Open Access Article
Open Access ArticleArgentate(I) and (III) complexes as intermediates in silver-mediated cross-coupling reactions†
Sebastian
Weske‡
a,
Richard A.
Hardin‡
b,
Thomas
Auth‡
a,
Richard A. J.
O’Hair
 *ac,
Konrad
Koszinowski
*ac,
Konrad
Koszinowski
 *a and
Craig A.
Ogle
*a and
Craig A.
Ogle
 *b
*b
aInstitut für Organische und Biomolekulare Chemie, Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany. E-mail: konrad.koszinowski@chemie.uni-goettingen.de
bDepartment of Chemistry, University of North Carolina-Charlotte, Charlotte NC 28223, USA. E-mail: cogle@uncc.edu
cSchool of Chemistry and Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, 30 Flemington Rd, Parkville, Victoria 3010, Australia. E-mail: rohair@unimelb.edu.au
First published on 30th April 2018
Abstract
Despite the potential of silver to mediate synthetically valuable cross-coupling reactions, the operating mechanisms have remained unknown. Here, we use a combination of rapid-injection NMR spectroscopy, electrospray-ionization mass spectrometry, and quantum chemical calculations to demonstrate that these transformations involve argentate(I) and (III) complexes as key intermediates.
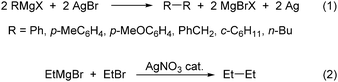
Organoargentates, unlike organocuprates, are not widely used in coupling reactions1,2 nor have they been as well characterized.3,4 This neglect is surprising given that early work demonstrated the potential of silver to mediate these transformations. Already in 1929, Gardner reported that reactions of Grignard reagents with stoichiometric amounts of silver salts gave rise to coupling reactions (eqn (1)).5 Over 40 years later Kochi demonstrated silver-catalyzed coupling (eqn (2)), and established that the mechanism is complex.6
Since then there have been several other reports on silver-catalyzed coupling reactions,1 but not much progress has been made in their mechanistic elucidation. In particular, the role of organoargentates(I) and organoargentates(III) in these reactions has received only little attention.4b,7–9 Here, we use a combination of: (1) rapid-injection NMR spectroscopy10 and electrospray-ionization (ESI) mass spectrometry to show that organoargentates(III) are formed in reactions of alkyl iodides with dimethylargentate; (2) gas-phase experiments and quantum chemical calculations to examine the elementary step of reductive elimination from organoargentates(III).11 The obtained mechanistic insight improves the fundamental understanding of the role of high-valent transition metals in cross-coupling reactions.12
Reaction of 2 equiv. of MeLi with silver(I) iodide at −78 °C afforded the dimethylargentate LiAgMe2·LiI (Scheme 1, X = I). Consistent with previous NMR-spectroscopic studies,3d the 1H NMR spectrum of LiAgMe2·LiI in THF-d8 at −100 °C displayed a doublet at −1.25 ppm, which exhibited an averaged 2JAg–H coupling of 7.30 Hz (Fig. S1, ESI†), while the 13C NMR spectrum gave a chemical shift of −8.63 ppm, with 1J109Ag–C of 92.45 Hz and 1J107Ag–C of 83.69 Hz (Fig. S2, ESI†).§ Upon warming to −70 °C, the dimethylargentate underwent an oxidative addition with allyl iodide (RI, 0.5 equiv.). The resulting transient intermediate [R(I)AgMe2]− was not detectable on the NMR time scale. Presumably, it immediately reacted with another equivalent of LiAgMe2·LiI to displace iodide for a methyl group and produce the hitherto elusive tetraalkylargentate(III) complex [RAgMe3]− (Fig. 1) along with monomethylsilver. [RAgMe3]− slowly decomposed at −70 °C to yield 1-butene via a reductive elimination (Scheme 1, eqn (3a)). The same product had also been observed for the reaction of LiCuMe2·LiI and allyl chloride.10c However, in that case the reactive organometallic species was not identified as the anionic ate complex, but the neutral intermediate [RCuMe2]. Interestingly, we did not find an analogous [RAgMe2] species in our present experiments.
