Open Access Article
Open Access ArticleAluminium-mediated carbon–carbon coupling of an isonitrile†
Stephanie J.
Urwin
,
Gary S.
Nichol
 and
Michael J.
Cowley
and
Michael J.
Cowley
 *
*
EaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, UK. E-mail: michael.cowley@ed.ac.uk
First published on 7th December 2017
Abstract
Cp*Al reacts with diphenylacetylene to form a Cp*-substituted 1,4-dialuminacyclohexene. The dialuminacyclohexene reacts with four equivalents of an isonitrile to couple the terminal carbon atoms, forming 6 new carbon–carbon bonds and resulting in a zwitterionic diamide ligand which contains a carbocationic backbone.
Metal-mediated couplings of unsaturated organic compounds such as alkynes or isonitriles are a powerful route to constructing new organic frameworks.1,2 In transition metal chemistry, such transformations, especially cyclotrimerisations,3,4 are abundant. Because of the high abundance and low cost and toxicity that aluminium has in comparison to transition metals, we have recently become interested in using aluminium to catalyse synthetically useful transformations.5
Low oxidation state aluminium compounds have a range of reported reactivity with alkynes and isonitriles. We were thus interested in the potential for Al(I) compounds to mediate (or catalyse) multicomponent reactions in which isonitriles and alkynes are coupled to form new organic frameworks.
The reported reactivity of Al(I) compounds with alkynes is variable, depending chiefly on the substituent at the aluminium centre. When smaller substituents – such as chloride or alkyl groups – are present at the aluminium centre, aluminacyclopropenes, the product of a [2+1] cycloaddition with an alkyne, are proposed (but not observed) as intermediate products.6,7 These strained 3-membered rings dimerise to produce the ultimately isolated 1,4-dialuminacyclohexadienes (e.g.A, Fig. 1).7 The reaction of Roesky's NacNacAl(I) compound with alkynes allows the isolation of aluminacyclopropenes such a B,8–10 presumably stable against dimerisation because of steric hindrance and a four-coordinate aluminium centre. In some cases, alternative dimerisation products such as D have been observed,11 which is proposed to result from the dimerisation of the unobserved (despite its extreme steric bulk) intermediate C.
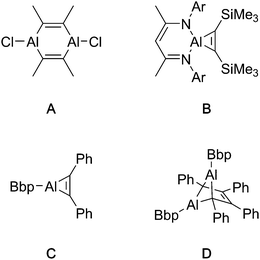 | ||
| Fig. 1 Isolated (A, B, D) and proposed (C) products of reactons between monomeric Al(I) compounds and alkynes. Ar = 2,6-diisopropylphenyl. Bbp = {2,6-[CH(SiMe3)2]2-C6H3}. | ||
We noticed that stabilisation of the Lewis acidic aluminium centre seems to be required for the synthesis of stable aluminacyclopropenes, which could serve as isolable precursors for aluminium-mediated cycloaddition chemistry. Also noting that the reactivity of the prototypical Al(I) compound (Cp*Al)4 with alkynes had not been reported, we set out to investigate.
Here, we report the synthesis of a 1,4-dialumina cyclohexadiene from the reaction of Cp*Al and diphenyl acetylene. Upon treatment with isonitriles, a complex insertion/cycloaddition reaction results in the formation of a norbornadienyl cation-based diamide ligand, which continues to support the central Al2C4 ring.
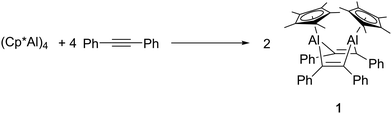
The reaction of (Cp*Al)4 with a range of alkynes at 80 °C was monitored by NMR spectroscopy. No reaction was observed with but-2-yne nor bis(trimethylsilyl) acetylene, for reasons which are not clear. However, with diphenylacetylene the formation of a new species was noted, evidenced by the formation of new Cp* resonances in the 1H NMR spectrum. A preparative-scale reaction between (Cp*Al)4 and 4 equivalents of diphenyl acetylene allowed the isolation of the new compound, 1, as a beige solid (Scheme 1).
