Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A synthesis-enabled relative stereochemical assignment of the C1–C28 region of hemicalide†
Bing Yuan
Han‡
,
Nelson Y. S.
Lam‡
 ,
Callum I.
MacGregor
,
Jonathan M.
Goodman
,
Callum I.
MacGregor
,
Jonathan M.
Goodman
 and
Ian
Paterson
and
Ian
Paterson
 *
*
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: ip100@cam.ac.uk
First published on 14th March 2018
Abstract
Through synthesising both candidate diastereomers of a model C1–C28 fragment of the potent cytotoxic marine polyketide hemicalide, an assignment of the relative configuration between the C1–C15 and C16–C26 regions has been achieved. By detailed NMR comparisons with the natural product, the relative stereochemistry between these two 1,6-related stereoclusters is elucidated as 13,18-syn rather than the previously proposed 13,18-anti relationship. A flexible and modular strategy using an advanced C1–C28 ketone fragment 22 is outlined to elucidate the remaining stereochemical features and achieve a total synthesis.
Extracted from the marine sponge Hemimycale sp., hemicalide (1, Fig. 1) was reported to display impressive picomolar IC50 values against a panel of human cancer cell lines. Initial studies pointed towards a novel antimitotic mechanism of action via microtubule destabilisation, but its low isolation yield (0.5 mg) precluded further biological evaluation.1 While extensive 1D and 2D-NMR experiments were able to ascertain the planar structure of hemicalide, the paucity of material rendered its full 3D structural elucidation elusive. Indeed, all 21 stereocentres were left unassigned in the patent application, leading to over 2 million possible permutations.
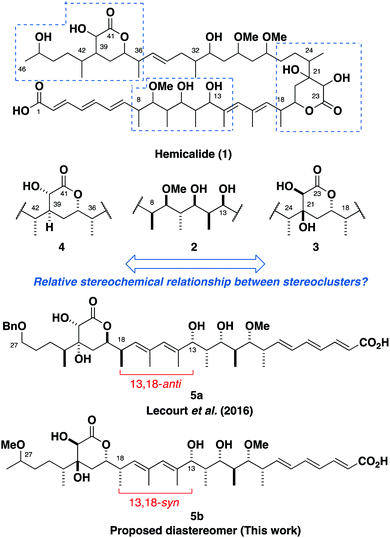 | ||
| Fig. 1 The planar structure for hemicalide (1) and the assigned relative configurations of each subunit.2–4,6 Previous endeavours towards the total synthesis of hemicalide have targeted the 13,18-anti diastereomer 5a. This work reassigns the relative configuration to be as represented in the 13,18-syn diastereomer 5b. | ||
Through a combination of computational NMR shift predictions and spectroscopic corroboration from synthesised model fragments, we, together with the Ardisson and Cossy/Meyer groups, have assigned the relative configuration of hemicalide for the C8–C13 stereohexad 2,2,3 C16–C26 dihydroxylactone 34,5 and C35–C42 hydroxylactone 4.6 To date, the relative and absolute configuration of the C26–C34 polyacetate region, and the isolated C45 stereocentre remained unassigned.6 While efforts have been reported by the Ardisson and Cossy/Meyer groups towards joining the fragments together, previously reported synthetic studies on the full C1–C27 fragment have targeted the 13,18-anti diastereomer 5a;5,7 only one of the two diastereomeric possibilities between the C1–C15 and the C16–C26 regions. Through the synthesis and detailed NMR spectroscopic comparisons of both candidate diastereomers, we herein report the likely relative configuration of the full C1–C28 region of hemicalide as in the 13,18-syn diastereomer 5b.
Our approach needed to be modular and highly stereoselective to allow for the facile synthesis of both enantiomers of each fragment. Given that the majority of the missing stereochemical information lies in the C27–C34 region, a flexible strategy was devised involving a late stage aldol/reduction sequence to forge the C27 and C29 stereocentres (Scheme 1). A cross-coupling was envisaged to connect the C34–C46 hydroxylactone 6 with each enantiomer of the C29–C33 aldehyde 7, as well as the C1–C15 and C16–C28 fragments 8 and 9 together.