 | ||
| Scheme 1 Formation of dimethylargentates LiAgMe2·LiX and their subsequent reactions with methyl or allyl iodide. | ||
 | ||
| Fig. 1 Stacked plot of the high-field sections of the 1H NMR spectra measured for the reaction of LiAgMe2·LiI with allyl iodide (RI) at −70 °C in THF-d8. | ||
In the 1H NMR spectrum (Fig. S3–S6, ESI†), [RAgMe3]− showed characteristic resonances at −0.01 and −0.16 ppm, which can be assigned to its Metrans and Mecis substituents, respectively. In both cases, averaged 2JAg–H couplings of approx. 5 Hz were observed. Very similar behavior had previously been found for the related tretraalkylcuprate(III) [RCuMe3]−.10c Further information could be obtained from the 13C NMR spectrum of [RAgMe3]− and, in particular, the 1JAg–C coupling constants. With 1J109Ag–C and 1J107Ag–C couplings of 52.5 and 45.6 (Metrans) and 48.9 and 42.4 Hz (Mecis), respectively, the methyl groups of [RAgMe3]− exhibited a smaller coupling to the silver nucleus than those in the dimethylargentate, in line with the expected change from sp to sp2d hybridization.13 The α-allyl carbon in [RAgMe3]− showed even smaller 1J109Ag–C and 1J107Ag–C couplings of 35.3 and 30.8 Hz, respectively, which point to a residual planarization in the allyl moiety and decreased s bonding character of the α-allyl carbon.
The reaction of LiAgMe2·LiI with allyl iodide also yielded small amounts of the tetramethylargentate(III) [AgMe4]− (Fig. 1). Presumably, [AgMe4]− originated from the substitution of the allyl group in [RAgMe3]− by a methyl group from LiAgMe2·LiI. [AgMe4]− could also be prepared from LiAgMe2·LiI and MeI (Scheme 1), but only in poor yield. The synthesis was greatly improved by the addition of 2 equiv. of PMe3 and the formation of neutral [(Me3P)AgMe3] (Fig. S7, ESI†), yielding [AgMe4]− upon reaction with MeLi (Fig. S8, ESI†). [AgMe4]− decomposed at temperatures above 0 °C and showed 1H and 13C NMR resonances and coupling constants much alike those of the methyl groups of its heteroleptic congener [RAgMe3]−. In contrast to the latter, [AgMe4]− gave only one set of signals, thus reflecting its higher symmetry. Moreover, its 1H NMR-spectroscopic signature closely resembles that of its [CuMe4]− homologue.10d
Next, we turned to negative-ion mode electrospray-ionization (ESI) mass spectrometry to analyze a THF solution of LiAgMe2·Li(CN). We observed a series of ate complexes including [AgnMen+1]− (n = 2–4), [Li2Ag3Me6]−, and [LiAg2Me4−n(CN)n]− (n = 1, 2), but no significant amounts of mononuclear [AgMe2]− (Fig. S9, ESI†).§¶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 These assignments were confirmed by both the accurate mass measurements (Table S1, ESI†) as well as the isotope patterns (Fig. S10–S26, ESI†). Organocuprates give similar ESI mass spectra.15 Most likely, the predominance of polynuclear ions in the mass-spectrometric experiments results from the shift of aggregation equilibria during the ESI process.15c,16 Upon the addition of methyl or allyl iodide, the spectra changed markedly and showed mononuclear Ag(III) ate anions as well as the corresponding higher aggregates as new species. The reaction with MeI afforded the lithium-bound dimer [LiAg2Me8]− as base peak (Fig. 2 and Fig. S20–S26, ESI†). The same ion as well as the related heteroleptic species [LiRAg2Me7]− and [LiR2Ag2Me6]− were observed for the reaction with allyl iodide (RI, Fig. S27–S32, ESI†). Argentate(III) complexes were also observed in control experiments with cyclopentyl methyl ether or tert-butyl methyl ether as solvents (Fig. S33–S39, ESI†).
