 Open Access Article
Open Access ArticleThiol–ene click chemistry towards easy microarraying of half-antibodies†
Rafael
Alonso
a,
Pilar
Jiménez-Meneses
 a,
Jaime
García-Rupérez
a,
Jaime
García-Rupérez
 b,
María-José
Bañuls
b,
María-José
Bañuls
 *a and
Ángel
Maquieira
*a and
Ángel
Maquieira
 *a
*a
aDepartamento de Química, Instituto Interuniversitario de Investigación de Reconocimiento Molecular y Desarrollo Tecnológico (IDM), Universitat Politècnica de València, Universitat de València, Camino de Vera s/n, 46022, Valencia, Spain. E-mail: mbpolo@upvnet.upv.es
bNanophotonics Technology Center, Universitat Politècnica de València, Valencia, Spain
First published on 29th May 2018
Abstract
A UV light-induced thiol–ene coupling reaction (TEC) between half-antibodies (hIgG) and vinyl functionalized glass surfaces was run for biosensing in the microarray format. The accomplished performance improved that obtained with whole antibodies.
The thiol–ene coupling reaction (TEC) fulfils all the desirable requirements of a click reaction.1 It is highly effective, proceeds in high yields under mild reaction conditions and does not generate side products. TEC is normally initiated by UV light, which induces the formation of a thiol radical that reacts with a carbon–carbon double bond and leads to a thioether. This reaction is extremely tolerant to a variety of functional groups and is orthogonal.2 Moreover, it can be performed in aqueous media, which allows this methodology to be used for biomolecules.3 All these features make the TEC reaction a very interesting methodology for the covalent immobilisation of biomolecules in microarray format and for its use in biosensing.
Liu et al. recently reported the use of thiol–ene click chemistry for an efficient and cysteine-selective thiol–ene click reaction-based bioconjugation strategy using colloidal nanoparticles.4 They demonstrated its applicability with thiolated organic compounds, aptamers and enzymes (HRP), but not for antibodies, which is surely due to the difficulty of making free thiol moieties available in them.
In line with our previous studies on the microarraying of thiolated oligonucleotides onto silicon-based surfaces by means of TEC,5 we envisioned the use of UV light-induced thiol–ene coupling to pattern antibodies microarrays rapidly and cleanly.
Immunoglobulin G antibodies (IgG) are the most prominent class of immunoglobulins employed in biosensing. They consist of four subunits, two heavy protein chains (H) and two light protein chains (L). The two halves are connected through the hinge region by a number of disulphide bonds, which depends on both the species6 and the antibody subclass.7 IgG immobilisation is key in the development of sensing devices to detect analytes, such as proteins or drugs. IgGs can be immobilised either randomly or in an oriented fashion.8 The latter is especially relevant for immunosensing applications since the antibody's paratopes must be available for antigens to be captured. Several approaches are reported for the oriented immobilisation of IgGs, of which the most relevant are those that employ protein A or G.9 Due to unique capabilities of the antibody microarray and its applicability in a range of biomedical projects, a series of different antibody microarrays have been developed, of which some have become commercially available.10 However, regardless the rapid technological advances in the last years, there are still technical issues that need to be overcome to ensure high-specificity and reproducibility of antibody arrays, to ensure high impact data and meaningful conclusions. The surface chemistry and the mode of antibody immobilization are within the experimental factors that may help to solve these problems.
Besides the whole antibody, antibody fragments like Fab’ and scFv fragments can also be successfully employed as probes for immunosensing.11 For the purpose of using the TEC approach to create antibody microarrays, we selected half-antibodies (hIgG) as capture probes. Then the disulphide bonds bridging the two halves of an IgG must be properly reduced, which results in two half-antibodies that bear as many free-thiol groups as the disulphide bonds that exist in the hinge region.12 hIgG have been reported to covalently link to maleimide-functionalised surfaces13 and to chemisorb onto gold14 and zinc15 surfaces. However, a direct, rapid and efficient attachment of hIgGs on glass or other Si-based surfaces has not yet been reported.
