Open Access Article
Open Access ArticlePorous dipeptide crystals as volatile-drug vessels†
S.
Bracco
 ,
D.
Asnaghi
,
D.
Asnaghi
 ,
M.
Negroni
,
M.
Negroni
 ,
P.
Sozzani
,
P.
Sozzani
 and
A.
Comotti
and
A.
Comotti
 *
*
Department of Materials Science, University of Milano Bicocca and INSTM Consortium, via R. Cozzi 55, 20125, Milano, Italy. E-mail: angiolina.comotti@unimib.it
First published on 6th December 2017
Abstract
Porous crystalline dipeptides absorb, reversibly from the gas phase, a series of volatile fluorinated ethers in use as anesthetics. Their vapor pressure was considerably reduced, with favorable guest capture and release. Variable channel sizes were customized for selective sorption and pressure thresholds were observed in the narrowest pores. 1H, 13C and 19F MAS NMR coupled with ab initio conformational analysis and grand canonical Monte Carlo simulations highlight the guest loading and arrangement adopted in the congruent nanochannels, suggesting how the anesthetics can accommodate in biochemical receptors.
In recent years intense development in the field of porous materials with nanometric and sub-nanometric pore dimensions has enabled the confinement and release of gases and volatile molecules with high efficiency.1 Indeed, in addition to conventional inorganic materials, such as zeolites,2 hybrid materials and fully organic frameworks have been proposed.3 Thus, the nature of the bonds sustaining the structures was extended to metal–organic, covalent–organic bonds as well as to soft supramolecular interactions, which produce porous materials denominated hydrogen bonded organic frameworks (HOFs), supramolecular organic frameworks (SOFs) and porous molecular crystals (PMCs).4 The easy self-assembly from their molecular constituents as well as the processability in solution make them particularly attractive. In this context, biological molecules, especially dipeptides composed by proteinogenic amino-acids, are able to self-assemble into porous architectures and have been proposed as absorptive materials.5 Such dipeptides are outstanding for their large variety of available channels. Supramolecular interactions as stable as charge assisted hydrogen bonding between ammonium and carboxylate end-groups support the porous structure. They are biodegradable and thus environmentally friendly, as opposed to the majority of porous materials. However, their use as volatile drug vessels has never been explored.
Halogenated ethers and alkanes have extensive applications in general anesthesia, although there are still difficulties in their storage and delivery without dispersion into the environment.6 Halothane, enflurane, desflurane and isoflurane, and the non-halogenated diethyl ether, are among the most commonly used substances as anesthetics. These compounds, administered by inhalation, interact with GABAA receptors in synaptic membranes and cause a reversible loss of consciousness.7 Moreover, most of them belong to the category of chlorofluorocarbon ethers (CFCs) and thus represent a constant danger for the environment.8
The use of biocompatible peptides in various association states make them suitable as drug delivery systems for hydrophobic drugs.9 However, crystalline porous dipeptides have not yet been exploited for hosting/releasing volatile drugs, although they are particularly intriguing for the subnanometric size of the available spaces. The reduction of the vapor pressure of commonly-used volatile anesthetics upon nanoconfinement may give rise to practical advantages in handling and storage,10 as well as in delivery for controlled administration.9 Moreover, the use of nanocrystals as hydrophobic-drug carriers may be attractive for parenteral or oral administration, thanks to the increased solubility and, thus, bioavailability. Herein we present adsorption/desorption isotherms of ultra-microporous dipeptide crystals and spectroscopic evidences for guest-anesthetic arrangement in the nanochannels (Fig. 1).
