Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective aqueous acetylation controls the photoanomerization of α-cytidine-5′-phosphate†
Christian
Fernández-García
,
Natalie M.
Grefenstette
and
Matthew W.
Powner
 *
*
Department of Chemistry, UCL, 20 Gordon St., London, WC1H 0AJ, UK. E-mail: matthew.powner@ucl.ac.uk
First published on 24th April 2018
Abstract
Nucleic acids are central to information transfer and replication in living systems, providing the molecular foundations of Darwinian evolution. Here we report that prebiotic acetylation of the non-natural, but prebiotically plausible, ribonucleotide α-cytidine-5′-phosphate, selectively protects the vicinal diol moiety. Vicinal diol acetylation blocks oxazolidinone formation and prevents C2′-epimerization upon irradiation with UV-light. Consequently, acetylation enhances (4-fold) the photoanomerization of α-cytidine-5′-phosphate to produce the natural β-pyrimidine ribonucleotide-5′-phosphates required for RNA synthesis.
Developing a chemical rationale for the origins of the canonical nucleotides is one of the key challenges at the heart of understanding the origins of life on Earth.1 In particular, RNA is thought to have played an essential role in the early evolution of life. Despite the remarkable progress made in elucidating prebiotic pathways towards ribonucleotide-2′,3′-cyclic phosphates,2–4 much less attention has been paid to understanding the origins of the canonical 5′-phosphorylation pattern observed in biological nucleotides.5 We have recently reported an oxidative phosphorylation of nucleotide precursors that delivers regiospecific 5′-phosphorylation in water.6 Our reaction pathway begins with prebiotically plausible acrolein 1 and 2-aminooxazole 2, and furnishes ribo-3-5P with excellent ribo-selectivity through a combination of kinetic and thermodynamic control (Fig. 1). Direct nucleophilic phosphorylation with inorganic phosphate in water sidesteps the requirement for electrophilic activation of phosphate, and as a direct consequence of exploiting the intrinsic nucleophilicity of phosphate, overcomes the widely cited “phosphorylation in water problem” during nucleotide synthesis.7 However, access to RNA through this route still requires an important stereochemical issue to be resolved. The reaction strategy relies upon (a tethered) simultaneous assembly of the nucleobase and sugar moieties of the cytidine nucleotide. The constrained architecture of intermediate ribo-3-5P provides the stereochemical control required to direct the stereoselective assembly of the ribo-diastereomer, but delivers the non-natural anomeric stereochemistry. Accordingly, pyrimidine ribonucleotide α-ribo-4 is readily synthesized in high yield upon reaction of ribo-3-5P with cyanoacetylene (5) in water (Fig. 1), and though α-ribo-4 has the natural 5′-phosphorylation pattern, it has non-natural C1′-stereochemistry which requires stereochemical inversion to access the canonical nucleotide.
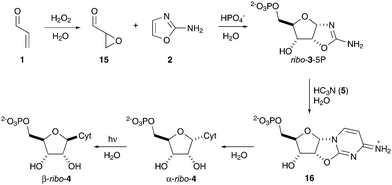 | ||
| Fig. 1 Previous work: synthesis of cytidine-5′-phosphates α-ribo-4 and β-ribo-4 by oxidative phosphorylation and nucleobase photoanomerization in water.2,6 Cyt = cytosine. | ||
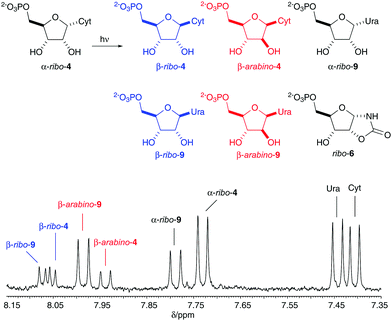
The absence of (biogenic) O2 and O3 UV-absorbance in a primitive atmosphere and the greater UV output from the young Sun,8,9 suggest that UV-light would be an important component of prebiotic environments and may have been the most abundant energy source available to drive prebiotic chemistry.8 Attempts to photoanomerize α-ribo-4 with UV irradiation to furnish β-ribo-4 is low yielding (4–6%),10 and also results in concomitant C2′-epimerization to give a complex mixture of ribo- and arabino-nucleotides (Fig. 2). Furthermore, photoanomerization of α-ribo-4 is severely prohibited by the formation of oxazolidinone 6, which gradually and irrevocably destroys the cytosine nucleobase through intramolecular reaction with the C2′-hydroxyl moiety of α-ribo-4.11
To overcome the poor photoanomerization of α-ribo-4, Sutherland and co-workers recently reported a remarkably efficient photoanomerization of non-natural nucleoside, α-2-thiocytidine, however, although certainly interesting, this strategy requires a non-aqueous reaction step (to yield a 2-thiopyrimidine intermediate) and ultimately requires synthesis of the non-natural 2′,3′-cyclic phosphates, rather than nucleotides with the canonical 5′-phosphorylation pattern observed in biology.4
With the specific goal of only undertaking all reactions in water, the most prebiotically plausible and biological relevant solvent, and retaining the 5′-phosphorylation pattern installed by oxidative phosphorylation,6 we consider the effect of nucleotide sugar moiety modification on photoanomerization en route to RNA. We have previously found that blocking the C2′-hydroxyl moiety of α-ribo-4 by non-natural 2′-phosphorylation, led to improved photoanomerization.12 However, to synthesize the targeted canonical nucleotide (β-ribo-4) the cis-2′,3′-diol moiety must be preserved, therefore 2′-phosphorylation diverts nucleotide synthesis away from the canonical structures observed in biology. Ideally, a temporary blockade of the C2′-hydroxyl moiety of α-ribo-4 would increase the efficiency of the photoanomerization, but then be removed (tracelessly) through a simple chemical transformation giving access to the target nucleotide structure (β-ribo-4). These considerations prompted us to further investigate the photochemistry of α-ribo-4 and its prebiotic derivatives.