More importantly, none of the reported approaches allows the site-directed immobilisation of probes. Thus we hypothesised the use of UV light-induced thiol–ene coupling for the reaction between the exposed sulfhydryl groups in hIgG and alkene-functionalised glass surfaces. The new free thiol moieties generated by the reduction of antibodies would be available for reaction via TEC with alkenes attached to surfaces, which would allow them to be immobilised in an oriented fashion, and be ready to be employed in biosensing (Fig. 1).
 | ||
| Fig. 1 Reduction of Immunoglobulin G antibodies (IgG) to half-antibodies (hIgG) and their use to generate planar microarrays on alkene-functionalised surfaces. | ||
Anti-bovine serum albumin polyclonal antibodies (IgG αBSA), anti-human C-reactive protein monoclonal antibodies (IgG αCRP) and anti-cardiac troponin I monoclonal antibodies (IgG αcTnI) were chosen as capture probes for microarraying. hIgG were obtained by treating the commercially available whole antibodies with tris(2-carboxyethyl)phosphine (TCEP).12,16 When employing other reductants, such as mercaptoethylamine (MEA), over-reduction occurred, and additional disulphide bonds between light and heavy chains were cleaved, which led to loss of recognition capability. For the case of TCEP several temperatures, times, and concentrations were assayed. The chosen conditions were those providing the best biorecognition capability of the immobilised hIgG. The hIgG generated by TCEP reduction were characterised by SDS-PAGE electrophoresis (ESI,† Fig. S1). Additionally, in order to determine the available number of free thiol groups after reduction, hIgG were subjected to Ellman's assay17 (for Experimental details, see ESI†). This experimental procedure showed that the polyclonal hIgG αBSA prepared by TCEP reduction bore 3.7 free sulphhydryl groups while monoclonal hIgG αcTnI had 2.6 thiol groups. These values agree with the average number of disulphide bridges in the hinge region for the rabbit polyclonal and mouse monoclonal IgG2a subtype, respectively.18 Besides, the maintenance of the recognition ability indicated that the link between H and L was not disrupted.
Due to the presence of free thiols after the cleavage of disulphide bonds, the new generated hIgG were used for UV light-induced thiol–ene coupling (TEC) on vinyl-functionalised glass surfaces. Glass slides were activated by UV light irradiation and were subsequently functionalised by immersion in a solution of triethoxyvinylsilane in toluene (2%; 2 h), which led to an alkene-coated surface. The water contact angle (WCA) increased from 24° for the non-functionalised surface to 77° for the organosilane-coated chips (ESI,† Fig. S2).
The bovine serum albumin (BSA)/rabbit polyclonal hIgG αBSA system was selected as the model system to optimise the methodology. Thus freshly protein A purified polyclonal IgG αBSA was reduced with TCEP to the corresponding hIgG. AlexaFluor 647-labelled hIgG αBSA was dispensed over the vinyl-functionalised glass chips at different concentrations (0–20 μg mL−1). Then chips were irradiated for 5 s at 254 nm and were subsequently washed. By measuring the fluorescence emission with a homemade surface fluorescence reader (SFR),19 the immobilised probe density was determined as 2.36 ± 0.18 pmol cm−2 which corresponds to the maximum theoretical immobilisation density for a half-antibody by assuming that the dimensions of an antibody are ca. 15 × 5 × 5 nm3.
Several hIgG αBSA solutions at different concentrations (between 25 and 500 μg mL−1) were microarrayed over the vinyl-functionalised glass chips, and irradiated with UV-light to induce TEC. Whole IgG αBSA antibodies were also immobilised for comparison purposes. After washing, chips were subsequently incubated for 30 min in the dark with different solutions of freshly prepared AlexaFluor 647-labelled BSA (BSA*). The fluorescence of the cleaned and dried chips was measured with the SFR. hIgG performed significantly better than the corresponding whole antibodies in all cases. The fluorescence intensity of the signal obtained for hIgG was 7-fold stronger than the signal obtained for the immobilised IgG. When comparing to the performance of a standard microarray created by immobilising the IgG onto an epoxylated surface, the fluorescence obtained for our microarray was up to 4-fold higher than for the case of the reference microarray. The control experiments done in the absence of UV-light showed that irradiation was needed for the TEC reaction to take place and to immobilize the hIgG (Fig. 2). Under these experimental conditions, the sensitivity assay showed that the system can detect up to 0.2 μg mL−1 of labelled BSA when the hIgG is immobilised at concentrations that equal or were higher than 50 μg mL−1 (Fig. 3). The reached sensitivity fell within the limit of detection (LOD) that was intrinsic to the detection system employed and the obtained BSA labelling ratio. The density of labelled protein retained on the surface by the immobilised hIgG resulted 1.54 picomol cm−2, which provides a 65% of biorecognition yield from the attached probes.
