Open Access Article
Open Access ArticleMechanochemical synthesis of cooperative spin crossover materials†
Jed H.
Askew
 and
Helena J.
Shepherd
and
Helena J.
Shepherd
 *
*
School of Physical Sciences, University of Kent, Canterbury, CT2 7NH, UK. E-mail: h.j.shepherd@kent.ac.uk
First published on 8th December 2017
Abstract
We describe the synthesis of switchable spin crossover materials via mechanochemistry for the first time. Three chemically diverse spin crossover materials have been produced using solvent-free grinding. Crucially, cooperative spin transition behavior and crystallinity is retained, presenting exciting opportunities for the discovery of new materials with switchable magnetic, optical and structural properties.
Spin crossover (SCO) materials have seen a surge in interest in recent years as a result of their optical, magnetic and structural bistability in technologically accessible temperature ranges, giving rise to a number of potential applications in sensing, display and actuator technologies.1,2 The bistability in these systems stems from their propensity to switch reversibly between high-spin (HS) and low-spin (LS) states in response to physical stimuli including temperature, pressure, light irradiation, guest molecules and magnetic field.3 There are hundreds of known SCO-active materials in the literature including molecular coordination complexes, 1D coordination polymers and 2-/3-dimensional metal organic frameworks. The cooperativity of the SCO phenomenon (abruptness of the transition, hysteretic effects etc.) is a result of electron–phonon coupling between SCO centers in the solid state through elastic interactions in the lattice.4 The degree of cooperativity is important for application, and has been shown to be sensitive to the crystallinity of the material, particle size and the presence of solvents in the lattice.2,5 Synthesis of bulk SCO materials has typically been carried out via traditional solution state chemistry, although more complex techniques for the production of nanoparticles and thin films have been developed in the last decade.6 Remarkably, all existing techniques use solvents and can be time-consuming; often requiring inert atmospheres and multiple synthetic steps. This aspect is particularly limiting in the search for new SCO-active materials. Herein, we present for the first time the rapid and facile synthesis of three chemically different classes of SCO material, using mechanochemical techniques. We show that microcrystalline samples of SCO-active molecular materials, 1D coordination networks and 3D MOFs can be produced using this technique and crucially the cooperative behaviour of the spin transition is maintained. Finally, we highlight how this technique can be used to accelerate SCO research.
Mechanochemistry refers to the reaction of materials through the application of mechanical energy, often through grinding in the solid state. While mechanochemistry has long been used in the synthesis of inorganic materials and composites, in recent years it has also been applied in the synthesis of molecular systems, coordination complexes and frameworks, co-crystals and supramolecular networks.7,8 Mechanistic explanations for the success of the mechanochemical approach are numerous and as yet no single model can be applied to the varied range of systems that can be produced in this manner. However, it is clear that the technique has the potential to produce both known and new materials across diverse areas of chemistry.
Triazole-based 1D SCO coordination polymers have become the subject of a huge number of studies owing to their high temperature range of operation and relative ease of chemical modification, making them extremely attractive from the point of view of application.9 [Fe(atrz)3]SO4 (where atrz = 4-amino-1,2-4-triazole, compound 1) is a member of this family and has been previously prepared using solution-state techniques.10 Like other members of this family it shows a pronounced colour change from purple in the LS state at ambient temperature, to white in the HS state above 350 K. For the first time, we have used mechanochemical techniques to prepare samples of 1; [NH4]2[Fe(SO4)2]·6H2O and atrz were ground in a pestle and mortar without solvent for 5 minutes.‡
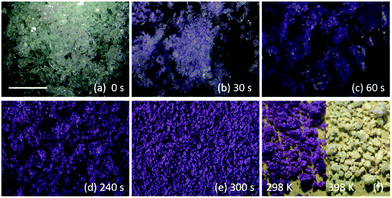
Within 30 seconds, traces of the purple colour indicative of the LS product began to appear. After ca. 4 minutes the entire sample was purple and no further colour change was observed at room temperature. The product became damp as a result of liberation of water molecules from the hydrated metal salt. It dried out during a further minute of grinding, and was subsequently further dried by heating.§ This general procedure was followed for the production of a series of SCO-active materials, experimental details and materials characterization are provided in the ESI.†Fig. 1 shows the reaction progression as a function of grinding time, and the characteristic purple (LS) → white (HS) colour change accompanying SCO on heating is clear in Fig. 1(f).