The 1H NMR spectrum of 1 is uncomplicated. The characteristic singlet signal for the Cp* group is found at δ 1.74, which is not significantly shifted from that of (Cp*Al)4 (δ 1.90). When integrated, a series of multiplets in the range δ 7.40–6.97, assigned to new phenyl groups, reveal that the ratio of Cp* to C6H5 substituents is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. In the 13C NMR spectrum, a single resonance is located at δ 148.4 and assigned to two equivalent alkene carbons, presumably derived from the diphenyl acetylene starting material. Together, these observations are suggestive of a possible aluminacyclopropene-type structure. However, EI mass spectrometric measurements reveal the molecular mass of 1 to be 680.36, which corresponds to a formula of Cp2*Al2(PhCCPh)2. Accordingly, a single-crystal X-ray structural determination identified 1 as a 1,4-dialuminacyclohexadiene.
2. In the 13C NMR spectrum, a single resonance is located at δ 148.4 and assigned to two equivalent alkene carbons, presumably derived from the diphenyl acetylene starting material. Together, these observations are suggestive of a possible aluminacyclopropene-type structure. However, EI mass spectrometric measurements reveal the molecular mass of 1 to be 680.36, which corresponds to a formula of Cp2*Al2(PhCCPh)2. Accordingly, a single-crystal X-ray structural determination identified 1 as a 1,4-dialuminacyclohexadiene.
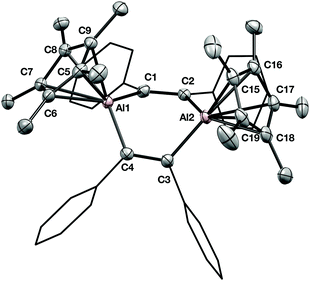
The solid-state structure of 1 is shown in Fig. 2. Immediately notable is the boat-shaped conformation of the central C4Al2 ring and the η5-coordination of the two Cp* groups. The bond distance between the aluminium centres and the Cp* ring carbons falls into the usual range for aluminium compounds with η5-Cp* ligands (mean Al–C distance for Al1: 2.293(10) Å; Al2: 2.285(10) Å). The boat conformation is in contrast to the only other crystallographically characterised examples of a 1,4-dialuminacyclohexadiene, which are both planar in the solid state.6,7 A 1,4-dialuminacyclohexane, generated from Cp*Al and an alkene, possesses a chair conformation.12
Although we attempted to monitor the conversion of Cp*Al and diphenyl acetylene to 1 by NMR spectroscopy, we were not able to identify any intermediates in this reaction. A likely mechanism would involve an initial [2+1] cycloaddition between monomeric Cp*Al and the alkyne generates an aluminacyclopropene, analogous to B or C, which then dimerises.7,11
We explored the reactivity of 1 towards Lewis bases, wondering if coordination of an external base to the aluminium centres could provoke dissociation of the ring into two base-stabilised cyclopropene units. Despite the η5-coordinated cyclopentadienyl rings of 1, it retains sufficient Lewis acidic character to coordinate even weak Lewis bases. Thus, treatment of 1 with Et2O, THF, or DME results in formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts with these solvents, which are chiefly characterised by the downfield-shifted resonances for the coordinated ethers (see ESI†). We then trialled reactions of 1 with stronger Lewis bases. Treatment of 1 with sterically small NHCs or N,N-dimethylaminopyridine resulted in the production of intractable mixtures. However, reactions with isonitriles were more fruitful.
2 adducts with these solvents, which are chiefly characterised by the downfield-shifted resonances for the coordinated ethers (see ESI†). We then trialled reactions of 1 with stronger Lewis bases. Treatment of 1 with sterically small NHCs or N,N-dimethylaminopyridine resulted in the production of intractable mixtures. However, reactions with isonitriles were more fruitful.
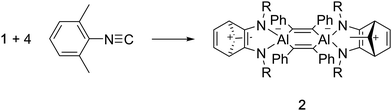
Compound 1 reacts with 2,6-dimethylphenylisonitrile at room temperature in benzene to yield the new compound 2 (Scheme 2). 1H NMR spectroscopy of 2 reveals three resonances in the Cp* Me region, in a ratio 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, which indicate that the Cp* group is no longer η5 nor undergoing rapid sigmatropic shifts. Two resonances in a ratio 12
6, which indicate that the Cp* group is no longer η5 nor undergoing rapid sigmatropic shifts. Two resonances in a ratio 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 were also present and assignable to the methyl groups of the isonitrile, indicating that four isonitrile groups had been incorporated. 13C NMR spectroscopy revealed an unusually low-field chemical shift for one of the Cp* ring carbons of δ 151.4.
