Open Access Article
Open Access ArticleOne-step synthesis of 2D-layered carbon wrapped transition metal nitrides from transition metal carbides (MXenes) for supercapacitors with ultrahigh cycling stability†
Wenyu
Yuan
 a,
Laifei
Cheng
*a,
Heng
Wu
a,
Yani
Zhang
a,
Shilin
Lv
a and
Xiaohui
Guo
*b
a,
Laifei
Cheng
*a,
Heng
Wu
a,
Yani
Zhang
a,
Shilin
Lv
a and
Xiaohui
Guo
*b
aScience and Technology on Thermostructural Composite Materials Laboratory, Northwestern Polytechnical University, 710072, Xi'an, P. R. China. E-mail: chenglf@nwpu.edu.cn
bCollege of Chemistry and Materials Science, Northwest University, Xi'an 710069, P. R. China. E-mail: guoxh2009@nwu.edu.cn
First published on 16th February 2018
Abstract
A novel one-step method to synthesize 2D carbon wrapped TiN (C@TiN) was proposed via using 2D metal carbides (MXenes) as precursors. This study provides a novel approach to synthesize carbon wrapped metal nitrides.
Supercapacitors (SCs) are considered to be one of the candidates for future energy storage devices owing to their high power density and long cycling life.1,2 Recently, great efforts have been focused on high-rate performance supercapacitors, which can work under high current densities. Transition metal nitrides (TMNs), including TiN, VN, MoN, Mo2N, Ni3N, etc., have been considered as promising candidates for high-rate SCs due to their high electrical conductivities (∼104 S cm−1) and high thermal stability, and have been used widely as electrodes in SCs.3–7 TiN, with a high electrical conductivity of 5.5 × 104 S cm−1, fits well with the requirements for high-rate performance supercapacitors.8,9 However, TiN suffers from poor electrochemical stability because of the generation of transition metal oxide layers in electrolytes, deteriorating the activity and resulting in poor stability and low specific capacitance for SCs.10–12 To improve the cyclic stability and activity, the use of a carbon wrapped structure, which can effectively prevent its electrochemical oxidation, was proved to be an efficient method and has received growing attention.13 Lei et al. designed a self-supported carbon coated TiN nanotube array to improve the cycling stability.14 Mai et al. recently reported TiN@C nanotube-based fiber electrodes through nitridation and a carbon coating process.15 A relatively high cyclic stability of ∼80% capacitance retention after 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles was achieved. However, the to-date-reported method for the synthesis of C@TMN structures mainly involves two steps: (1) the synthesis of metal nitrides and (2) the deposition of carbon layers. It is of critical significance to develop an efficient, simple process for carbon wrapped metal nitrides to replace the present complex method.
000 cycles was achieved. However, the to-date-reported method for the synthesis of C@TMN structures mainly involves two steps: (1) the synthesis of metal nitrides and (2) the deposition of carbon layers. It is of critical significance to develop an efficient, simple process for carbon wrapped metal nitrides to replace the present complex method.
Furthermore, the architecture usually affects the resulting performance. To date, 1D C@TMN architectures, including nanotubes,14 nanoarrays,5 nanowires,16etc., have been mostly discussed. However, to the best of our knowledge, no studies on 2D carbon wrapped TMN structures have been reported because it is very hard for TMNs to form 2D layered structures via the traditional exfoliation strategy.17 A 2D layered structure is always regarded to have a fast ion transport rate and large SSA, beneficial to the surface chemical reactions and the absorption–desorption process.18,19 Previous works demonstrated that 2D nanostructures can be successfully synthesized via the chemical conversion of 2D precursors.20,21 To synthesize a 2D layered C@TMN structure, 2D transition metal carbides (MXenes) with their excellent properties in energy storage and conversion fields, which recently received much attention,22–24 were chosen as precursors. Thus, we developed a novel synthesis method to drive metal carbides into a C@TiN structure via one-step nitridation of 2D carbides. To the best of our knowledge, no studies have been reported on MXene-derived TMNs to date. The in situ generated carbon layers not only enhance the charge transfer efficiency, but also can effectively prevent the oxidation of metal nitrides in electrolytes, resulting in improved specific capacitance, rate capacitance and cycling stability. This universal approach is promising to be applied to synthesize more carbon wrapped TMNs for energy storage and conversion fields.