Despite the increasingly routine nature of mechanochemical synthesis in modern chemistry, there are no reports of these techniques being used to make switchable SCO materials. The most likely reason for this is the widely-held assumption that grinding results in significant reduction in cooperativity of the spin transition. There are many examples in the literature of powdered and nano-scale SCO materials exhibiting weaker cooperativity than associated bulk samples. This is attributed to amorphisation and a reduction in the coherent domain length of individual particles, through increased defects and a more significant surface contribution.11 Cooperativity can be assessed through evaluation of the abruptness of the SCO curve and hysteresis widths via magnetometry.12 Previous studies on the effects of grinding SCO materials involved grinding solution-synthesized molecular SCO materials for several hours. This resulted in a pronounced reduction in cooperative behaviour and the eventual suppression of all SCO properties.13,14 Thus, careful comparison of SCO properties arising from mechanochemical and solution-state synthetic protocols (via SQUID magnetometry) is required to ensure functionality of the produced materials is not deleteriously affected by grinding.
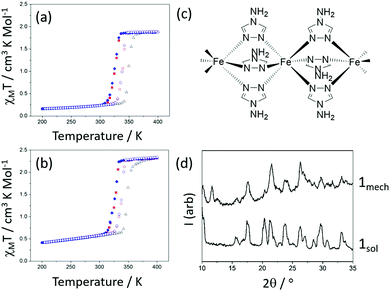
The SCO behaviour of solution-synthesized 1 (denoted 1sol) as a function of temperature is shown in Fig. 2(a); the spin transition is abrupt and accompanied by a hysteresis of 23 K for the first cycle (T½↑ = 350 K, T½↓ = 327), and 14 K on subsequent cycles. By comparison, the mechanochemically synthesized sample after annealing (denoted 1mech) has very similar hysteresis widths of 21 K for the first cycle (T½↑ = 348 K, T½↓ = 327 K), and 14 K on subsequent cycles (Fig. 2(b)). Clearly, despite grinding the sample for several minutes during cooperative SCO behaviour; the spin transitions in the two samples are virtually indistinguishable. The degree of crystallinity is comparable in both 1mech and 1sol, as demonstrated in the powder diffraction patterns in Fig. 2(d).
There are however additional peaks in the powder diffraction pattern, which we attribute to irreversible structural differences, possibly caused by water loss from the lattice. This is discussed further in the ESI.† Raman spectroscopy (shown in the ESI†) of the sample, in both HS and LS states, reveals typical changes in the spectrum associated with SCO.15 A sample of the related [Fe(atrz)3](BF4)2 complex was also synthesized using mechanochemistry and shows similar cooperativity to the equivalent solution-state product. More details are provided in the ESI.†
One of the most frequently studied Fe2+ SCO molecular materials is [Fe(phen)2(NCS)2] (where phen = 1,10-phenanthroline, compound 2). At room temperature it is in the HS state and undergoes SCO to the LS state around 178 K, as shown in Fig. 3(a).16 The abruptness of the transition has been shown to be sensitive to varying crystallinity that results from differing preparation methods. It is known that the SCO-inactive complex [Fe(phen)3](NCS)2 (2pre) can be converted to compound 2via thermolysis.17,18 Mechanochemistry was used to synthesize 2pre and it was subsequently thermally converted to the active compound 2mech. The SCO properties of both the precursor 2pre and 2mech are shown in Fig. 3(b). While 2pre is not SCO-active, 2mech shows very similar SCO behaviour to the literature values (T½↑ = T½↓ = 174 K). Fig. 3(d) shows the evolution from 2pre to 2mech with powder diffraction; additional peaks in the 2pre diffraction pattern are attributed to the presence of by-products from the mechanochemical reaction. Thermal gravimetric analysis of the conversion from 2pre to 2mech is shown in the ESI.† Again, it is clear that 2mech is crystalline and that cooperative SCO behaviour is retained.
