Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The chaotropic effect as an orthogonal assembly motif for multi-responsive dodecaborate-cucurbituril supramolecular networks†
Wenjing
Wang
a,
Xiaoqiang
Wang
a,
Jin
Cao
b,
Jun
Liu
c,
Bin
Qi
a,
Xiaohai
Zhou
a,
Shuai
Zhang
d,
Detlef
Gabel
d,
Werner M.
Nau
 d,
Khaleel I.
Assaf
d,
Khaleel I.
Assaf
 *d and
Haibo
Zhang
*d and
Haibo
Zhang
 *a
*a
aCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, China. E-mail: haibozhang1980@gmail.com
bHubei Gedian Humanwell Pharmaceutical Excipents Co., Ltd, Gedian 436070, China
cHunan University of Arts and Science, Changde 415000, China
dDepartment of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen, Germany. E-mail: k.assaf@jacobs-university.de
First published on 3rd January 2018
Abstract
In aqueous solution, host–guest complexation is frequently driven by the hydrophobic effect, which also constitutes a popular approach in the design of supramolecular assemblies. Herein, we report an orthogonal assembly motif, the chaotropic effect, which exploits the tendency of chaotropic anions to associate with hydrophobic surfaces.
Supramolecular chemistry provides a tool for designing novel functional materials. For example, supramolecular polymers, combining the outstanding properties of traditional polymers and the controllable features of supramolecular systems, show very interesting and versatile properties due to their dynamic and designable nature.1 They have potential applications, among others, for cargo delivery, micropollutant removal, biomaterial scaffolds, and materials science.2 For supramolecular materials in water, the building blocks are often low-molecular weight monomeric units brought together by the hydrophobic effect, assisted and directed by additional noncovalent interactions, such as hydrogen bonding, electrostatic interactions or metal–ligand coordination.3 Macrocyclic host molecules have become particularly attractive in the formation of supramolecular networks, because they allow the site-specific formation of both exclusion and inclusion complexes.4
Dodecaborate clusters are inorganic anions, which have an icosahedral structure with a permanent double negative charge.5 As efficient 10B-enriched reagents, they have numerous applications in medicinal chemistry and materials science, especially in boron neutron capture therapy.6 Recently, a high propensity for inclusion complex formation of dodecaborate clusters with large cyclodextrins (CDs) has been observed in aqueous solution.7 The driving force, modulated by dispersion interactions, was attributed to the chaotropic effect, which describes an intrinsic affinity of water-structure breaking (chaotropic) anions to hydrophobic surfaces.7a,8 The new noncovalent binding motif, which is orthogonal to the hydrophobic effect chemically and thermodynamically,7a,9 can be extended to the outer surface of cucurbit[n]urils (CBn, n = 5–8), see Fig. 1. CBn, a series of pumpkin-shaped glycoluril oligomers, are water-soluble macrocycles with unique recognition properties.10 They are capable of encapsulating a wide range of organic guest molecules in their hydrophobic cavities.11 Recently, CBn have also been used for constructing supramolecular assemblies and engineering functional materials.12
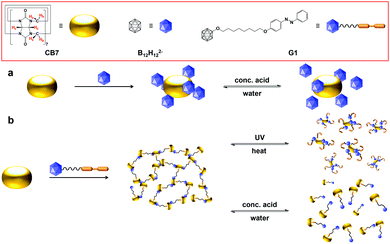 | ||
| Fig. 1 (a) The self-assembly of CB7 and B12H122− in aqueous solution. (b) Multi-responsive assembly formed between CB7 and G1 in aqueous solution. | ||
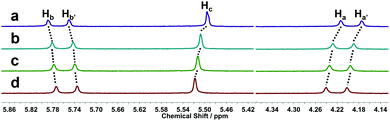
In this work, we introduce, for the first time, the chaotropic effect as a new assembly motif, which exploits the tendency of dodecaborate cluster anions, as superchaotropic anions, to bind to the hydrophobic exterior of CBn macrocycles. Complex formation between B12H122− and CB7 was established using 1H NMR spectroscopy (Fig. 2). In the 1H NMR spectra, the B–H protons are not informative, due to the extreme broadness of the B–H resonance (from −0.5 to +2.0 ppm).7 Instead, we followed the complexation-induced chemical shift of the CBn protons. Sizable shifts of all protons (Ha, Hb and Hc, see Fig. 1, top left) were observed, in particular upfield shifts of Ha and Hc, which are co-equatorial and equatorial, respectively, and a downfield shift of Hb, positioned co-axially (Fig. 2); this signalled the formation of an exclusion complex, because the CB protons are generally barely affected by the formation of the inclusion complex. Job plot analysis (Fig. S1, ESI†) indicated higher-order complexation stoichiometries, while the analysis of the ITC data (Fig. S2, ESI†) indicated that the data could be fitted, for example, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 binding model and the extracted apparent binding constants were 104–105 M−1. Aggregate formation at low millimolar concentration (see below) provided visible evidence of the affinity, but prevented an accurate determination of the complexation thermodynamics.