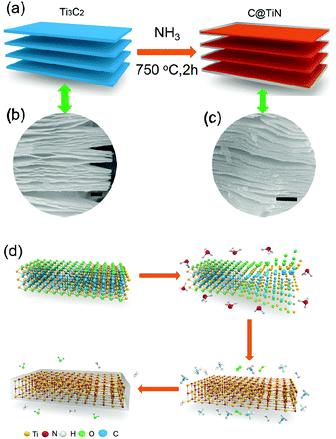
The one-step synthetic procedure for C@TiN is illustrated in Fig. 1a. After one-step nitridation, the TiN nanosheets wrapped by the carbon layers were produced, meanwhile the 2D-layered structure could be well preserved. The layered structure has been well preserved during the nitridation process (Fig. 1b and c). The thickness of the C@TiN layer is ∼20 nm. The total nitridation mechanism is illustrated in Fig. 1d. NH3 molecules can destroy the Ti–C bonds, followed by the formation of Ti–N bonds. The carbon atoms can react with hydrogen atoms in NH3 molecules, and generate CH4 molecules. Then CH4 molecules are decomposed into carbon atoms and hydrogen atoms at high temperature. The generated carbon atoms are deposited on the surface of TiN nanosheets, resulting in a 2D-layered C@TiN structure, meanwhile some of the hydrogen was oxidized to produce H2O molecules. These reaction processes can happen simultaneously during the nitridation process, thus realizing the one-step synthesis of 2D-layered C@TiN hybrids from 2D metal carbides (MXenes). The total reaction equation can be summarized as the following:
| Ti3C2O2 + 3NH3 → 3TiN + 2C + 2H2O↑ + 5/2H2↑ |
The 2D layered structure was further identified using TEM, as shown in Fig. 2a. A 2D C@TiN nanosheet can be clearly observed. The HRTEM image further clearly shows that the well-crystallized TiN (inset in Fig. 2b) is wrapped by a layer of amorphous carbon with a thickness of ∼2–3 nm. The HAADF-STEM image (Fig. S1, ESI†) clearly shows a carbon shell at the surface of the TiN. The EDX mapping further suggests a carbon wrapped TiN structure. XRD was further carried out to analyze the phase transformation during the nitridation process, as shown in Fig. 2c. The (002) characteristic peak of Ti3C2, located at 9.01°, indicates the successful synthesis of Ti3C2 MXenes.24 After calcination under an NH3 atmosphere, the Ti3C2 MXenes were totally transformed into TiN. The main crystalline phase of C@TiN can be assigned to the TiN phase (JCPDS: 38-1420), in which the main diffraction peaks are at 36.8° (111), 42.7° (200), and 62.0° (220), which are in line with the pure TiN.25 Considering that the amorphous carbon cannot be identified using XRD, the Raman spectra were further recorded to detect the amorphous carbon. The two peaks at 1352 cm−1 (D band) and 1583 cm−1 (G band) are assigned to the defects and the in-plane vibration of sp2 carbon atoms, indicating the existence of carbon.26,27 The ID/IG value reached 0.79, suggesting that the carbon was amorphous.28 The band at 557 cm−1 and the broadened band at 200–300 cm−1 are caused by TiN.29,30 Thus, we could confirm that Ti3C2 MXenes have been totally transformed into TiN and carbon after the NH3 thermal treatment at high temperature. Besides Ti3C2 MXenes, more 2D metal carbide MXenes can be used to synthesize carbon wrapped metal nitrides. The total reaction equation can be concluded as the following:
 | ||
| Fig. 2 (a) The TEM of C@TiN. (b) HRTEM of C@TiN. The inset is the SAED of TiN. (c–f) The XRD, Raman spectra, N2 absorption/desorption isotherm and pore size distribution of Ti3C2 and C@TiN. | ||
The N2 adsorption–desorption isotherms of C@TiN are shown in Fig. 2e.31 Compared with Ti3C2 (7.96 m2 g−1), the SSA of 2D-layered C@TiN was significantly improved, it was up to 63.00 m2 g−1. Moreover, the C@TiN shows a unique hierarchically porous structure (Fig. 2f). There are numerous micropores (1.1%), mesopores (73.3%), and macropores (25.6%) within the C@TiN hybrids. The high percentage of mesopores can efficiently promote the transport of ions and electrons.32,33 The pore size distribution of the 2D-layered C@TiN hybrids was mainly focused around 1.3 and 4.1 nm, as shown in Fig. 2f.