 | ||
| Fig. 1 Chemical structures of the halogenated ethers used as guests (above); portion of the crystal structure of L-valyl-L-alanine (VA) along the channels axis (below). | ||
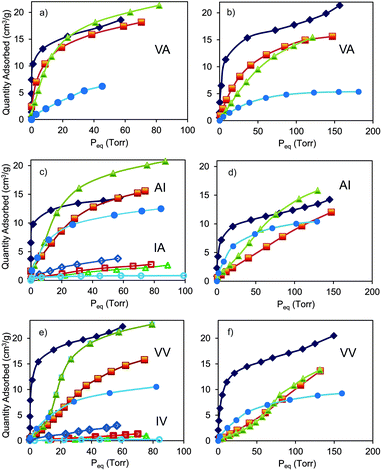
In particular, we investigated porous dipeptides L-valyl-L-alanine (VA), L-alanyl-L-isoleucine (AI), L-valyl-L-valine (VV), L-isoleucyl-L-valine (IV) and L-isoleucyl-L-alanine (IA), whose hexagonal crystal structures (space group P61) exhibit one-dimensional channels.5 The family of volatile halogenated ethers and alkanes was chosen as probing guests, given the high vapour pressure already at 273 K. Pores with tailored cross-sections from 5.3 Å to 3.5 Å, as modulated by aliphatic groups lining the inner surface of the walls, offer a great versatility for studying vapour absorptive properties, especially, selectivity and capacity towards these guests. Indeed, pore size plays a key role in the selective recognition of the anesthetics: dipeptide crystals with channels larger than 4 Å such as VA, AI and VV efficiently absorb the guests whilst IA and IV with pore sizes of about 3.5 Å exclude the anesthetics.
The vapor isotherms are reported in Fig. 2. The adsorption in VA channels (d = 5.1–5.3 Å, as calculated by an exploring sphere of 2 Å radius)11 exhibited Langmuir-type profiles for all the halogenated guests showing maximum absorption values of 170–200 mmol mol−1 at 273 K and 80–100 Torr, which correspond to a virtually complete loading of available volume and more than 20% by weight. The unit cell parameters of the samples upon loading remain substantially unaltered, indicating the zeolitic behavior of the molecular crystalline material. Langmuir-type isotherms for enflurane are also shown in the smaller channels of AI (4.7–5.0 Å) and VV (4.0–4.2 Å) owing to the limited steric requirements of the chlorine on the terminal methyl group, which allows easy adjustment in the channels. On the contrary, the isotherms of the isoflurane isomer bearing the chlorine substituent on the methylene group and halothane (containing F, Cl and Br halogens) in VV do not follow a Langmuir profile, this different behavior is particularly accentuated in the case of halothane. In fact, in the channels of VV, halothane isotherms exhibited a minimal adsorption at low pressures followed by an increased uptake above 10 Torr. Thus, at low pressures a selective absorption of enflurane with respect to halothane is exhibited. This behavior is fully reproducible over a few cycles and can be attributed to the flexibility of the structure that expanded upon crossing a pressure threshold. On the contrary, in IA and IV nanochannels with cross-sections of about 3.5 Å the halogenated molecules are excluded for their exceeding steric encumbrance. Interestingly, dipeptides containing the same aminoacids, alanine and isoleucine, in opposite sequences, AI and IA, generate different cross-sections (from 4.7–5.0 Å to 3.5 Å, respectively), that can differentiate the adsorption properties of the same guest (Fig. 2). Desflurane, bearing exclusively fluorine atoms as halogens and exhibiting the highest volatility (b.p. = 23 °C), shows in all dipeptides modest absorption values at 273 K whilst diethylether is absorbed with high efficiency in the hydrocarbon environment (ESI†). Desorption branches show complete reversibility of the isotherms and no hysteresis, indicating possible reuse of dipeptides without regeneration (ESI†).
From adsorption isotherms at various temperature, we evaluated the isosteric heats of adsorption (see ESI†) by applying the Clausius–Clapeyron equation. We found out values in the range of 35–50 kJ mol−1, which demonstrate the strong affinity between the dipeptide hosts and the guests owing to multiple interactions installed within the narrow channels. These adsorption energies are comparable to the highest values reported for adsorbed anesthetics in porous materials.10c
Fast magic angle spinning 1H NMR spectra provided direct evidence of the inclusion of the anesthetics in the crystalline channels of VA, AI and VV upon sorption from the gas phase (Fig. 3) (see ESI†). An interesting feature is the upfield shift (δ = −0.2 ppm) of included halogenated ethers with respect to the pure compounds, owing to the magnetic susceptibility generated by the dipeptide environment.12 Specifically, a sample with an excess of enflurane in VA allowed us to distinguish the signals of free and included enflurane. The signals of the included halogenated molecules have a linewidth wider than that of free molecules owing to stronger 1H homonuclear coupling, indicating that guest molecules undergo restricted motion. Quantitative analysis of the amount of included anesthetics with respect to the dipeptide hosts allowed us to measure the maximum loading of the channels. Enflurane, isoflurane and halothane in VA were found to be 0.19, 0.16 and 0.19 mol mol−1, respectively, in agreement with the amount measured from adsorption isotherms at 273 K (0.18, 0.16 and 0.19 mol mol−1, respectively).