12 were also present and assignable to the methyl groups of the isonitrile, indicating that four isonitrile groups had been incorporated. 13C NMR spectroscopy revealed an unusually low-field chemical shift for one of the Cp* ring carbons of δ 151.4.
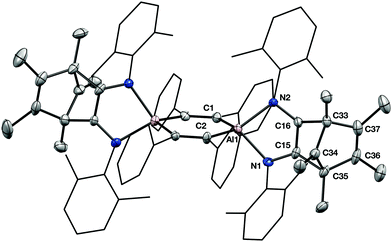
An X-ray crystallographic study reveals that 2 retains the central cyclohexadiene core of 1, but that a complex reaction with two equivalents of isonitrile has generated an unusual 2,3-diamidonorbornadienyl cation based ligand. The norbornadienyl cation fragment is derived from the former Cp* ligand and the two carbon atoms of the isonitrile units (Fig. 3). The insertion of two isonitriles into the Cp*–Al bond in this way is similar to the CO insertion chemistry observed for sterically crowded tris-cyclopentadienyl complexes of the lanthanides.13
Immediately apparent on close examination of the structure of 2 is the planar, 3-coordinate carbon centre C34, which has a sum of angles of 359.9(12) (the other cationic centre, C76, has very similar metrical parameters so this discussion will use values relating to C34 alone). There was no crystallographic indication of a C–H at this position, matching NMR spectroscopic evidence from 1H–13C correlation experiments, which indicate the three-coordinate carbon has no bonded hydrogens, and allow its assignment to the resonance at δ 151.4.
As is usual in norbornadienyl cations, C34 is not equidistant from the C36–C37 and C15–C16 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. C34 closely approaches the C15–C16 double bond (C34–C15 1.988(11); C34–C16 1.962(11) Å). This interaction lengthens the C15–C16 C
C double bonds. C34 closely approaches the C15–C16 double bond (C34–C15 1.988(11); C34–C16 1.962(11) Å). This interaction lengthens the C15–C16 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond significantly (1.401(10) Å) in comparison to the C36–37 bond (1.327(11) Å) or those found in other aluminium compounds supported by 1,2 diamidoalkene-based ligands.14
C bond significantly (1.401(10) Å) in comparison to the C36–37 bond (1.327(11) Å) or those found in other aluminium compounds supported by 1,2 diamidoalkene-based ligands.14
The formal positive charges located at C34 and C76 are balanced by the formally anionic, four coordinate aluminium centres, which now occupy a planar Al2C4 ring. Despite the carbocationic fragment, compound 2 is stable in CD2Cl2 solution for several days, indicating that, as in the related lanthanide analogues, stabilisation by the integral anionic centres is effective.
The aluminium mediated C–C coupling of two isonitrile units and their concomitant insertion into the Cp*Al bond is unusual. Isonitriles are typically poor electrophiles, though they do react with nucleophilic organometallics or metal hydrides (e.g. organolithiums). With simple alanes, coordination of isonitriles to the aluminium centre is reported,15,16 though adducts do not transfer alkyl or aryl substituents to the isonitrile carbon. Cp3Al has also been observed to form stable adducts with isonitrile ligands.17
The reactivity of isonitriles with cyclic aluminium compounds in which the aluminium centre is incorporated into a cyclic system is diverse. 1 reacts with isonitriles to couple their carbon centres; this is similar to the reactivity observed in the Al–Al bonded adduct of Tokitoh's Bbp ‘masked dialene’, which couples two isonitriles units to form an alkyne.18 Power's related compound is derived from activation of unsaturated C–C bonds,19 which is also observed in reactions of Inoue's dialuminene.20 Aluminacyclopropenes like B react to insert isonitriles into the Al–C bond and generate 4-membered rings with exocyclic imine functionality.21,22 Roesky's NacNac Al(I) compound also mediates the C–C coupling of isonitriles to form an unusual 4-membered N-heterocyclic carbene. We are currently exploring Al-mediated C–C coupling reactions with the aim of targeting the development of catalytic processes.