6 binding model and the extracted apparent binding constants were 104–105 M−1. Aggregate formation at low millimolar concentration (see below) provided visible evidence of the affinity, but prevented an accurate determination of the complexation thermodynamics.
To verify the formation of exclusion complexes between dodecaborate anions and CBn, we screened various dodecaborate clusters (B12Cl11OH2−, B12Cl122− and B12H122−) with CB7 and CB6 for single-crystal formation. The XRD structure of the complex between CB7 and B12H11OH2− shows that each CB7 unit interacts with eight B12H11OH2− clusters, four above and four below the equator with non-centrosymmetrical distribution (Fig. 3). Two clusters at the same layer are adjacent and connected by a hydrogen bond between the hydroxyl groups (a B–O–H⋯(O–H)–B motif with a O⋯O bond distance of 2.35 Å). The crystal packing further revealed short contacts between the partially negatively charged hydrogens on the clusters and the carbonyl carbons of CB7 (H⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 2.5–2.6 Å). A database search (Cambridge Crystallographic Data Centre) to identify the abundance and occurrence of similar intermolecular interactions (H⋯C
O: 2.5–2.6 Å). A database search (Cambridge Crystallographic Data Centre) to identify the abundance and occurrence of similar intermolecular interactions (H⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) revealed only three examples (codes: EZIHAX, PERXEP, and WICHEV), all of which involve carborane derivatives. Another unconventional interaction was observed in the crystal structure, in which the partially negative hydrogen atoms on the cluster interact with the hydrogen atoms on the exterior of CB7 (H⋯H approximately 2.25–2.58 Å). We also obtained single crystals of the complex between CB7 and B12Cl122− (Fig. S3, ESI†), and single crystals of the complex between CB6 and B12H122− (Fig. S4, ESI†). All XRD structures proved the formation of exclusion complexes between dodecaborate clusters and CBn, and thereby confirmed the observations in aqueous solution by 1H NMR spctroscopy. Dodecaborate clusters are chaotropic dianions,7a they are hydrophilic and their sodium salts are highly water soluble. The general tendency of chaotropic anions to associate with hydrophobic surfaces in aqueous solution has been recently established for cyclodextrins7a and referred to as the chaotropic effect, complementing the hydrophobic effect which brings together two hydrophobic partners. This accounts also for the tendency of dodecaborate clusters to associate with CBn macrocycles, although in this case exclusion complexes rather than inclusion complexes are formed. The desolvation of chaotropic anions leads to the restoration of the hydrogen bonding network. This is an enthalpically favorable and entropically penalized process,7a which allows these anions to associate with hydrophobic concave and convex surfaces with overall negative free energies.