We have previously demonstrated that C2′-acetylation can play an important role controlling the 3′–5′-specific ligation of RNA under prebiotic constraint,13 and C2′-acetylation has recently been implicated in (prebiotic) proofing of RNAs during energy-dissipative recycling.14 Therefore, we considered how C2′-acetylation would effect the photoanomerization of prebiotic α-ribo-4 to biological β-ribo-4. The simplicity and prebiotic plausibility of acyl-transfer reactions suggested that, if direct acetylation of the 2′,3′-diol moiety of non-natural α-ribo-4 could be readily and selectivity achieved in water, then acetylation would be an excellent prebiotic strategy to facilitate photoanomerization of ribo-4 by prohibiting the formation of oxazolidinone ribo-6 (Fig. 2). Subsequent slow ester hydrolysis (or rapid ester ammonolysis) would liberate the free hydroxyl moieties from these simple esters, rendering acetylation ideally suited to act as a traceless directing group in an aqueous environment.13 We have previously reported the selective acetylation of β-ribo-4, and, pleasingly, we observed that incubation of α-ribo-4 (100 mM) with N-acetylimidazole15 (4–10 eq.) in water at near-neutral pH furnished 2′,3′-(di-O-acetyl)-α-cytidine-5′-phosphate (α-ribo-7) in 60–91% yield (Fig. 3 and ESI,† Fig. S3), even with only 2 eq. of N-acetylimidazole significant 2′,3′-di-O-acetylation (29%) is observed. Imidazoles are widely exploited in prebiotic activation chemistry and thought to have potentially played a key role in the origins of life,16 however we observed comparable acetylation of α-ribo-4 with thioacetic acid and ferricyanide17 (ESI,† Fig. S1).
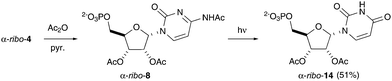
Importantly, acetylation of the cytosine moiety of α-ribo-4 was not observed in water, even with a large excess of acetylating agent (20 eq.). By comparison, under traditional acetylation conditions (pyridine, Ac2O) high yielding conversion to triacetylated nucleotide α-ribo-8 was found to occur with concomitant nucleobase modification (Fig. 4 and ESI,† Fig. S4). The difference in product distribution further suggests that selective aqueous acetylation, which does not prevent the Watson–Crick base pairing of canonical nucleotides, may have played a role in the first steps towards establishing biochemistry.
 | ||
| Fig. 4 Acetylation of α-cytidine-5′-phosphate α-ribo-4, in pyridine (pyr.) and subsequent rapid deamination upon UV irradiation (λ = 254 nm) in water. | ||
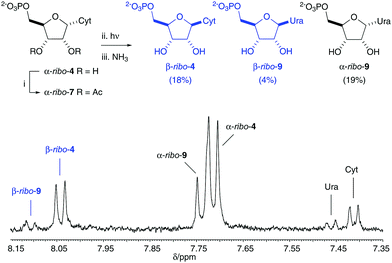
The selective di-O-acetylation of α-ribo-4 in water spurred us to investigate the UV irradiation of α-ribo-7. Accordingly, α-ribo-7 (2.5–5 mM) was irradiated with UV light (principle emission at λ = 254 nm) for 16 h in aqueous solution at pH 6.5 or 8.0. The products of irradiation were then subjected to ammoniacal deacetylation, and we were pleased to observe 22% (pH 6.5) and 17% (pH 8.0) anomerization to yield the natural cytidine and uridine ribonucleotides (β-ribo-4/β-ribo-9, Fig. 2, blue). Acetylation inhibited the formation of oxazolidinone ribo-6 by blocking the C2′-hydroxyl group, but remarkably, acetylation also completely suppressed C2′-epimerization. It is of note suppressing C2′-epimerization preserves the ribo-stereochemistry of α-ribo-7 (Fig. 3), and prevents formation of arabino-isomers during photoanomerization.