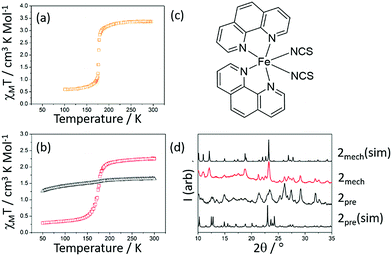 | ||
| Fig. 3 (a) χMT versus T for 2sol adapted from ref. 16 (b) χMT versus T for 2pre (black) and 2mech (red), (c) structure of 2 and (d) powder diffraction patterns of [Fe(phen)3](NCS)2 (simulated from single crystal data, 2pre(sim)) 2pre, 2mech and [Fe(phen)2(NCS)2] (simulated from single crystal data, 2mech(sim)). | ||
A series of bimetallic (Fe-MI and Fe-MII) cyanide-bridged 2- and 3-D coordination polymers known as the Hofmann-like networks represent another class of SCO materials that have been studied extensively. Their sensitivity to guest molecules, huge potential for chemical modification and often high-temperature, cooperative SCO behaviour has attracted increasing attention.19 [Fe(pz){Au(CN)2}2] (where pz = pyrazine, compound 3) is an example of one of these Hofmann-like networks, adopting an infinite 3D structure through ditopic pyrizine ligands, which bridge between iron tetracyanoaurate layers. When produced through solution-state synthetic procedures (3sol) it has an abrupt SCO above room temperature, as shown in Fig. 4(a) (T½↑ = 369, T½↓ = 349 K).20 The mechanochemically synthesized complex (denoted 3mech) shows very similar SCO behaviour (T½↑ = 370, T½↓ = 354 K).
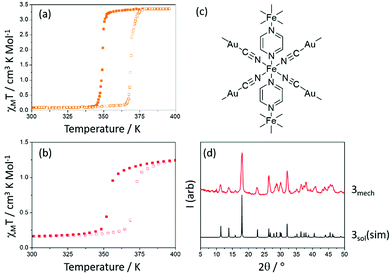 | ||
| Fig. 4 (a) χMT versus T for 3sol adapted from ref. 18 (b) χMT versus T for 3mech (c) structure of 3 and (d) PXRD of complex 3 (simulated from single crystal data20), and 3mech. | ||
It must be added at this stage, that due to the nature of the mechanochemical procedure, the final product may well contain unreacted starting materials and by-products from the reaction. While washing the product may remove these impurities, SCO materials are notoriously sensitive to the presence of solvents. For this reason, in this study we aim to demonstrate that SCO-active materials can be produced without any solvent, either in synthesis or processing of the material. As a consequence the accuracy of the absolute values of χMT presented here for systems elaborated through mechanochemistry may be variable depending on the specific impurities present. Of more importance to the current study are the abruptness and hysteresis effects as they relate to cooperativity in the solid state. Further discussion of the effects of washing mechanochemically prepared samples and experimental examples are presented in the ESI.† Values for the transition temperatures of each of the mechanochemically synthesized samples and the equivalent solution-state material are summarised in Table 1. Transition temperatures for all compounds are very similar for each sample, and more importantly, hysteresis widths (ΔT½) of the samples made via mechanochemistry are identical (in the cases of 1 and 2) or very similar (in the case of 3) to those synthesized in solution. While the width of the hysteresis is often used as a proxy for cooperativity in SCO systems, it is also important to look at how abrupt the transition is. Hence Table 1 also shows values for the “smoothness” of the transition (defined as the difference in the temperatures for which 80% and 20% of the molecular complexes are in the HS state), a measure which has previously been used to quantitatively compare how abrupt a given transition is.21 In the case of 1mech, the transition is slightly more abrupt than that synthesized by solution-state methods (smoothness values of 9 and 12 K respectively). By contrast, the reverse is true for 2 and 3, with smoothness increasing by 12 and 9 K respectively between solution and mechanochemical synthesis protocols. We attribute these differences to reduction in the coherent domain length of particles in the mechanochemically synthesized products. The dependence of hysteresis and smoothness on particle size has been shown to vary for different families of SCO complexes. In triazole-based coordination polymers (such as 1), hysteresis width and abruptness of the transition is maintained below 10 nm.22,23 While in complex 2, the transition becomes much more gradual as size of particles decreases in the nanometre range.18,24 A similar reduction in abruptness and hysteresis width has also been observed for the family of bimetallic frameworks to which 3 belongs.25,26 All observations shown in Table 1 agree with these studies, and point to the reduction in hysteresis and increased smoothness being a consequence of particle size effects rather than something intrinsic to the mechanochemical procedure. Full characterisation of particle size and morphology of 1 and 2 is currently underway, and we believe this method may present a promising new route towards the size-selective synthesis of SCO materials.