Once the BSA/polyclonal hIgG αBSA model system was fully optimised, the methodology was applied to detect analytes of clinical interest, C-reactive protein (CRP) and cardiac troponin I (cTnI). CRP is an annular pentameric protein found in blood plasma, whose levels rise in response to inflammation. The sensitivity of the CRP assay using the corresponding monoclonal hIgG was determined by preparing microarrays and incubating them with different concentrations of AlexaFluor 647 labelled CRP (CRP*, in 10% diluted human serum, following the procedure described for the BSA system). In this case, system performance was assessed by employing a commercially available microarray scanner to avoid the LOD constraints associated with the SFR (when employing the microarray scanner for the BSA/hIgG αBSA model system the sensitivity reached was 2.3 ng mL−1). The minimum concentration detected under these experimental conditions was 2 ng mL−1 for a hIgG αCRP microarray generated by TEC when employing a 50 μg mL−1 concentration of half-antibody (Fig. 4).
The immunodetection of cTnI was also carried out. cTnI is a cardiac and skeletal muscle protein considered to be the most sensitive and, significantly, the most specific marker in myocardial infarction diagnosis.20 Due to the fact that cTnI is most unstable to buffer changes, and it could not, therefore, be labelled with the Alexa Fluor fluorophore, a sandwich-type immunoassay had to be performed. Thus the detection of cTnI was done by creating microarrays of the hIgG of monoclonal αcTnI by the TEC methodology as described above, and by incubating with different cTnI concentrations in 10% diluted human serum, and by finally developing with a labelled detection antibody. This optimised methodology obtained a sensitivity of 10 ng mL−1 of cTnI (Fig. 5).
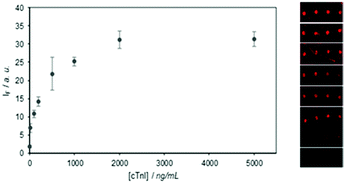 | ||
| Fig. 5 Sensitivity assay for cTnI using a sandwich immunoassay with hIgG αcTnI (50 μg mL−1) immobilised by TEC as the capture agent. | ||
Finally, in order to test the selectivity of the optimised systems, microarrays were created following the same biofunctionalisation protocol with a row of each hIgG αBSA, hIgG αCRP and hIgG αcTnI. Some chips were incubated only with labelled BSA, others with labelled CRP, others with cTnI, and others with a mixture of the three targets, in 10% diluted human serum. Fluorescence was recorded after the development step with the labelled detection antibody for cTnI. All the results demonstrated the specificity of capture (Fig. 6).
To the best of our knowledge, we herein report the first example of a UV light-induced thiol–ene coupling reaction between the free thiol groups present in half-antibodies and vinyl-functionalised surfaces to construct microarrays. The performance of these half-antibody microarrays generated by TEC dramatically improved the response compared to whole antibody microarrays, which is likely due to the fixed orientation of hIgG under these conditions. The methodology described herein allowed us to successfully determine interesting analytes (CRP and cTnI in this case), perform multiplexing experiments, and represent a non-reported approach for the effective immobilisation of antibodies under very mild, rapid and biocompatible conditions. The approach is applicable to a wide range of materials that can be functionalised with organosilane chemistry, and can selectively pattern antibodies on the surface by using selective irradiation through a photomask.
The authors acknowledge the funding from the European Commission through the project H2020-634013-PHOCNOSIS. This work was also partly supported by MINECO CTQ/2013/45875-R, CTQ/2016/75749-R, FEDER, and GVA PROMETEO II 2014/40.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef PubMed.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540 CrossRef PubMed.
- A. Dondoni, Angew. Chem., Int. Ed., 2008, 47, 8995 CrossRef PubMed; D. Weinrich, M. Koehn, P. Jonkheijm, U. Westerlind, L. Dehmelt, H. Engelkamp, P. C. M. Christianen, J. Kuhlmann, J. C. Maan, D. Nuesse, H. Schroeder, R. Wacker, E. Voges, R. Breinbauer, H. Kunz, C. M. Niemeyer and H. Waldmann, ChemBioChem, 2010, 11, 235 CrossRef PubMed.
- Y. Liu, W. Hou, H. Sun, C. Cui, L. Zhang, Y. Jiang, Y. Wu, Y. Wang, J. Li, B. S. Sumerlin, Q. Liu and W. Tan, Chem. Sci., 2017, 8, 6182 RSC.