We next turned our attention to the role of the 5′-phosphate in the irradiation of α-ribo-7. Selective tri-O-acetylation of α-cytidine (α-ribo-10) was readily observed upon reaction N-acetylimidazole (10 eq.) in water at pH 8 to furnish 2′,3′,5′-(triacetyl)-α-cytidine (α-ribo-11) in 90% yield (65% isolated yield, Fig. 5). However, interestingly, subsequent irradiation – at initial pH 6.5 – now lead to significant levels of ester hydrolysis and concomitant formation of oxazolidinone by-product ribo-12 (33%; ESI,† Fig. S9). This hydrolysis had not been observed upon irradiation of α-ribo-7 at pH 6.5, and was significantly suppressed at pH 8.0, which is in line with the buffering capacity of the 5′-phosphate moiety. We recognized that Dewar pyrimidine intermediates are observed during the photochemical irradiation of pyrimidine nucleotides,2,11,18 and therefore suspected that hydrolysis was incurred upon pH fluctuation during irradiation. Photochemical irradiation was suspected to yield transient concentrations of hydroxide capable of hydrolyzing the ester moieties, for example, upon Dewar pyrimidine 13 C5-protonation (Fig. 5). This suggested that efficient acetyl protection of the 2′-hydroxyl moiety of cytidine required a sufficient buffering capacity during irradiation to prevent photochemical induced pH fluctuations. To test this hypothesis we next introduced phosphate buffer into the irradiation of α-ribo-11, and we were pleased to observe that near complete ester stability was now observed during a comparable irradiation (ESI,† Fig. S9).
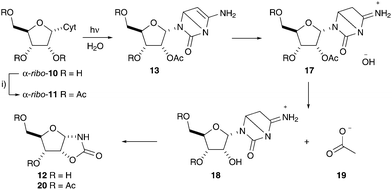 | ||
| Fig. 5 Photochemical induced ester hydrolysis and oxazolidinone formation upon irradiation of cytidine nucleotides.15 (i) N-Acetyl imidazole, H2O, pH 8, 90%. Cyt = cytosine. | ||
Finally, having observed the suppression of C2′-epimerization upon diol acetylation, we investigated the effect of nucleobase acetylation. Nucleobase acetylation had not been observed upon prebiotic (aqueous) acetylation of α-ribo-4, but was facile under non-aqueous acetylation protocols. Numerous investigations into prebiotic chemistry have proposed non-aqueous reaction steps, often to overcome perceived problematic effects of reaction modulation by water and the so-called “water problem”.19 Therefore we were intrigued to investigate the effect of the non-aqueous acetylation on photoanomerization. Interestingly, irradiation of α-ribo-8 lead to remarkably low photoanomerization yield with only trace yield observed after 16 h (Fig. 4, ESI,† Fig. S10). However, we observed unprecedentedly high nucleobase photohydrolysis to uridine nucleotide α-ribo-14 (51%), alongside significant nucleobase loss (19%) and oxazolidinone ribo-6 (6%) after 16 h.
We recognize the contingency of changing nucleotide concentration during acetylation and irradiation. High concentrations are not realistic in the context of the earth Earth's oceans, however are reasonable to consider in fresh water environments, where the process of dilution with water is not difficult to imagine in a prebiotic context. Moreover the UV screening effect of the water column further suggests fresh water, rather than a deep ocean, environment for prebiotic nucleotide photochemistry. Therefore, finally we carried out the one-pot acetylation-photoanomerization-deacetylation (ESI,† Fig. S8), which was observed to yield comparable anomerization results. Again no arabino-nucleotides were observed to form during irradiation. The influx of high-pH eluent, to liberate or introduce simple nucleophiles (such as ammonia),13 are also not thought to be unreasonable and provide a simple, effective and potentially prebiotic method to liberate the vicinal diol of β-ribo-4. Given the observed propensity of nucleobase acetylation to inhibit anomerization, it is of note that aqueous (prebiotically plausible) acetylation yielded only α-ribo-7 and not α-ribo-8. Moreover, as life is a chemical system that has evolved to exploit reactions, products and processes that occur in water, it is should not be seen as a surprise that aqueous reactions (more so than reactions in organic solvents) reflect the structure and pattern of biological chemistry. It is necessary that prebiotic chemistry embraces water as a solvent, and changes the mindset that invokes water as a “problem”. Prebiotic chemistry must come to terms with the principle that water is a part of the solution, and not the problem.