| Sample | T ½↓ (K) | T ½↑ (K) | ΔT½ (K) | Smoothnessa/K |
|---|---|---|---|---|
| a Defined in ref. 21 as the difference in the temperatures for which 80% and 20% of the molecular complexes are in the HS state. b Values calculated based on data presented in ref. 16. c Values calculated based on data presented in ref. 20. | ||||
| 1sol | 325 | 338 | 13 | 12 |
| 1mech | 325 | 338 | 13 | 9 |
| 2sol | 178 | 178 | 0 | 4 |
| 2mech | 174 | 174 | 0 | 16 |
| 3sol | 349 | 369 | 20 | 1 |
| 3mech | 354 | 370 | 16 | 10 |
In addition to cooperativity and size-control, some current challenges in the field of SCO include the discovery of active new materials, enhancement of switching and sensing properties and issues associated with technological application. The mechanochemical procedure is extremely rapid compared to solution-state techniques, and thus shows great potential for discovery of novel materials through the ability to screen many more combinations of ligand and metal salt. It is also trivial to introduce stoichiometric proportions of potential guest molecules (either liquid or solid) into the mechanochemical procedure to further assess the influence of host–guest interactions on SCO properties. There are no limitations associated with solubility/compatibility of solvent mixtures, and mechanochemistry has even been used to synthesize materials and polymorphs that are not accessible at all via solution.27 All of these advantages open the door to development of new materials with improved SCO properties for technological application. As SCO materials move closer towards such real world application, the ability to scale-up syntheses and reduce reliance on solvents (green chemistry) will become ever more important. In this regard mechanochemistry can offer significant opportunities as there are already several commercial routes to scaling up including ball-milling and extrusion.28 Having shown that it is possible to synthesize SCO materials while retaining cooperative properties via mechanochemistry we anticipate that many of the advantages brought by the technique will be exploited in the quest for new, technologically useful smart materials.
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Senthil Kumar and M. Ruben, Coord. Chem. Rev., 2017, 346, 176–205 CrossRef CAS.
- M. D. Manrique-Juárez, S. Rat, L. Salmon, G. Molnár, C. M. Quintero, L. Nicu, H. J. Shepherd and A. Bousseksou, Coord. Chem. Rev., 2016, 308, 395–408 CrossRef.
- M. A. Halcrow, Spin-Crossover Materials: Properties and Applications, Wiley-Blackwell, 1st edn, 2013 Search PubMed.
- P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419–427 RSC.
- A. B. Gaspar and B. Weber, in Molecular Magnetic Materials: Concepts and Applications, ed. B. Sielucka and D. Pinkowisz, Wiley VCH, 2016, pp. 231–252 Search PubMed.
- H. J. Shepherd, G. Molnár, W. Nicolazzi, L. Salmon and A. Bousseksou, Eur. J. Inorg. Chem., 2013, 653–661 CrossRef CAS.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- T. Friščić, Chem. Soc. Rev., 2012, 41, 3493 RSC.
- O. Roubeau, Chem. – Eur. J., 2012, 18, 15230–15244 CrossRef CAS PubMed.
- L. G. Lavrenova, O. G. Shakirova, V. N. Ikorskii, V. A. Varnek, L. A. Sheludyakova and S. V. Larionov, Russ. J. Coord. Chem., 2003, 29, 22–27 CrossRef CAS.
- J. Larionova, L. Salmon, Y. Guari, A. Tokarev, K. Molvinger, G. Molnár and A. Bousseksou, Angew. Chemie – Int. Ed., 2008, 47, 8236–8240 Search PubMed.