14 These assignments were confirmed by both the accurate mass measurements (Table S1, ESI†) as well as the isotope patterns (Fig. S10–S26, ESI†). Organocuprates give similar ESI mass spectra.15 Most likely, the predominance of polynuclear ions in the mass-spectrometric experiments results from the shift of aggregation equilibria during the ESI process.15c,16 Upon the addition of methyl or allyl iodide, the spectra changed markedly and showed mononuclear Ag(III) ate anions as well as the corresponding higher aggregates as new species. The reaction with MeI afforded the lithium-bound dimer [LiAg2Me8]− as base peak (Fig. 2 and Fig. S20–S26, ESI†). The same ion as well as the related heteroleptic species [LiRAg2Me7]− and [LiR2Ag2Me6]− were observed for the reaction with allyl iodide (RI, Fig. S27–S32, ESI†). Argentate(III) complexes were also observed in control experiments with cyclopentyl methyl ether or tert-butyl methyl ether as solvents (Fig. S33–S39, ESI†).
The gas-phase fragmentation of the mass-selected homoleptic mononuclear complex [AgMe4]− solely led to the reductive elimination of ethane (Fig. S40, ESI†).§ Its heteroleptic counterpart [RAgMe3]− also underwent reductive elimination (Fig. 3) and preferentially released the cross-coupling product RMe (eqn (3a)), whereas the homo-coupling product Me2 (eqn (3b)) was formed only to a much smaller extent (Fig. 3). This result is consistent with the solution-phase NMR experiment, but is in stark contrast with gas-phase fragmentation of the related allylcuprate, [RCuMe3]−, for which the dominant channel affords the homo-coupling product.15c This finding for the first time shows that silver-mediated cross-coupling may substantially differ from the corresponding copper-based reactions. Fragmentation of the lithium-bound dimers resulted in formation of the mononuclear complexes (eqn (4)) and reductive eliminations (eqn (5), Fig. S41–S43, ESI†). For the heteroleptic complexes, cross-coupling (eqn (5a)) again strongly prevailed over homo-coupling (eqn (5b)). Secondary fragmentation reactions were also observed (eqn (6) and (7)).
 | ||
| Fig. 3 Mass spectrum of mass-selected [R107AgMe3]− (m/z 193) and its fragment ions produced upon collision-induced dissociation at ELAB = 7.5 eV. | ||
To obtain further insight into the reductive elimination as the product-forming step, we carried out quantum chemical calculations.§ The exothermic release of ethane from [AgMe4]− proceeds via a C2-symmetric transition structure, which is predicted to be 166 kJ mol−1 higher in energy than the reactant (Fig. 4). The resulting ion–molecule complex easily dissociates into [AgMe2]− and the coupling product. According to a natural population analysis (NPA),17 the release of ethane is accompanied by a decrease of the charge of the silver center from 0.56 to 0.25 (Fig. 4), consistent with a reduction from Ag(III) to Ag(I).18,19 For the lithium-bound dimer [LiAg2Me8]− the energy of the transition structure associated with the reductive elimination of ethane is calculated at 127 kJ mol−1 (Fig. S44, ESI†), a value significantly lower than for the mononuclear complex. Apparently, the interaction with the lithium center lowers the barrier substantially. This effect can be rationalized by Li+ withdrawing electron density from the ate complex and thereby raising the propensity of the silver(III) center to regain its preferred oxidation state of +I by reductive elimination. A similar trend has been found for the corresponding cuprate(III) complexes [CuMe4]− and [LiCu2Me8]−.15c The competing dissociation of [LiAg2Me8]−, eqn (4), requires an energy of 173 kJ mol−1 and, thus, is energetically more demanding than the reductive elimination. However, the latter reaction is entropically favored (Fig. S44, ESI†), which explains why it is found to compete with the former in the experiment (Fig. S41, ESI†). Thus, the results of the quantum chemical calculations are in full accordance with those of the gas-phase fragmentation experiments.