The chemical components and elemental chemical state were further characterized using XPS and are displayed in Fig. S2 (ESI†). The elements O, Ti, N, and C can be clearly identified in the survey XPS spectra, as shown in Fig. S2a (ESI†). The oxygen in C@TiN is caused by the functional groups on the surface of MXene precursors. The high-resolution C1s electron XPS spectra are displayed in Fig. S2b (ESI†). The peak at 281.9 eV in the Ti3C2 precursors (Fig. S3, ESI†), which is assigned to the C–Ti bond, disappeared after nitridation, indicating that the C–Ti bond has been totally destroyed.34 The peak at 284.5 eV is corresponding to the C–C bond. Some functional groups exist in the carbon layers owing to the peak at 286.2 eV (C–O bond). The peaks at 285.7 eV and 288.2 eV are assigned to the C–N and C–N–Ti bonds, respectively.35 The three main peaks at 396.3, 398.4, and 401.0 eV are assigned to the N–Ti, N–C, and C–N–Ti bonds, respectively.17 The chemical bonds between carbon and TiN can lead to strong integration, which is helpful for the stability. The high-resolution Ti2p electron XPS spectra further suggest the successful synthesis of TiN.6,36
The electrochemical performances of C@TiN, Ti3C2 and TiN were evaluated using CV, galvanostatic charge–discharge (GCD), and EIS techniques in a three-electrode system using 2 M KOH as the electrolyte. The effects of calcination conditions on the electrochemical performance are discussed here. Fig. 3a shows the CV curves of these samples at a scan rate of 10 mV s−1. All samples delivered rectangular shaped curves for typical electrochemical double layer capacitance (EDLC) performance. Among the four C@TiN samples, C@TiN-750-2 delivered the highest electrochemical performance owing to the high SSA, high pore volume and high ratio of mesopores (Table S1, ESI†). C@TiN calcinated at 850 °C suffers from heavy aggregation (Fig. S4, ESI†), which leads to the low SSA and pore volume. Furthermore, the CV curves of C@TiN-750-2 under various scan rates have been studied and are shown in Fig. S5a (ESI†). We found that even under a high scan rate of 500 mV s−1, the CV curve did not obviously change, indicating a high rate capacitance. Fig. 3b shows the GCD profiles of C@TiN, TiN and Ti3C2, at a current density of 1 A g−1. Similar to the results in Fig. 3a, C@TiN-750-2 shows the longest discharge time with a low IR drop of 0.0053 V among all samples. The GCD profiles of C@TiN-750-2 measured at the current density ranging from 0.5 to 30 A g−1 are shown in Fig. S5b (ESI†). Interestingly, the IR drop did not obviously increase even under a high current density of 20 A g−1, suggesting a high rate performance. The gravimetric specific capacitances of 6 samples at different current densities were calculated from the corresponding discharge curves, and the results are shown in Fig. 3c. The specific capacitances of C@TiN-750-1, C@TiN-750-2, C@TiN-850-1, C@TiN-850-2, TiN, and Ti3C2 under a current density of 0.5 A g−1 are 176, 238, 100, 59, 30, and 20 F g−1, respectively. The specific capacitance retention of C@TiN-750-2 under a high current density of 30 A g−1 reached 72.3%, which is much higher than those of C@TiN-750-1 (41.9%), C@TiN-850-1 (15.0%), C@TiN-850-2 (9.0%), TiN (2.5%) and Ti3C2 (1.2%). Fig. 3d shows the Nyquist plots of the 6 samples. C@TiN-750-2 possessed the largest slope among all samples due to the high ratio of mesopores. C@TiN-750-2 shows the lowest equivalent series resistance (ESR) of 1.28 Ω and a low charge transfer resistance of 0.18 Ω, suggesting a high electrical conductivity of C@TiN-750-2.