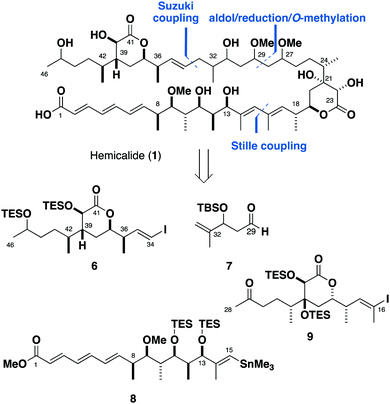 | ||
| Scheme 1 Retrosynthetic analysis of hemicalide (1) reveals key fragments 6–9. Note that only the relative configuration within each of the three stereoclusters is suggested. | ||
Our first quest towards determining the complete stereochemistry of hemicalide began with the C1–C28 region, consisting of the C1–C15 and the C16–C28 stereoclusters. With computational methods proving useful in elucidating the relative configuration within the isolated fragments,2,4 we initially sought to employ our DP4f methodology2 to elucidate their relative stereochemical relationship. However, due to the size and flexibility of the virtual fragments involved, it proved to be too computationally demanding to accurately model the C1–C28 truncate. As such, we turned towards synthesising both candidate diastereomers of the full C1–C28 region to elucidate the relative configuration between these two stereoclusters. We envisioned that meaningful NMR differences could be observed for each diastereomer despite the relatively remote 1,6-related chiral environments between C13 and C18 adjacent to the connecting E,E-diene.8
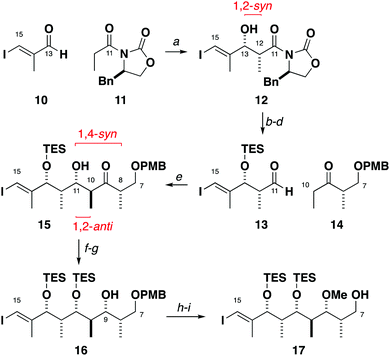
The stereohexad in the C1–C15 region 8 was installed by asymmetric boron-mediated aldol reactions. Starting from aldehyde 10, an Evans aldol reaction (Bu2BOTf, DIPEA)9 with oxazolidinone 11 gave adduct 12 as a single diastereomer, setting up the 1,2-syn relationship at C12 and C13 (Scheme 2). TES protection and auxiliary cleavage (LiBH4) followed by a Swern oxidation gave aldehyde 13. Using our standard conditions (c-Hex2BCl, Et3N),10 ethyl ketone 14 was engaged with aldehyde 13 to yield the 1,2-anti-1,4-syn adduct 15 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). The C11 hydroxyl group was TES protected before submitting to a controlled reduction (DIBAL) to afford alcohol 16 (>20
1 dr). The C11 hydroxyl group was TES protected before submitting to a controlled reduction (DIBAL) to afford alcohol 16 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, see the ESI† for confirmation of stereochemistry). Methylation (Me3O·BF4, Proton Sponge®) and deprotection of the PMB ether (DDQ) gave 17. As the absolute configuration is set by 11 and 14, the enantiomer of 17 was obtained in a similar fashion by employing ent-11 and ent-14 instead.
1 dr, see the ESI† for confirmation of stereochemistry). Methylation (Me3O·BF4, Proton Sponge®) and deprotection of the PMB ether (DDQ) gave 17. As the absolute configuration is set by 11 and 14, the enantiomer of 17 was obtained in a similar fashion by employing ent-11 and ent-14 instead.