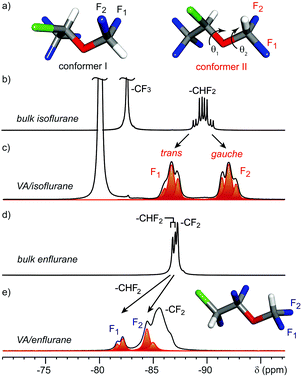
However, the conformational arrangement of anesthetics in confined spaces has never been investigated. 19F MAS NMR spectra of isoflurane in the all the dipeptides show a dramatic change with respect to the spectra in the liquid state (Fig. 4). Indeed, the 19F NMR spectra of isoflurane confined in VA showed two distinct triplets separated by about 5.3 ppm for the two fluorine nuclei of the CHF2 group (Fig. 4c), in contrast to a single multiplet recorded for pure isoflurane in the bulk wherein fast exchange among conformers occurs (Fig. 4b). Such large separation must be ascribed to the conformational arrangement of the two fluorine atoms and the γ-gauche effect that produces an upfield shift of 5 ppm when the observed nucleus changes from a trans to a gauche conformation.13
A theoretical study allowed us to establish the most stable conformers of isoflurane. We explored the dihedral angles θ1 and θ2 (C–C–O–C and F1–C–O–C, respectively) and observed the stability of two conformational minima in the energy map with values as close as Δ = 0.5 kcal mol−1 (Fig. 4a). In conformer I, the F–C–O–C dihedral angles for F1 and F2 are −63° and 58° (i.e. both in gauche conformation); on the contrary in conformer II the F1–C–O–C and F2–C–O–C dihedral angles are 177° and −62°, respectively (i.e. F1 in trans and F2 in gauche conformations).14 The experimental spectrum clearly indicates that the conformer II (with the torsional angle θ2 setting F1 in trans and F2 in gauche conformation) provides the only feasible arrangement of isoflurane confined in the narrow dipeptide channels: in fact, the behavior of included isoflurane is common to all the observed dipeptide crystals, such as AI and VV (Fig. S25, ESI†). Indeed, the lateral steric encumbrance is reduced in conformer II because at least one of the two fluorine atoms is set in the more elongated trans arrangement, whereas in conformer I both fluorines protrude laterally in gauche arrangement. Conformer II also displayed a θ1 torsional angle close to a trans arrangement, setting the main chain in the most elongated conformation. 13C CP MAS spectra decoupled from 1H or 19F (see Fig. S26 and S28, ESI†) are in agreement with the stretched conformation preferred for the steric requirements imposed by the crystalline channels. In the case of enflurane, a different behavior is observed for the CHF2 group. Actually, the two fluorine nuclei resonate downfield with respect to those of the structural isomer isoflurane (Fig. 4e). This is due to the conformation of the two fluorine atoms, which are set at F–C–O–C dihedral angles of −105° and 135° (ESI†) and thus do not undergo γ-gauche effect when enflurane is included in the VA channels. The GCMC simulations of enflurane and isoflurane isotherms in VA show that the energetically stable conformers occupy the channels with a maximum loading corresponding to that of the experimental isotherms and the guests are not periodically distributed along the channels.
Dynamic light scattering analysis of a suspension of crystals proved that their particle size falls in the nanometric range with a distribution centered at about 30 nm. This evidence enforces the perspectives for the use of dipeptides in nanomedicine, because this particle size is suitable for blood stream transport and cell internalization.