The cycling performance of the C@TiN samples was also studied via cyclic GCD tests under a current density of 2 A g−1, as shown in Fig. 4a. The specific capacitance of bare TiN sharply decreased during the beginning 2000 cycles owing to the oxidation of TiN via the following equation:13
| TiN + 2H2O → TiO2 + 1/2N2 + 4H+ + 4e−. |
The capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles is only 29.2%. The C@TiN samples showed much better stability in electrolytes. C@TiN-750-2 delivered the highest cyclic stability with an ultra-high capacitance retention of 93.1%. Its specific capacitance and cycling stability are superior to most reported TiN electrodes. Furthermore, we investigated the CV curves, the Nyquist plots and the XPS Ti2p spectra after 10
000 cycles is only 29.2%. The C@TiN samples showed much better stability in electrolytes. C@TiN-750-2 delivered the highest cyclic stability with an ultra-high capacitance retention of 93.1%. Its specific capacitance and cycling stability are superior to most reported TiN electrodes. Furthermore, we investigated the CV curves, the Nyquist plots and the XPS Ti2p spectra after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, as shown in Fig. 4b–d. The rectangular shaped CV curve after 10
000 cycles, as shown in Fig. 4b–d. The rectangular shaped CV curve after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles suggested a typical EDLC performance, indicating that the structure has not changed obviously. The ESR and the charge transfer resistance almost remained the same, meanwhile the slope at the low-frequency region slightly decreased. The XPS Ti2p spectra suggest that no obvious intensity enhancement of the Ti–O bond was observed after cyclic tests and the main bonds have been well preserved. No obvious oxidation occurs during the electrochemical process. These results suggested that the carbon could significantly improve the cyclic stability of TiN in electrolytes. Two reasons are responsible for the high cyclic stability of C@TiN: (1) the carbon layers can effectively prevent the electrochemical oxidation reaction of TiN and (2) the carbon shell acts as a cage for TiN and prevents the structural damage and mass loss during cycling.
000 cycles suggested a typical EDLC performance, indicating that the structure has not changed obviously. The ESR and the charge transfer resistance almost remained the same, meanwhile the slope at the low-frequency region slightly decreased. The XPS Ti2p spectra suggest that no obvious intensity enhancement of the Ti–O bond was observed after cyclic tests and the main bonds have been well preserved. No obvious oxidation occurs during the electrochemical process. These results suggested that the carbon could significantly improve the cyclic stability of TiN in electrolytes. Two reasons are responsible for the high cyclic stability of C@TiN: (1) the carbon layers can effectively prevent the electrochemical oxidation reaction of TiN and (2) the carbon shell acts as a cage for TiN and prevents the structural damage and mass loss during cycling.
To evaluate the energy and power density, a solid-state supercapacitor was prepared using two parallel C@TiN samples as electrodes and KOH/PVA gel as the electrolyte. Fig. S6a (ESI†) shows that the shape of CV curves does not change under different potential windows, indicating that the supercapacitor can be operated under wide operating potential windows. The rectangular shaped CV curve under an ultrahigh scan rate of 2000 mV s−1 suggests a high-rate performance. The GCD curves are shown in Fig. S6c (ESI†) and the calculated specific capacitances of the SCs are 61.2, 56.9, 53.0, 52.1, and 51.3 F g−1, respectively. The Ragone plots of the C@TiN supercapacitors derived from the GCD curves are displayed in Fig. S6d (ESI†). The energy density is 5.46 W h kg−1 at a power density of 111 W kg based on the total mass of two electrodes. The energy density still remained at 4.56 W h kg−1 at a high power density of 2161 W kg−1. The power density increased ∼2000%, and the energy density only decreased ∼16%, suggesting high-rate performance and high stability under high current densities. Table S2 (ESI†) compares the electrochemical performances of the synthesized C@TiN and reported TiN-based electrodes, and the C@TiN in this work is superior to the reported TiN-based SC materials.