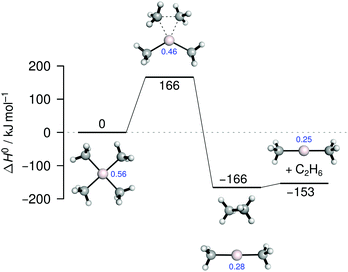 | ||
| Fig. 4 Energy diagram for the gas-phase reductive elimination of ethane from [AgMe4]− obtained from quantum chemical calculations. NPA charges of Ag are shown in blue. | ||
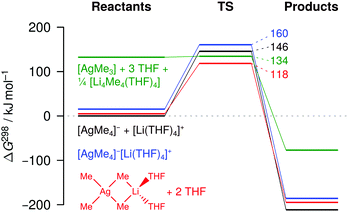
We also used theoretical methods to investigate the occurrence of reductive eliminations from [AgMe4]− and related systems in THF. Apart from the free ions, we considered solvent-separated and contact-ion pairs (Fig. 5, Fig. S45 and Table S2, ESI†). Our calculations predict that ion pairing does not strongly affect the Gibbs energy of the reactant argentate(III) complex at 298 K, but that the interaction with Li+ in the contact-ion pair substantially stabilizes the transition state, thus facilitating the overall reaction. This reactivity-enhancing effect of lithium essentially equals that already found for the gaseous [LiAg2Me8]− complex discussed above. For comparison, we also included the neutral species AgMe3 in our analysis, taking into account that the formation of this species from [AgMe4]−[Li(THF)4]+ generates 0.25 equiv. of [Li4Me4(THF)4] as the most stable form of MeLi in THF (Fig. 5; for the reactivity of other neutral systems, see Fig. S46, ESI†).20 Similar to previous theoretical results of Nakamura and coworkers, we find AgMe3 to eliminate ethane without any significant barrier.8 Nevertheless, the overall reaction proceeding via neutral [AgMe3] is still less favorable than the reaction via the contact-ion pair because the formation of [AgMe3] requires a large amount of energy. Thus, our calculations indicate that silver-mediated C–C coupling in solution indeed involves argentate complexes as central intermediates, whose reactivity is strongly influenced by Li+ counter-ion effects.
In conclusion, we have shown that Ag(I) and Ag(III) ate complexes are crucial intermediates in C–C coupling. Remarkably, the silver-mediated coupling reactions with allyl halides differed significantly from the previously studied copper-mediated reactions. For copper, the cross-coupling product evolves from the neutral allyl-containing intermediate [RCuMe2]. In contrast, we did not observe the analogous silver complex in the present experiments. This finding suggests that the silver-mediated formation of the cross-coupling product proceeds from anionic Ag(III) ate complexes. Our gas-phase experiments directly show the feasibility of this reaction. This mode of reactivity deviates from that of the analogous cuprate, which preferentially affords the homo-coupling instead of the cross-coupling product. Preliminary quantum chemical calculations on the competition between the reductive elimination elementary steps for isolated [RAgMe3]− and [RCuMe3]− anions reveal that fundamentally different mechanisms operate. Clearly, organoargentates are more than just a costly variant of the all popular organocuprates. The improved understanding of their mode of action also promises to boost their use in synthesis.
S. W., T. A., and K. K. gratefully acknowledge support from the DFG (KO 2875/6-1). R. A. J. O. thanks the Humboldt foundation for the award of a senior fellowship.
Conflicts of interest
There are no conflicts to declare.References
- Reviews on organosilver chemistry: (a) J.-M. Weibel, A. Blanc and P. Pale, Chem. Rev., 2008, 108, 3149 CrossRef CAS PubMed; (b) Silver in Organic Chemistry, ed. M. Harmata, Wiley, Hoboken, 2010 Search PubMed; (c) J. Cossy, in Grignard Reagents and Transition Metal Catalysts, ed. J. Cossy, Walter de Gruyter, Berlin/Boston, 2016, ch. 7 Search PubMed.
- Reviews on organocuprate chemistry: (a) R. M. Gschwind, Chem. Rev., 2008, 108, 3029 CrossRef CAS PubMed; (b) The Chemistry of Organocopper Compounds, ed. Z. Rappoport, I. Marek, Wiley, Hoboken, 2009 Search PubMed; (c) N. Yoshikai and E. Nakamura, Chem. Rev., 2012, 112, 2339 CrossRef CAS PubMed.