In conclusion, dipeptide crystals, with their ultra-micropores, offer suitable room for hosting anesthetic molecules and tuning their volatility. The diversity in the size and shape of the space available for the guests in the numerous dipeptide structures allowed us to select the most appropriate dipeptide crystals for specific guests and show the remarkable selectivity. The guests included in the dipeptide crystals were detected by 13C, 19F and fast-1H MAS NMR spectroscopy, which provided a detailed analysis of the arrangement of the guest molecules in the cavities and the amount of loaded anesthetics, in agreement with the adsorption isotherms. This investigation may shed light on the way volatile anesthetics adapt to variable-size subnanometric channels, mimicking biological receptors. Moreover, the biodegradability and biocompatibility of nanoporous dipeptide materials encourages their use in biomedical applications.
Cariplo Foundation 2016, PRIN 2015-CTEBBA and INSTM Consortium IN-RL14-2016 are acknowledged for financial support. The authors would like to thank D. Piga for helpful discussion.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Kitagawa, Angew. Chem., Int. Ed., 2015, 54, 10686 CrossRef CAS PubMed.
- D. W. Breck, Zeolite Molecular Sieves. Structure, Chemistry and Use, Wiley, New York, 1974, p. 593 Search PubMed.
- (a) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (b) S. Das, P. Heasman, T. Ben and S. Qiu, Chem. Rev., 2017, 117, 1515 CrossRef CAS PubMed; (c) Z. Zhang and M. J. Zaworotko, Chem. Soc. Rev., 2014, 43, 5444 RSC; (d) N. Huang, P. Wang and D. Jiang, Nat. Rev. Mater., 2016, 1, 1 Search PubMed; (e) P. J. Waller, F. Gandara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053 CrossRef CAS PubMed.
- (a) H. Wang, B. Li, H. Wu, T.-L. Hu, Z. Yao, W. Zhou, S. Xiang and B. Chen, J. Am. Chem. Soc., 2015, 137, 9963 CrossRef CAS PubMed; (b) S. A. Dalrymple and G. K. H. Shimizu, J. Am. Chem. Soc., 2007, 129, 12114 CrossRef CAS PubMed; (c) N. B. Mckewon, J. Mater. Chem., 2010, 20, 10588 RSC; (d) P. Sozzani, S. Bracco, A. Comotti, L. Ferretti and R. Simonutti, Angew. Chem., Int. Ed., 2005, 44, 1816 CrossRef CAS PubMed; (e) M. Baroncini, S. d’Agostino, G. Bergamini, P. Ceroni, A. Comotti, P. Sozzani, I. Bassanetti, F. Grepioni, T. M. Hernandez, S. Silvi, M. Venturi and A. Credi, Nat. Chem., 2015, 7, 634 CrossRef CAS PubMed; (f) A. Comotti, S. Bracco, A. Yamamoto, M. Beretta, T. Hirukawa, N. Tohnai, M. Miyata and P. Sozzani, J. Am. Chem. Soc., 2014, 136, 618 CrossRef CAS PubMed; (g) A. Pulido, L. Chen, T. Kaczorowski, D. Holden, M. A. Little, S. Y. Chong, B. J. Slater, D. P. McMahon, B. Bonillo, C. J. Stackhouse, A. Stephenson, C. M. Kane, R. Clowes, T. Hasell, A. I. Cooper and G. M. Day, Nature, 2017, 543, 657 CrossRef CAS PubMed; (h) W. Yang, A. Greenaway, X. Lin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, P. Hubberstey, S. Kitagawa, N. R. Champness and M. Schröder, J. Am. Chem. Soc., 2010, 132, 14457 CrossRef CAS PubMed; (i) M. Mastalerz and I. M. Oppel, Angew. Chem., Int. Ed., 2012, 51, 5252 CrossRef CAS PubMed; (j) R. S. Patil, D. Banerjee, C. Zhang, P. K. Thallapally and J. L. Atwood, Angew. Chem., Int. Ed., 2016, 55, 4523 CrossRef CAS PubMed; (k) V. N. Yadav, A. Comotti, P. Sozzani, S. Bracco, T. Bonge-Hansen, M. Hennum and C.-H. Gorbitz, Angew. Chem., Int. Ed., 2015, 54, 15684 CrossRef PubMed; (l) Y. Liu, C. Hu, A. Comotti and M. D. Ward, Science, 2011, 333, 436 CrossRef CAS PubMed; (m) I. Bassanetti, F. Mezzadri, A. Comotti, P. Sozzani, M. Gennari, G. Calestani and L. Marchio, J. Am. Chem. Soc., 2012, 134, 9142 CrossRef CAS PubMed.