In summary, we delivered a novel one-step approach to synthesize 2D-layered carbon wrapped TMNs for supercapacitors. The optimal specific capacitance reached 238 F g−1 under the current density of 0.5 A g−1. Even under a current density of 30 A g−1, the capacitance retention was still as high as 72.3%, indicating a high rate capacitance. Furthermore, owing to the existence of carbon layers, the C@TiN delivered an ultrahigh cyclic stability (high capacitance retention of 93.1% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under the current density of 2 A g−1). This novel one-step method can be further applied to synthesize other carbon wrapped TMNs and the 2D layered carbon wrapped TMNs are potential candidates for other energy storage and conversion fields.
000 cycles under the current density of 2 A g−1). This novel one-step method can be further applied to synthesize other carbon wrapped TMNs and the 2D layered carbon wrapped TMNs are potential candidates for other energy storage and conversion fields.
The authors would like to acknowledge the financial support provided by the National Key R&D Program of China (No. 2017YFB1103500), the National Natural Science Foundation of China (No. 51302220, 51672218 and 51632007), Northwestern Polytechnical University (NPU) Foundation for Fundamental Research (No. 3102015ZY086), and the Fund of the State Key Laboratory of Solidification Processing in NPU (No. SKLSP201613).
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P.-L. Taberna, C. P. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef CAS.
- M.-S. Balogun, W. Qiu, W. Wang, P. Fang, X. Lu and Y. Tong, J. Mater. Chem. A, 2015, 3, 1364–1387 CAS.
- D. R. Deng, F. Xue, Y. J. Jia, J. C. Ye, C. D. Bai, M. S. Zheng and Q. F. Dong, ACS Nano, 2017, 11, 6031–6039 CrossRef CAS PubMed.
- P. Yang, D. Chao, C. Zhu, X. Xia, Y. Zhang, X. Wang, P. Sun, B. K. Tay, Z. X. Shen, W. Mai and H. J. Fan, Adv. Sci., 2016, 3, 1500299 CrossRef PubMed.
- X. Lu, G. Wang, T. Zhai, M. Yu, S. Xie, Y. Ling, C. Liang, Y. Tong and Y. Li, Nano Lett., 2012, 12, 5376–5381 CrossRef CAS PubMed.
- S. Yang, J. Kim, Y. J. Tak, A. Soon and H. Lee, Angew. Chem., Int. Ed., 2016, 55, 2058–2062 CrossRef CAS PubMed.
- Z. Cui, C. Zu, W. Zhou, A. Manthiram and J. B. Goodenough, Adv. Mater., 2016, 28, 6926–6931 CrossRef CAS PubMed.
- Z. Li, J. Zhang, B. Y. Guan and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 16003–16007 CrossRef CAS PubMed.
- B. Avasarala and P. Haldar, Electrochim. Acta, 2010, 55, 9024–9034 CrossRef CAS.
- B. M. Gray, A. L. Hector, M. Jura, J. R. Owen and J. Whittam, J. Mater. Chem. A, 2017, 5, 4550–4559 CAS.
- B. Avasarala and P. Haldar, Int. J. Hydrogen Energy, 2011, 36, 3965–3974 CrossRef CAS.
- X. Lu, T. Liu, T. Zhai, G. Wang, M. Yu, S. Xie, Y. Ling, C. Liang, Y. Tong and Y. Li, Adv. Energy Mater., 2014, 4, 1300994 CrossRef.