O) revealed only three examples (codes: EZIHAX, PERXEP, and WICHEV), all of which involve carborane derivatives. Another unconventional interaction was observed in the crystal structure, in which the partially negative hydrogen atoms on the cluster interact with the hydrogen atoms on the exterior of CB7 (H⋯H approximately 2.25–2.58 Å). We also obtained single crystals of the complex between CB7 and B12Cl122− (Fig. S3, ESI†), and single crystals of the complex between CB6 and B12H122− (Fig. S4, ESI†). All XRD structures proved the formation of exclusion complexes between dodecaborate clusters and CBn, and thereby confirmed the observations in aqueous solution by 1H NMR spctroscopy. Dodecaborate clusters are chaotropic dianions,7a they are hydrophilic and their sodium salts are highly water soluble. The general tendency of chaotropic anions to associate with hydrophobic surfaces in aqueous solution has been recently established for cyclodextrins7a and referred to as the chaotropic effect, complementing the hydrophobic effect which brings together two hydrophobic partners. This accounts also for the tendency of dodecaborate clusters to associate with CBn macrocycles, although in this case exclusion complexes rather than inclusion complexes are formed. The desolvation of chaotropic anions leads to the restoration of the hydrogen bonding network. This is an enthalpically favorable and entropically penalized process,7a which allows these anions to associate with hydrophobic concave and convex surfaces with overall negative free energies.
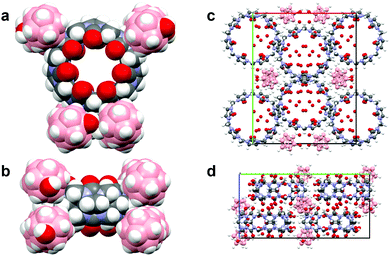 | ||
| Fig. 3 Views of the CB7/B12H11OH2− complex XRD structure. The CB7 component has crystallographically-imposed twofold symmetry and that the B12H11OH2− component is in a general position. | ||
The XRD structures of the exclusion complexes reveal additional details, which can be rationalized from the electrostatic potential (ESP) maps of the dodecaborate anions and CBn macrocycles (Fig. 4). For the dodecaborate, the double negative charge is evenly dispersed over the cluster sphere. On the other hand, the ESP of CBn reveals the negatively charged carbonyl regions (in red) and the electron-deficient equatorial region (in blue), which offer docking points for the cluster dianions through ion–dipole interactions. Scrutiny of the literature reveals that the tendency of some anions to associate with the outer walls of CBn macrocycles has already been documented,13 but not appreciated as a generic recognition motif, operative selectively for chaotropic anions. For example, Tao and coworkers have shown that coordination polymers based on CBn can be achieved in the presence of inorganic anions (e.g., ZnCl22−, PtCl62−, and CdCl42−).13a–e Fang et al. also reported that CBn and polyoxometalates form hybrid organic–inorganic assemblies.13f
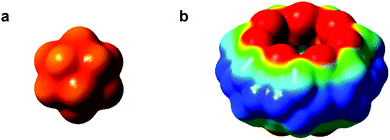 | ||
| Fig. 4 DFT computed electrostatic potential maps for B12H122− and CB7. Electron-rich and -deficient regions shown in red and blue, respectively. | ||
The symmetrical nature of both, dodecaborates and CBn, allows the assembly motif to be expanded into three dimensions, into a supramolecular network. We presume therefore, in aqueous solution, that the chaotropic effect drives the borate cluster anions to the walls of the CBn macrocycles where they dock according to the electrostatic potentials and ultimately form aggregates (Fig. 1). The large size of the borate cluster anions therefore allows multiple interactions to occur, which phenomenologically leads to a “salting-out effect” of an organic solute. This is against chemical intuition, because salting-out effects are otherwise characteristic for cosmotropic ions.
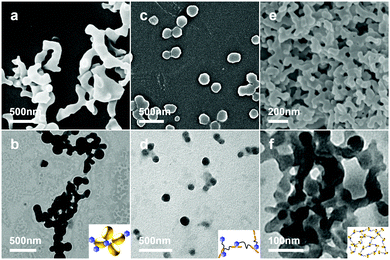
The CBn aggregation induced by dodecaborate anions was observed at low millimolar concentrations (>0.1 mM) of the clusters and CBn. Fig. 5a and b show the SEM and TEM images of the formed aggregates. The multiple connectivity of the assemblies is in line with the dodecaborate clusters acting as bridges that link several CB7 units together. Similar aggregates were observed when Na2B12Cl12 was mixed with CB7 (Fig. S5, ESI†). Interestingly, the CB7/B12H122− self-assembly can be reversibly controlled by changing the pH (Fig. 1). The aggregates can be partially disassembled by concentrated acid (Fig. S6a and b, ESI†) and re-generated upon dilution or pH-neutralization (Fig. S6c and d, ESI†). The partial disassembly of the aggregates in strongly acidic solution is attributed to a competitive binding of acid (hydronium ions) to the dodecaborate clusters.