We gratefully acknowledge financial support from the Engineering and Physical Science Research Council (EP/K004980/1) and the Simons Foundation (318881).
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. F. Joyce, Nature, 2002, 418, 214–221 CrossRef CAS PubMed; L. E. Orgel, Biochem. Mol. Biol., 2004, 39, 99–123 Search PubMed; A. Eschenmoser, Angew. Chem., Int. Ed., 2011, 50, 12412–12472 CrossRef PubMed; J. W. Szostak, J. Syst. Chem., 2012, 3, 2 CrossRef; K. Ruiz-Mirazo, C. Briones and A. de la Escosura, Chem. Rev., 2014, 114, 285–366 CrossRef PubMed.
- M. W. Powner, B. Gerland and J. D. Sutherland, Nature, 2009, 459, 239–242 CrossRef CAS PubMed.
- S. Stairs, A. Nikmal, D.-K. Bučar, S.-L. Zheng, J. W. Szostak and M. W. Powner, Nat. Commun., 2017, 8, 15270 CrossRef CAS PubMed.
- J. Xu, M. Tsanakopoulou, C. J. Magnani, R. Szabla, J. E. Šponer, J. Šponer, R. W. Góra and J. D. Sutherland, Nat. Chem., 2016, 9, 303–309 CrossRef PubMed.
- S. Islam and M. W. Powner, Chem, 2017, 2, 470–501 Search PubMed; C. Fernández-García, A. J. Coggins and M. W. Powner, Life, 2017, 7, 31 CrossRef CAS PubMed.
- C. Fernández-García, N. M. Grefenstette and M. W. Powner, Chem. Commun., 2017, 53, 4919–4921 RSC.
- M. Karki, C. Gibard, S. Bhowmik and R. Krishnamurthy, Life, 2017, 7, 32 CrossRef PubMed; M. Gull, Challenges, 2014, 5, 193–242 CrossRef.
- S. Ranjan and D. D. Sasselov, Astrobiology, 2016, 16, 68–88 CrossRef CAS PubMed.
- R. J. Rapf and V. Vaida, Phys. Chem. Chem. Phys., 2016, 18, 20067–20084 RSC; J. Kasting, Science, 1993, 259, 920–926 CrossRef CAS PubMed; I. Ribas, G. F. Porto de Mello, L. D. Ferreira, E. Hébrard, F. Selsis, S. Catalán, A. Garcés, J. D. do Nascimento and J. R. de Medeiros, Astrophys. J., 2010, 714, 384–395 CrossRef.
- R. A. Sanchez and L. E. Orgel, J. Mol. Biol., 1970, 47, 531–543 CrossRef CAS PubMed.
- M. W. Powner, C. Anastasi, M. A. Crowe, A. L. Parkes, J. Raftery and J. D. Sutherland, ChemBioChem, 2007, 8, 1170–1179 CrossRef CAS PubMed.
- M. W. Powner and J. D. Sutherland, ChemBioChem, 2008, 9, 2386–2387 CrossRef CAS PubMed.
- F. R. Bowler, C. K. W. Chan, C. D. Duffy, B. Gerland, S. Islam, M. W. Powner, J. D. Sutherland and J. Xu, Nat. Chem., 2013, 5, 383–389 CrossRef CAS PubMed.
- A. Mariani and J. D. Sutherland, Angew. Chem., Int. Ed., 2017, 56, 6563–6566 CrossRef CAS PubMed.
- C. Fernández-García and M. W. Powner, Synlett, 2016, 78–83 Search PubMed.
- R. Lohrmann and L. E. Orgel, Nature, 1973, 244, 418 CrossRef CAS PubMed; T. J. Oró, B. Basile, S. Cortes, C. Shen and T. Yamrom, Origins Life, 1984, 14, 237–242 CrossRef; T. Wu and L. E. Orgel, J. Am. Chem. Soc., 1992, 114, 5496 CrossRef PubMed; J. C. Blain and J. W. Szostak, Annu. Rev. Biochem., 2014, 83, 615–640 CrossRef PubMed; A. Vaźquez-Salazar, G. Tan, A. Stockton, R. Fani, A. Becerra and A. Lazcano, Origins Life Evol. Biospheres, 2017, 47, 345–354 CrossRef PubMed.
- R. Liu and L. E. Orgel, Nature, 1997, 389, 52 CrossRef CAS PubMed.
- A. A. Shaw and M. D. Shetlar, J. Am. Chem. Soc., 1990, 112, 7736–7742 CrossRef CAS.
- S. A. Benner, H.-J. Kim and M. A. Carrigan, Acc. Chem. Res., 2012, 45, 2025–2034 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc01929k |
| This journal is © The Royal Society of Chemistry 2018 |