- For argentates(I), see: (a) C. Eaborn, P. B. Hitchcock, J. D. Smith and A. C. Sullivan, J. Chem. Soc., Chem. Commun., 1984, 870 RSC; (b) M. Y. Chiang, E. Böhlen and R. Bau, J. Am. Chem. Soc., 1985, 107, 1679 CrossRef CAS; (c) R. R. Burch and J. C. Calabrese, J. Am. Chem. Soc., 1986, 108, 5359 CrossRef CAS; (d) D. E. Bergbreiter, T. J. Lyncha and S. Shimazu, Organometallics, 1983, 2, 1354 CrossRef CAS.
- For stable isolated argentates(III) bearing fluorinated organyl substituents, see: (a) W. Dukat and D. Naumann, Rev. Chim. Miner., 1986, 23, 589 CAS; (b) R. Eujen, B. Hoge and D. J. Brauer, Inorg. Chem., 1997, 36, 1464 CrossRef CAS PubMed; (c) R. Eujen, B. Hoge and D. J. Brauer, Inorg. Chem., 1997, 36, 3160 CrossRef CAS PubMed.
- H. Gardner and P. Borgstrom, J. Am. Chem. Soc., 1929, 51, 3375 CrossRef.
- K. Kochi and M. Tamura, J. Am. Chem. Soc., 1971, 93, 1483 CrossRef.
- K. Murakami, K. Hirano, K. Yorimitsu and K. Oshima, Angew. Chem., Int. Ed., 2008, 47, 5833 CrossRef CAS PubMed.
- For theoretical studies on silver-mediated carbon-carbon coupling reactions, see: W. Nakanishi, M. Yamanaka and E. Nakamura, J. Am. Chem. Soc., 2005, 127, 1446 CrossRef CAS PubMed.
- Cationic Ag(III) complexes have recently been demonstrated to be involved in catalytic cross-coupling reactions: M. Font, F. Acuña-Parés, T. Parella, J. Serra, J. M. Luis, J. Lloret-Fillol, M. Costas and X. Ribas, Nat. Commun., 2014, 5, 4373 CAS.
- (a) S. H. Bertz, S. Cope, M. Murphy, C. A. Ogle and B. J. Taylor, J. Am. Chem. Soc., 2007, 129, 7208 CrossRef CAS PubMed; (b) S. H. Bertz, S. Cope, D. Dorton, M. Murphy and C. A. Ogle, Angew. Chem., Int. Ed., 2007, 46, 7082 CrossRef CAS PubMed; (c) E. R. Bartholomew, S. H. Bertz, S. Cope, M. Murphy and C. A. Ogle, J. Am. Chem. Soc., 2008, 130, 11244 CrossRef CAS PubMed; (d) E. R. Bartholomew, S. H. Bertz, S. K. Cope, M. D. Murphy, C. A. Ogle and A. A. Thomas, Chem. Commun., 2010, 46, 1253 RSC.
- For gas-phase studies on the reductive elimination of other high-valent transition-metal complexes, see: (a) K. L. Vikse, M. A. Henderson, A. G. Oliver and J. S. McIndoe, Chem. Commun., 2010, 46, 7412 RSC; (b) J. Hývl and J. Roithová, Org. Lett., 2014, 16, 200 CrossRef PubMed; (c) E. P. A. Couzijn, I. J. Kobylianskii, M.-E. Moret and P. Chen, Organometallics, 2014, 33, 2889 CrossRef CAS.
- J. Hickman and M. S. Sanford, Nature, 2012, 484, 177 CrossRef PubMed.
- 1 J coupling constants correlate with the s character of the binding electrons: R. K. Harris, Nuclear Magnetic Resonance Spectroscopy, Longman Science & Technical, Essex, 1986 Search PubMed.