In contrast to common chaotropic anions, borate clusters have the advantage that they can be easily functionalized and tethered to organic residues, an option based on which we expanded our study. In particular, this allowed us to assemble responsive supramolecular structures by exploiting the chaotropic and hydrophobic effects as orthogonal interactions. In detail, we have tethered dodecaborate to an azobenzene (AZO) moiety, which is known to be encapsulated by CB7 (hydrophobic effect under inclusion complex formation) and to undergo trans-to-cis photoisomerization (Fig. 1b).14 The ditopic guest G1 showed the ability to self-assemble on its own, because the dodecaborate cluster has also an affinity to the AZO group (chaotropic effect). SEM and TEM images showed that G1 formed spherical nanoparticles (Fig. 5c and d), while DLS measurement resulted in an average hydrodynamic diameter of 125 nm (Fig. S12, ESI†).
Multi-responsive assemblies were prepared by mixing G1 and CB7 in aqueous solution. The hydrophobic effect drives the trans-azobenzene residue into the cavity of CB7, while the chaotropic effect allows dodecaborate anions to dock to the exterior of CB7 according to the binding motif established above. Fig. 1b shows one possible cross-sectional view of various structures of the anticipated multi-responsive assembly, and its reversible changes as controlled by light and pH. The orthogonal self-assembled supramolecular networks grew with time (hours). Due to the self-aggregation of G1 and the CB7/G1 assembly in water, NMR spectroscopy was unsuitable for further characterization. However, the 1H NMR spectra of 4-phenylazophenol in the presence of CB7 verified that the AZO residue in its trans configuration forms an inclusion complex with CB7 (Fig. S13, ESI†). Shear viscosity of the CB7/G1 complex increased steeply with a low shear rate and decreased gradually after that, and shear stress of the complex was higher than that of single components, which indicated the formation of the complex (Fig. S14, ESI†). The morphology of the CB7/G1 assembly was obtained by SEM and TEM (Fig. 5e and f). In comparison to the CB7/B12H122− aggregates, the CB7/G1 aggregates are more compact, due to the additional inclusion complexation.
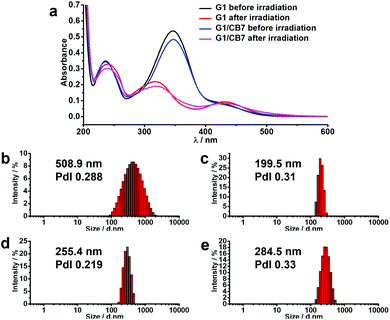
The propensity of the AZO moiety to undergo trans-to-cis photoisomerization in G1 and the CB7/G1 assemblies was confirmed by UV-Vis spectra before and after 350 nm irradiation, see Fig. 6a, which provided evidence that both G1 and CB7/G1 undergo trans-to-cis photoisomerization. As an additional independent line of evidence for the retained photoactivity, we have now also prepared a highly water-soluble model compound (G2, ESI†). The photoisomerization was also observed by 1H NMR spectroscopy (ESI,† Fig. S16), in which only the trans form of azobenzene could be encapsulated in the cavity of CB7. Although both isomers are hydrophobic, only the elongated trans form displays a good shape fitting and adapted electronic properties to allow an axial threading through both CB7 portals.14a
The size measured by DLS of the CB7/G1 aggregate showed an average hydrodynamic diameter of 500 nm (Fig. 6b), which decreased to 200 nm upon irradiation for 30 minutes (Fig. 6c). Moreover, SEM and TEM images showed that, after irradiation by UV light, the former networks transformed into spherical nanoparticles, hinting to the anticipated change in the structure of the assemblies (see ESI,† Fig. S17a and b). When the disassembled system was exposed to visible light, the average hydrodynamic diameter of the system increased with irradiation time (Fig. 6d and e). Furthermore, when the system was heated to 60 °C, larger aggregates were formed (Fig. S19, ESI†).
