F. Achkasov, S. A. Myz, E. V. Boldyreva and V. V. Boldyrev, Dokl. Chem., 2014, 457, 154 CrossRef CAS
.
- Z. Rahman, C. Agarabi, A. S. Zidan, S. R. Khan and M. A. Khan, AAPS PharmSciTech, 2011, 12, 693 CrossRef CAS PubMed
.
- A. L. Grzesiak, M. Lang, K. Kim and A. J. Matzger, J. Pharm. Sci., 2003, 92, 2260 CrossRef CAS PubMed
.
- J. Li, S. A. Bourne and M. R. Caira, Chem. Commun., 2011, 47, 1530 RSC
.
- N. Chieng, M. Hubert, D. Saville, T. Rades and J. Aaltonen, Cryst. Growth Des., 2009, 9, 2377 CAS
.
- J. G. Osorio, E. Hernández, R. J. Romañach and F. J. Muzzio, Powder Technol., 2016, 297, 349 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc02187b |
| This journal is © The Royal Society of Chemistry 2018 |