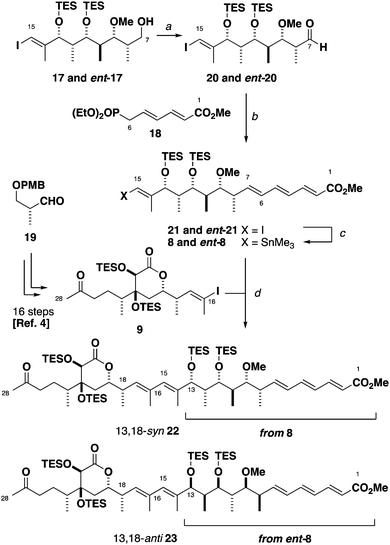
The phosphonate 18 (Scheme 3) was prepared in three steps from sorbic acid. We previously reported in the enantiomeric series the synthesis of the C16–C28 ketone 9 in 16 steps from aldehyde 19.4 Dess–Martin oxidation of alcohol 17 provided aldehyde 20, which was subjected to an HWE olefination with phosphonate 18 to afford the C1–C15 vinyl iodide as a single geometric isomer. At this juncture, the planned cross-coupling step required the appendage of a stannane handle onto either the C1–C15 or the C16–C28 vinyl iodide (21 and 9 respectively). As the C16 vinyl stannane proved to be highly prone to decomposition, we instead converted the vinyl iodide 21 to the corresponding stannane 8 under Wulff–Stille conditions (Pd(PPh3)2Cl2, (Me3Sn)2).11 With the two key fragments in hand, a modified Stille coupling12 afforded the advanced C1–C28 ketone 22. By repeating the Stille coupling with ent-8, the 13,18-anti diastereomer 23 was also obtained in an analogous manner. Encouragingly, NMR comparisons in CDCl3 indicated clear differences between the protected 13,18-syn and 13,18-anti diastereomers despite the distal nature of the two stereoclusters.
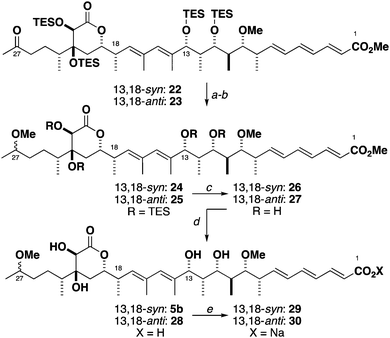
Initial exploratory studies showed that deprotecting the C16–C28 ketone resulted in the concomitant hemiacetal engagement with the C27 carbonyl. Furthermore, the natural product contains a methyl ether at C27 and its presence is expected to aid a more representative NMR correlation. As such, we looked to transform the C27 carbonyl to the corresponding methyl ether. Attempts at effecting a stereoselective reduction on both the full truncate, as well as the C16–C28 lactone and intermediates thereof proved to be ineffective. Guided by previous reports6 that the configuration of the distal C46 hydroxyl group had little influence with NMR shifts on the C36–C46 fragment, we separately subjected 22 and 23 to a non-selective reduction (NaBH4) and methylation (Me3O·BF4, Proton Sponge®) to afford the C27 methyl ethers 24 and 25 (Scheme 4). While the C27 epimers were inseparable by chromatography, the NMR assignment proved to be straightforward. Global silyl deprotection was found to be problematic. After extensive experimentation, careful treatment of the protected model truncates with TASF followed by HF·py/py reliably afforded tetraols 26 and 27. Ester hydrolysis using Ba(OH)213 provided the acids 5b and 28.
We found that the NMR chemical shifts for the C1–C7 region were highly dependent on the protonation state of the C1 carboxylic acid (see the ESI†), though this did not appear to significantly affect the signals for the remainder of the molecule. The effect of the protonation state on the 13C NMR was most noticeable for C1–C3, where presumed proton exchange kinetics resulted in peak broadening in acids 5b and 28. To verify this hypothesis, and noting that the natural product was proposed to be isolated as the carboxylate salt,3in situ treatment of the free acid with Na2CO3 effected complete acid deprotonation, which sharpened the carbon signals at C1-C3 in the sodium salts 29 and 30.