As the exclusion complexes of dodecaborate clusters with CB7 can be partially disassembled at low pH values in a reversible manner, a multi-responsive material is obtained. The SEM and TEM images of CB7/G1 dissolved in formic acid (88%) showed the disassembly of the aggregate and transformation into spherical nanoparticles with 300–500 nm size (see ESI,† Fig. S17c and d). The addition of acid to the CB7/G1 system breaks down the CB7/dodecaborate exclusion complexation (partially “neutralizes” the chaotropic effect), while irradiation by UV light leads to the dissociation of the inclusion complexation between the AZO-residue and CB7 (“neutralizes” the hydrophobic effect). The nanoparticles formed in formic acid solution displayed a different size compared to those formed upon irradiation. ESI-MS conducted in formic acid (88%) indicated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation of G1 with CB7, providing evidence for the existence of non-aggregated, stoichiometric complexes (Fig. S20 (ESI†), m/z 1521.20). When the partially disassembled system was aged for a month with evaporation of the acid, the networks reassembled (Fig. S21, ESI†).
1 complexation of G1 with CB7, providing evidence for the existence of non-aggregated, stoichiometric complexes (Fig. S20 (ESI†), m/z 1521.20). When the partially disassembled system was aged for a month with evaporation of the acid, the networks reassembled (Fig. S21, ESI†).
In summary, new assemblies between dodecaborate anions and CBn have been reported, which exploit the chaotropic effect in the context of functional materials. In the assemblies, dodecaborate anions formed exclusion complexes with CB7 driven by the chaotropic effect, leaving the cavity accessible to bind other organic guest molecules by the hydrophobic effect. Novel multi-responsive dodecaborate-cucurbituril supramolecular networks were developed based on orthogonal hydrophobic–chaotropic interactions allowing exclusion and inclusion complexation. The multi-responsive assemblies were conveniently assembled in aqueous solution at ambient temperature, establishing a new platform to prepare supramolecular polymer networks under mild conditions.
This work was financially supported by the Fundamental Research Funds for the Central Universities (no. 2042016HF1054) and Wuhan University Experiment Technology Project Funding (no. WHU-2016-SYJS-06). We are grateful to Prof. Dr Dongsheng Guo (Nankai University) for fruitful discussion. And we are grateful to Dr Wenjing Liu (Nanjing Normal University) and Dr Zhengguo Lin (Beijing Institute of Technology) for assistance of crystallography. K. I. A. and W. M. N. are grateful to the DFG for the support within grant NA-686/8. The work was initiated through a fellowship of the China Scholarship Council (no. 201406275086) supporting the research of H. Z. at Jacobs University Bremen.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS PubMed; (b) L. Brunsveld and E. W. Meijer, Chem. Rev., 2001, 101, 4071 CrossRef CAS PubMed; (c) L. Voorhaar and R. Hoogenboom, Chem. Soc. Rev., 2016, 45, 4013 RSC; (d) C. Heinzmann, C. Weder and L. Montero de Espinosa, Chem. Soc. Rev., 2016, 45, 342 RSC.
- (a) Q.-D. Hu, G.-P. Tang and P. K. Chu, Acc. Chem. Res., 2014, 47, 2017 CrossRef CAS PubMed; (b) A. Alsbaiee, D. E. Helbling and W. R. Dichtel, Nature, 2016, 529, 190 CrossRef CAS PubMed; (c) S. Zhang, Nat. Biotechnol., 2003, 21, 1171 CrossRef CAS PubMed; (d) S. I. Stupp, Science, 1997, 276, 384 CrossRef CAS PubMed.
- (a) X. Dai and W. Liu, Adv. Mater., 2015, 27, 3566 CrossRef CAS PubMed; (b) F. Luo and J. P. Gong, Adv. Mater., 2015, 27, 2722 CrossRef CAS PubMed; (c) L. R. Hart, H. M. Colquhoun and W. Hayes, Polym. Chem., 2014, 5, 3680 RSC; (d) E. A. Appel and M. W. Tibbitt, Nat. Commun., 2015, 6, 6295 CrossRef CAS PubMed; (e) A. P. Constantinou and T. K. Georgiou, Polym. Chem., 2016, 7, 2045 RSC; (f) A. Noro and Y. Matsushita, Macromolecules, 2013, 46, 8304 CrossRef CAS.