We thank the European Commission (FP7-PEOPLE-2013-CIG-631483) and the University of Edinburgh for funding.
Conflicts of interest
There are no conflicts to declare.Notes and references
- V. P. Boyarskiy, N. A. Bokach, K. V. Luzyanin and V. Y. Kukushkin, Chem. Rev., 2015, 115, 2698–2779 CrossRef CAS PubMed.
- A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed.
- V. Gandon, C. Aubert and M. Malacria, Chem. Commun., 2006, 2209–2217 RSC.
- G. Domínguez and J. Pérez-Castells, Chem. Soc. Rev., 2011, 40, 3430–3444 RSC.
- A. Bismuto, S. P. Thomas and M. J. Cowley, Angew. Chem., Int. Ed., 2016, 55, 15356–15359 CrossRef CAS PubMed.
- R. Ahlrichs, M. Häser, H. Schnöckel and M. Tacke, Chem. Phys. Lett., 1989, 154, 104–110 CrossRef CAS.
- C. Üffing, A. Ecker, R. Köppe, K. Merzweiler and H. Schnöckel, Chem. – Eur. J., 1998, 4, 2142–2147 CrossRef.
- C. Cui, S. Koepke, R. Herbst-Irmer, H. W. Roesky, M. Noltemeyer, H.-G. Schmidt and B. Wrackmeyer, J. Am. Chem. Soc., 2001, 123, 9091–9098 CrossRef CAS PubMed.
- H. Zhu, J. Chai, H. Fan, H. W. Roesky, C. He, V. Jancik, H.-G. Schmidt, M. Noltemeyer, W. A. Merrill and P. P. Power, Angew. Chem., Int. Ed. Engl., 2005, 44, 5090–5093 CrossRef CAS PubMed.
- H. Zhu, R. B. Oswald, H. Fan, H. W. Roesky, Q. Ma, Z. Yang, H. Schmidt, M. Noltemeyer, K. Starke and N. S. Hosmane, J. Am. Chem. Soc., 2006, 128, 5100–5108 CrossRef CAS PubMed.
- T. Agou, K. Nagata, T. Sasamori and N. Tokitoh, Chem. – Asian J., 2014, 9, 3099–3101 CrossRef CAS PubMed.
- B. Buchin, T. Steinke, C. Gemel, T. Cadenbach and R. A. Fischer, Z. Anorg. Allg. Chem., 2005, 631, 2756–2762 CrossRef CAS.
- W. J. Evans, J. M. Perotti, S. A. Kozimor, T. M. Champagne, B. L. Davis, G. W. Nyce, C. H. Fujimoto, R. D. Clark, M. A. Johnston and J. W. Ziller, Organometallics, 2005, 24, 3916–3931 CrossRef CAS.
- Y. Zhao, Y. Liu, L. Yang, J. G. Yu, S. Li, B. Wu and X. J. Yang, Chem. – Eur. J., 2012, 18, 6022–6030 CrossRef CAS PubMed.
- A. Meller and H. Batka, Monatsh. Chem., 1970, 628, 627–628 CrossRef.
- N. B. Kingsley, K. Kirschbaum, J. A. Teprovich, R. A. Flowers and M. R. Mason, Inorg. Chem., 2012, 51, 2494–2502 CrossRef CAS PubMed.
- P. J. Shapiro, A. Vij, G. P. A. Yap and A. L. Rheingold, Polyhedron, 1995, 14, 203–209 CrossRef CAS.
- K. Nagata, T. Agou, T. Sasamori and N. Tokitoh, Chem. Lett., 2015, 44, 1610–1612 CrossRef CAS.
- R. J. Wright, A. D. Phillips and P. P. Power, J. Am. Chem. Soc., 2003, 125, 10784–10785 CrossRef CAS PubMed.
- P. Bag, A. Porzelt, P. J. Altmann and S. Inoue, J. Am. Chem. Soc., 2017, 139, 14384–14387 CrossRef CAS PubMed.
- X. Li, X. Cheng, H. Song and C. Cui, Organometallics, 2007, 26, 1039–1043 CrossRef CAS.
- X. Li, C. Ni, H. Song and C. Cui, Chem. Commun., 2006, 1763–1765 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1583369 and 1583370. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc08415c |
| This journal is © The Royal Society of Chemistry 2018 |