Accessing highly functionalized cyclopentanoids via a cascade palladation approach: unprecedented benzylic C–H activation towards cyclopentenoindanes†
Abstract
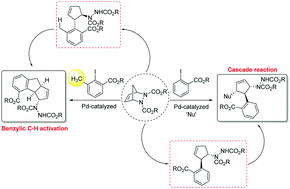
A Pd-catalyzed synthesis of 3,4,5-trisubstituted cyclopentenes from diazabicyclic olefins and o-iodobenzoates has been developed. The hitherto unknown cascade process involves three stages: carbopalladation, oxypalladation and a Tsuji–Trost reaction. We have also developed a facile route involving a novel benzylic C–H activation towards cyclopentenoindane moieties.


 Please wait while we load your content...
Please wait while we load your content...