- (a) X. Yan, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2014, 136, 4460 CrossRef CAS PubMed; (b) H. Chen, X. Ma and H. Tian, Angew. Chem., Int. Ed., 2014, 53, 14149 CrossRef CAS PubMed; (c) Q. Yan and J. Yuan, Polym. Chem., 2013, 4, 1216 RSC; (d) S. Dong and F. Huang, Acc. Chem. Res., 2014, 47, 1982 CrossRef CAS PubMed; (e) N. Song and Y.-W. Yang, Chem. Commun., 2014, 50, 8231 RSC.
- (a) C. Douvris and J. Michl, Chem. Rev., 2013, 113, PR179 CrossRef PubMed; (b) K. Karki and D. Roccatano, Inorg. Chem., 2012, 51, 4894 CrossRef CAS PubMed.
- (a) J. Plešek, Chem. Rev., 1992, 92, 269 CrossRef; (b) W. Tjarks, Chem. Commun., 2007, 4978 RSC.
- (a) K. I. Assaf, K. Rissanen, D. Gabel and W. M. Nau, Angew. Chem., Int. Ed., 2015, 54, 6852 CrossRef CAS PubMed; (b) K. I. Assaf and W. M. Nau, Org. Biomol. Chem., 2016, 14, 7702 RSC; (c) K. I. Assaf, Org. Lett., 2016, 18, 932 CrossRef CAS PubMed.
- R. Fernandez-Alvarez and Pavel Matějíček, Langmuir, 2017 DOI:10.1021/acs.langmuir.7b03306.
- F. Biedermann, W. M. Nau and H.-J. Schneider, Angew. Chem., Int. Ed., 2014, 53, 11158 CrossRef CAS PubMed.
- (a) W. A. Freeman and W. L. Mock, J. Am. Chem. Soc., 1981, 103, 7367 CrossRef CAS; (b) J. Kim and K. Kim, J. Am. Chem. Soc., 2000, 122, 540 CrossRef CAS; (c) J. Lagona and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS PubMed; (d) K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394 RSC.
- (a) M. Florea and W. M. Nau, Angew. Chem., Int. Ed., 2011, 50, 9338 CrossRef CAS PubMed; (b) S. J. Barrow and O. A. Scherman, Chem. Rev., 2015, 115, 12320 CrossRef CAS PubMed; (c) D. Shetty and K. Kim, Chem. Soc. Rev., 2015, 44, 8747 RSC.
- (a) E. A. Appel and O. A. Scherman, J. Am. Chem. Soc., 2010, 132, 14251 CrossRef CAS PubMed; (b) L. Chen and X. Zhang, Polym. Chem., 2016, 7, 1397 RSC.
- (a) H. Cong, K. Chen and Z. Tao, Eur. J. Inorg. Chem., 2014, 2262 CrossRef CAS; (b) N.-N. Ji and Z. Tao, Eur. J. Inorg. Chem., 2014, 1435 CrossRef CAS; (c) Y. Zhao, L.-L. Liang and Q.-J. Zhu, CrystEngComm, 2013, 15, 7987 RSC; (d) L.-L. Liang, Z. Tao and J.-X. Liu, CrystEngComm, 2013, 15, 3943 RSC; (e) L. L. Liang, X. L. Ni and Z. Tao, Inorg. Chem., 2013, 52, 1909 CrossRef CAS PubMed; (f) X. Fang and P. Kögerler, J. Am. Chem. Soc., 2009, 131, 432 CrossRef CAS PubMed; (g) T. Goel, A. C. Bhasikuttan and J. Mohanty, Chem. Commun., 2016, 52, 7306 RSC.
- (a) M. Baroncini and S. Silvi, Chem. – Eur. J., 2014, 20, 10737 CrossRef CAS PubMed; (b) J. Wu and L. Isaacs, Chem. – Eur. J., 2009, 15, 11675 CrossRef CAS PubMed; (c) S. Choi and K. Kim, Chem. Commun., 2003, 2176 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, characterization, binding studies, crystallographic studies and morphological studies. CCDC 1574822, 1574912 and 1574823. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc08078f |
| This journal is © The Royal Society of Chemistry 2018 |