At this stage, we were able to compare both the 1H and 13C NMR chemical shifts of our model truncates 5b and 28 with the 13,18-anti truncate 5a of Lecourt et al.5 and hemicalide itself. Notably the 13,18-anti acid 28 correlated well with the spectroscopic data reported for 5a (see the ESI†). The differences did not appear to be particularly diagnostic in the 1H NMR spectra for both acids 5b and 28, however, distinct differences were noted when comparing their 13C NMR data to the natural product (Fig. 2).1 In particular, the chemical shift differences for the 13,18-syn acid 5b did not exceed ±0.01 ppm for 1H NMR, and ±0.1 ppm for 13C NMR. This was in contrast to the 13,18-anti acid 28, where differences up to ±0.04 ppm (H11) and ±0.6 ppm (C11) were observed for the 1H and 13C NMR shifts respectively. Overall, the absolute and maximum errors recorded for the 13,18-syn diastereomers (Table 1, entries 1 and 2) were noticeably smaller than the corresponding 13,18-anti diastereomers (Table 1, entries 4 and 5) and to previously published values for 5a (entry 3).5 Interestingly, while the natural product was proposed to be isolated as the carboxylate salt,3 the correlation for both diastereomers of salts 29 and 30 (entries 2 and 5) was poorer than for the corresponding acids 5b and 28 (entries 1 and 4), particularly in the C1–C7 triene region (see the ESI†). This suggests that hemicalide was likely isolated as the acid rather than the carboxylate salt.
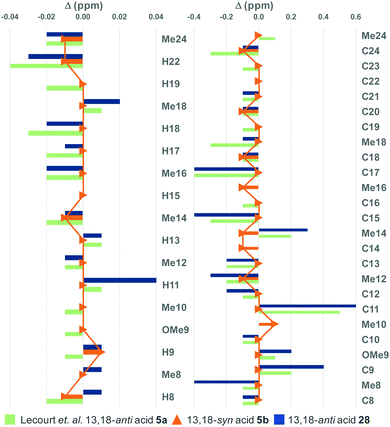 | ||
| Fig. 2 Bar graph highlighting the 1H (left) and 13C (right) NMR chemical shift differences between the model acids and hemicalide (1) between H8/C8 and Me24, overlaid with a line graph for acid 5b. 1H signals contained within the 22H multiplet in the natural product are omitted for comparison. See the ESI† for expanded bar graphs comparing acids 5a, 5b, 28 with salts 29 and 30. | ||
| Entry | Sum |Δ| 1H | Max |Δ| 1H | Sum |Δ| 13C | Max |Δ| 13C | |
|---|---|---|---|---|---|
| a Absolute errors taken for NMR shifts between C8–Me24. b |Δ| = δ(experimental shift) – δ(reported shift), errors in ppm. | |||||
| 1 | 13,18-syn acid 5b | 0.05 | 0.01 | 0.8 | 0.1 |
| 2 | 13,18-syn salt 29 | 0.08 | 0.02 | 1.7 | 0.2 |
| 3 | Lecourt et al. acid 5a | 0.26 | 0.04 | 3.8 | 0.5 |
| 4 | 13,18-anti acid 28 | 0.22 | 0.04 | 4.1 | 0.6 |
| 5 | 13,18-anti salt 30 | 0.22 | 0.04 | 4.3 | 0.7 |
In conclusion, we have firmly established the relative configuration between the C8–C13 and C16–C24 stereoclusters in hemicalide, where NMR correlations of advanced fragments decisively supported the reassigned 13,18-syn relationship. Additionally, NMR comparisons in the C1–C7 triene region indicated that hemicalide was likely isolated as the acid rather than the carboxylate salt. Our highly flexible construction of the advanced C1–C28 ketone 22 also enables the synthesis of the enantiomer ent-22. We hope to then achieve a bioassay-guided determination of hemicalide's absolute configuration, as well as ascertaining preliminary structure–activity relationships in a drug development context.14
We thank the Woolf Fisher Trust (NYSL), the EPSRC (CIM) for financial support and the National Mass Spectrometry Centre (Swansea) for mass spectra.