- Organo(I)argentates have been prepared in the gas-phase via decarboxylation and their reactivity has been examined: (a) R. A. J. O'Hair, Chem. Commun., 2002, 20 RSC; (b) N. J. Rijs and R. A. J. O’Hair, Organometallics, 2010, 29, 2282 CrossRef CAS; (c) N. J. Rijs, N. Yoshikai, E. Nakamura and R. A. J. O’Hair, J. Am. Chem. Soc., 2012, 134, 2569 CrossRef CAS PubMed.
- (a) A. Putau and K. Koszinowski, Organometallics, 2010, 29, 3593 CrossRef CAS ; addition/correction: A. Putau and K. Koszinowski, Organometallics, 2010, 29, 6841 Search PubMed; (b) A. Putau and K. Koszinowski, Organometallics, 2011, 30, 4771 CrossRef CAS; (c) A. Putau, H. Brand and K. Koszinowski, J. Am. Chem. Soc., 2012, 134, 613 CrossRef CAS PubMed.
- The nanodroplets formed in the ESI process constantly lose solvent molecules due to evaporation and, thus, become enriched in the analyte, see: (a) A. Wortmann, A. Kistler-Momotova, R. Zenobi, M. C. Heine, O. Wilhelm and S. E. Pratsinis, J. Am. Soc. Mass Spectrom, 2007, 18, 385 CrossRef CAS PubMed; (b) N. G. Tsierkezos, J. Roithová, D. Schröder, M. Ončák and P. Slavíček, Inorg. Chem., 2009, 48, 6287 CrossRef CAS PubMed.
- E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735 CrossRef.
- Calculated partial charges are well-known to deviate from formal oxidation states: (a) G. Aullón and S. Alvarez, Theor. Chem. Acc., 2009, 123, 67 CrossRef; (b) P. Karen, Angew. Chem., Int. Ed., 2015, 54, 4716 CrossRef CAS PubMed.
- For a discussion about the oxidation states of related Cu(III) species, see: R. Hoffmann, S. Alvarez, C. Mealli, A. Falceto, T. J. Cahill III, T. Zeng and G. Manca, Chem. Rev., 2016, 116, 8173 CrossRef CAS PubMed.
- A. Ogle, B. K. Huckabee, H. C. Johnson IV, P. F. Sims, S. D. Winslow and A. A. Pinkerton, Organometallics, 1993, 12, 1960 CrossRef.
- (a) C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158 CrossRef CAS; (b) S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- C. Riplinger, P. Pinski, U. Becker, E. F. Valeev and F. Neese, J. Chem. Phys., 2016, 144, 024109 CrossRef PubMed.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and computational details, additional spectra, measured and theoretical m/z ratios, additional energy diagrams, calculated ΔG298 values. See DOI: 10.1039/c8cc01707g |
| ‡ These authors contributed equally. |
| § Rapid-injection NMR experiments were carried out as previously described.10 For the mass-spectrometric experiments, sample solutions were injected (flow rate of 8 μL min−1) into the ESI source (ESI voltage of 3.5 kV, N2 as nebulizer and dry gas, 60 °C) of a micrOTOF-Q II mass spectrometer (Bruker Daltonik). In gas-phase fragmentation experiments, ions were mass-selected (isolation width of 1.0–2.0 u), accelerated to a kinetic energy of ELAB, and allowed to collide with N2 gas. Molecular structures were obtained by PBE0-D3BJ21 geometry optimizations. For all structures, DLPNO-CCSD(T)22 single-point energies (SPEs) were calculated. To determine G298 values in THF, solvation energies ΔGsolv were calculated for each structure as difference between the PBE0-D3BJ SPE and the SPE obtained from a C-PCM23 PBE0-D3BJ calculation. Additional SMD24 PBE0-D3BJ calculations of ΔGsolv support the validity of the ΔG298(C-PCM) results (Fig. S47 and Table S2, ESI†). For further details, see the ESI.† |
| ¶ Control experiments showed that both Ag(CN) and AgI afforded similar ESI mass spectra upon reaction with MeLi. The sample solutions formed from Ag(CN) were more stable and directly comparable to those formed from Cu(CN) reported in ref. 15c. |
| This journal is © The Royal Society of Chemistry 2018 |