- M. A. Halcrow, Chem. Soc. Rev., 2011, 40, 4119 RSC.
- E. W. Müller, H. Spiering and P. Gütlich, Chem. Phys. Lett., 1982, 93, 567–571 CrossRef.
- M. S. Haddad, W. D. Federer, M. W. Lynch and D. N. Hendrickson, J. Am. Chem. Soc., 1980, 102, 1468–1470 CrossRef CAS.
- J. A. Wolny, R. Diller and V. Schünemann, Eur. J. Inorg. Chem., 2012, 2635–2648 CrossRef CAS.
- B. Gallois, J. A. A. Real, C. Hauw and J. Zarembowitch, Inorg. Chem., 1990, 29, 1152–1158 CrossRef CAS.
- E. C. Ellingsworth, B. Turner and G. Szulczewski, RSC Adv., 2013, 3, 3745–3754 RSC.
- J. Laisney, A. Tissot, G. Molnár, L. Rechignat, E. Rivière, F. Brisset, A. Bousseksou and M. L. Boillot, Dalton Trans., 2015, 44, 17302–17311 RSC.
- M. C. Muñoz and J. A. Real, Coord. Chem. Rev., 2011, 255, 2068–2093 CrossRef.
- I. A. Gural'skiy, B. O. Golub, S. I. Shylin, V. Ksenofontov, H. J. Shepherd, P. R. Raithby, W. Tremel and I. O. Fritsky, Eur. J. Inorg. Chem., 2016, 3191–3195 CrossRef.
- P. Guionneau, M. Marchivie, G. Bravic, J.-F. Létard and D. Chasseau, Spin Crossover in Transition Metal Compounds II. Topics in Current Chemistry, Springer Berlin Heidelberg, Berlin, Heidelberg, 2004, vol. 234, pp. 97–128 Search PubMed.
- J. R. Galán-Mascarós, E. Coronado, A. Forment-Aliaga, M. Monrabal-Capilla, E. Pinilla-Cienfuegos and M. Ceolin, Inorg. Chem., 2010, 49, 5706–5714 CrossRef PubMed.
- M. Giménez-Marqués, M. Luisa García-Sanz de Larrea and E. Coronado, J. Mater. Chem. C, 2015, 7946, 7946–7953 RSC.
- F. Javier Valverde-Muñoz, A. B. Gaspar, S. I. Shylin, V. Ksenofontov and J. Real, Inorg. Chem., 2015, 54, 7906 CrossRef PubMed.
- V. Martínez, I. Boldog, A. B. Gaspar, V. Ksenofontov, A. Bhattacharjee, P. Gütlich and J. A. Real, Chem. Mater., 2010, 22, 4271–4281 CrossRef.
- F. Volatron, L. Catala, E. Rivière, A. Gloter, O. Stéphan and T. Mallah, Inorg. Chem., 2008, 47, 6584–6586 CrossRef CAS PubMed.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2016, 3, 13–19 CrossRef PubMed.
- D. E. Crawford and J. Casaban, Adv. Mater., 2016, 28, 5747–5754 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, variable temperature Raman spectroscopy, FTIR spectra and discussion of the effect of washing the mechanochemically prepared samples. See DOI: 10.1039/c7cc06651a |
| ‡ CAUTION: Do not use mechanochemical techniques for the preparation of potentially explosive materials. Perchlorate salts and tetrazole ligands have the potential to explode when handled dry, particularly on contact or grinding, and should thus not be used in routine mechanochemical experiments. Care should also be taken when preparing cyanometallate complexes to avoid accidental release of cyanide. |
| § Compounds 1mech, 2pre and 3mech were prepared by neat grinding of powdered precursors in the absence of solvent. In general, the reagents were manually ground using an agate or glass pestle and mortar for between two and five minutes. 1mech was heated at 473 K for 10 hours to remove residual water. 2pre was converted to 2mechvia heating at 473 K for 10 hours. 3mech was used as prepared. Full details are provided in the ESI.† |
| This journal is © The Royal Society of Chemistry 2018 |