Conflicts of interest
There are no conflicts to declare.Notes and references
- I. Carletti, C. Debitus and G. Massiot, Molécules polykétides comme agents anticancéreux, Pat. Appl. Pub., WO2011051380A1(FR), 2011Chem. Abstr., 2011, 154, 5130950 Search PubMed.
- S. G. Smith and J. M. Goodman, J. Am. Chem. Soc., 2010, 132, 12946 CrossRef CAS PubMed.
- E. Fleury, M.-I. Lannou, O. Bistri, F. Sautel, G. Massiot, A. Pancrazi and J. Ardisson, J. Org. Chem., 2009, 74, 7034 CrossRef CAS PubMed.
- C. I. MacGregor, B. Y. Han, J. M. Goodman and I. Paterson, Chem. Commun., 2016, 52, 4632 RSC.
- C. Lecourt, S. Boinapally, S. Dhambri, G. Boissonnat, C. Meyer, J. Cossy, F. Sautel, G. Massiot, J. Ardisson, G. Sorin and M.-I. Lannou, J. Org. Chem., 2016, 81, 12275 CrossRef CAS PubMed.
- S. Specklin, G. Boissonnat, C. Lecourt, G. Sorin, M.-I. Lannou, J. Ardisson, F. Sautel, G. Massiot, C. Meyer and J. Cossy, Org. Lett., 2015, 17, 2446 CrossRef CAS PubMed.
- G. Sorin, E. Fleury, C. Tran, E. Prost, N. Molinier, F. Sautel, G. Massiot, S. Specklin, C. Meyer, J. Cossy, M.-I. Lannou and J. Ardisson, Org. Lett., 2013, 15, 4734 CrossRef CAS PubMed.
- I. Paterson, S. M. Dalby, J. C. Roberts, G. J. Naylor, E. A. Guzmán, R. Isbrucker, T. P. Pitts, P. Linley, D. Divlianska, J. K. Reed and A. E. Wright, Angew. Chem., Int. Ed., 2011, 50, 3219 CrossRef CAS PubMed.
- D. A. Evans, J. Bartroli and T. L. Shih, J. Am. Chem. Soc., 1981, 103, 2127 CrossRef CAS.
- I. Paterson, G. J. Florence, K. Gerlach, J. P. Scott and N. Sereinig, J. Am. Chem. Soc., 2001, 123, 9535 CrossRef CAS PubMed.
- W. D. Wulff, G. A. Peterson, W. E. Bauta, K.-S. Chan, K. L. Faron, S. R. Gilbertson, R. W. Kaesler, D. C. Yang and C. K. Murray, J. Org. Chem., 1986, 51, 277 CrossRef CAS.
- A. Fürstner, J.-A. Funel, M. Tremblay, L. C. Bouchez, C. Nevado, M. Waser, J. Ackerstaff and C. C. Stimson, Chem. Commun., 2008, 2873 RSC.
- I. Paterson, K. S. Yeung, R. A. Ward, J. G. Cumming and J. D. Smith, J. Am. Chem. Soc., 1994, 116, 9391 CrossRef CAS.
- For related recent work from our group, see: (a) I. Paterson and N. Y. S. Lam, J. Antibiot., 2018, 71, 215 CrossRef CAS PubMed; (b) N. Anžiček, S. Williams, M. P. Housden and I. Paterson, Org. Biomol. Chem., 2018 10.1039/c7ob03204h.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental and characterisation details; NMR correlation tables and bar graphs. See DOI: 10.1039/c8cc00933c |
| ‡ Authors contributed to this manuscript equally. |
| This journal is © The Royal Society of Chemistry 2018 |