- F. Grote, H. Zhao and Y. Lei, J. Mater. Chem. A, 2015, 3, 3465–3470 CAS.
- P. Sun, R. Lin, Z. Wang, M. Qiu, Z. Chai, B. Zhang, H. Meng, S. Tan, C. Zhao and W. Mai, Nano Energy, 2017, 31, 432–440 CrossRef CAS.
- Y. Han, X. Yue, Y. Jin, X. Huang and P. K. Shen, J. Mater. Chem. A, 2016, 4, 3673–3677 CAS.
- X. Xiao, H. Yu, H. Jin, M. Wu, Y. Fang, J. Sun, Z. Hu, T. Li, J. Wu, L. Huang, Y. Gogotsi and J. Zhou, ACS Nano, 2017, 11, 2180–2186 CrossRef CAS PubMed.
- E. Pomerantseva and Y. Gogotsi, Nat. Energy, 2017, 2, 17089 CrossRef CAS.
- R. Mas-Balleste, C. Gomez-Navarro, J. Gomez-Herrero and F. Zamora, Nanoscale, 2011, 3, 20–30 RSC.
- P. Huang, C. Lethien, S. Pinaud, K. Brousse, R. Laloo, V. Turq, M. Respaud, A. Demortière, B. Daffos, P. L. Taberna, B. Chaudret, Y. Gogotsi and P. Simon, Science, 2016, 351, 691–695 CrossRef CAS PubMed.
- C. Liu, S. Zhao, Y. Lu, Y. Chang, D. Xu, Q. Wang, Z. Dai, J. Bao and M. Han, Small, 2017, 13, 1603494 CrossRef PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall’Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef CAS PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- A. Achour, M. Chaker, H. Achour, A. Arman, M. Islam, M. Mardani, M. Boujtita, L. Le Brizoual, M. Djouadi and T. Brousse, J. Power Sources, 2017, 359, 349–354 CrossRef CAS.
- A. C. Ferrari, J. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. Novoselov and S. Roth, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095 CrossRef CAS.
- Z. Wen, X. Wang, S. Mao, Z. Bo, H. Kim, S. Cui, G. Lu, X. Feng and J. Chen, Adv. Mater., 2012, 24, 5610–5616 CrossRef CAS PubMed.
- M.-S. Balogun, M. Yu, C. Li, T. Zhai, Y. Liu, X. Lu and Y. Tong, J. Mater. Chem. A, 2014, 2, 10825–10829 CAS.
- N. K. Ponon, D. J. Appleby, E. Arac, P. King, S. Ganti, K. S. Kwa and A. O’Neill, Thin Solid Films, 2015, 578, 31–37 CrossRef CAS.
- W. Zhang, X. Xu, C. Zhang, Z. Yu, Y. Zhou, Y. Tang, P. Wu and S. Guo, Small Methods, 2017, 1, 1700167 CrossRef.
- T. Kim, G. Jung, S. Yoo, K. S. Suh and R. S. Ruoff, ACS Nano, 2013, 7, 6899–6905 CrossRef CAS PubMed.
- Y. Shao, M. F. El-Kady, L. J. Wang, Q. Zhang, Y. Li, H. Wang, M. F. Mousavi and R. B. Kaner, Chem. Soc. Rev., 2015, 44, 3639–3665 RSC.
- B. Ahmed, D. H. Anjum, M. N. Hedhili, Y. Gogotsi and H. N. Alshareef, Nanoscale, 2016, 8, 7580–7587 RSC.
- Z. Yang, M. Xu, Y. Liu, F. He, F. Gao, Y. Su, H. Wei and Y. Zhang, Nanoscale, 2014, 6, 1890–1895 RSC.
- A. Achour, J. Ducros, R. Porto, M. Boujtita, E. Gautron, L. Le Brizoual, M. Djouadi and T. Brousse, Nano Energy, 2014, 7, 104–113 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc09017j |
| This journal is © The Royal Society of Chemistry 2018 |