- J. Escorihuela, M. J. Bañuls, R. Puchades and A. Maquieira, Chem. Commun., 2012, 48, 2116 RSC; J. Escorihuela, M.-J. Bañuls, R. Puchades and A. Maquieira, Bioconjugate Chem., 2012, 23, 2121 CrossRef PubMed; J. Escorihuela, M. J. Bañuls, S. Grijalvo, R. Eritja, R. Puchades and A. Maquieira, Bioconjugate Chem., 2014, 25, 618 CrossRef PubMed; D. Gonzalez-Lucas, M.-J. Bañuls, R. Puchades and A. Maquieira, Adv. Mater. Interfaces, 2016, 3, 1500850 CrossRef; M.-J. Bañuls, P. Jimenez-Meneses, A. Meyer, J.-J. Vasseur, F. Morvan, J. Escorihuela, R. Puchades and A. Maquieira, Bioconjugate Chem., 2017, 28, 496 CrossRef PubMed; D. Gonzalez-Lucas, M.-J. Bañuls, J. Garcia-Ruperez and A. Maquieira, Microchim. Acta, 2017, 184, 3231 CrossRef.
- V. Crivianu-Gaita, A. Romaschin and M. Thompson, Biochem. Biophys. Rep., 2015, 2, 23 Search PubMed.
- G. Vidarsson, G. Dekkers and T. Rispens, Front. Immunol., 2014, 5, 520 Search PubMed.
- P. Jonkheijm, D. Weinrich, H. Schroder, C. M. Niemeyer and H. Waldmann, Angew. Chem., Int. Ed., 2008, 47, 9618 CrossRef PubMed; F. Rusmini, Z. Zhong and J. Feijen, Biomacromolecules, 2007, 8, 1775 CrossRef PubMed.
- Y. Jung, J. Y. Jeong and B. H. Chung, Analyst, 2008, 133, 697 RSC; N. Tajima, M. Takai and K. Ishihara, Anal. Chem., 2011, 83, 1969 CrossRef PubMed.
- Z. Chen, T. Dodig-Crnkovic, J. Schwenk and S. Tao, Clin. Proteomics, 2018, 15, 7 CrossRef PubMed.
- V. Crivianu-Gaita and M. Thompson, Biosens. Bioelectron., 2016, 85, 32 CrossRef PubMed.
- A. Makaraviciute, C. D. Jackson, P. A. Millner and A. Ramanaviciene, J. Immunol. Methods, 2016, 429, 50 CrossRef PubMed.
- H. Y. Song, J. Hobley, X. Su and X. Zhou, Plasmonics, 2014, 9, 851 CrossRef.
- H. Sharma and R. Mutharasan, Anal. Chem., 2013, 85, 2472 CrossRef PubMed; S. Wu, L. Hong, X. M. Liang, X. Wu, B. Wang and Q. Zhang, Anal. Chem., 2014, 86, 4271 CrossRef PubMed.
- M. Pal, S. Lee, D. Kwon, J. Hwang, H. Lee, S. Hwang and S. Jeon, Anal. Chim. Acta, 2017, 952, 81 CrossRef PubMed.
- D. J. Cline, S. E. Redding, S. G. Brohawn, J. N. Psathas, J. P. Schneider and C. Thorpe, Biochemistry, 2004, 43, 15195 CrossRef PubMed.
- G. L. Ellman, Arch. Biochem. Biophys., 1959, 82, 70 CrossRef PubMed.
- L. J. Harris, S. B. Larson, K. W. Hasel and A. McPherson, Biochemistry, 1997, 36, 1581 CrossRef PubMed.
- D. Mira, R. Llorente, S. Morais, R. Puchades, A. Maquieira and J. Martí, Proc. SPIE, 2004, 5617, 364 CrossRef.
- A. B. Storrow, R. M. Nowak, D. B. Diercks, A. J. Singer, A. H. B. Wu, E. Kulstad, F. LoVecchio, C. Fromm, G. Headden, T. Potis, C. J. Hogan, J. W. Schrock, D. P. Zelinski, M. R. Greenberg, R. H. Christenson, J. C. Ritchie, J. S. Chamberlin, K. R. Bray, D. W. Rhodes, D. Trainor and P. C. Southwick, Clin. Biochem., 2015, 48, 260 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for the reduction of antibodies, surface chemical modification, photoimmobilisation procedures as well as SDS-PAGE electrophoresis and Ellman's assay. See DOI: 10.1039/c8cc01369a |
| This journal is © The Royal Society of Chemistry 2018 |