- (a) C. H. Gorbitz and E. Gundensen, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1996, 52, 1764 CrossRef; (b) C. H. Gorbitz, Chem. – Eur. J., 2007, 13, 1022 CrossRef PubMed; (c) A. Comotti, S. Bracco, G. Distefano and P. Sozzani, Chem. Commun., 2009, 284 RSC; (d) A. Comotti, A. Fraccarollo, S. Bracco, M. Beretta, G. Distefano, M. Cossi, L. Marchese, C. Riccardi and P. Sozzani, CrystEngComm, 2013, 15, 1503 RSC; (e) D. V. Soldatov, I. L. Moudrakoski and J. A. Ripmeester, J. Am. Chem. Soc., 2006, 128, 6737 CrossRef CAS PubMed; (f) R. V. Alfonso, J. Durão, A. Mendes, A. M. Damas and L. Gales, Angew. Chem., Int. Ed., 2010, 49, 3034 CrossRef PubMed; (g) G. Distefano, A. Comotti, S. Bracco, M. Beretta and P. Sozzani, Angew. Chem., Int. Ed., 2012, 51, 9258 CrossRef CAS PubMed; (h) S. Lim, H. Kim, N. Selvapalam, K.-J. Kim, S. J. Cho, G. Seo and K. Kim, Angew. Chem., Int. Ed., 2008, 47, 3352 CrossRef CAS PubMed.
- J. F. Hendrick, E. I. Eger, J. M. Sonner and S. L. Shafer, Anesth. Analg., 2008, 107, 494 Search PubMed.
- (a) P. S. Garcia, S. E. Kolesky and A. Jenkins, Curr. Neuropharmacol., 2010, 8, 2 CrossRef CAS PubMed; (b) P. S. Miller and A. Radu Aricescu, Nature, 2014, 512, 270 CrossRef CAS PubMed.
- (a) S. M. Ryan and N. J. Claus, Anesth. Analg., 2010, 111, 92 CrossRef PubMed; (b) S. Y. Jeffrey and J. White, Anesth. Prog., 2012, 59, 154 CrossRef PubMed; (c) J. Sherman, C. Le, V. Lamers and M. Eckelman, Anesth. Analg., 2012, 114, 108 CrossRef PubMed; (d) M. P. Sulbaek Andersen, S. P. Sander, O. J. Nielsen, D. S. Wagner, T. J. Sanford Jr and T. J. Wallington, Br. J Anaesth., 2010, 6, 760 CrossRef PubMed.
- (a) Y. Chen, C. Tang, J. Zhang, M. Gong, B. Su and F. Qiu, Int. J. Nanomed., 2015, 10, 847 Search PubMed; (b) M. Vallet-Regì, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548 CrossRef PubMed.
- (a) B. F. Abrahams, A. D. Dharma, P. S. Donnelly, T. A. Hudson, C. J. Kepert, R. Robson, P. D. Southon and K. F. White, Chem. – Eur. J., 2017, 23, 7871 CrossRef CAS PubMed; (b) T. H. Chen, W. Kaveevivitchai, A. J. Jacobson and O. S. Miljanic, Chem. Commun., 2015, 51, 14096 RSC; (c) N. Gargiulo, A. Peluso, P. Aprea, Y. Hua, D. Filipovi, D. Caputo and M. Eic, RSC Adv., 2014, 4, 49478 RSC.
- The average diameter (d) of the dipeptide channels was established by the exploration of a 2 Å sphere of available volume (V) and considering a cylinder of height equal to c unit cell parameter, according to the formula d = 2 (V/cπ)1/2.
- (a) S. Brown, Prog. Nucl. Magn. Reson. Spectrosc., 2007, 50, 199 CrossRef CAS; (b) S. Bracco, A. Comotti, P. Valsesia, M. Beretta and P. Sozzani, CrystEngComm, 2010, 12, 2318 RSC; (c) S. Bracco, A. Comotti, L. Ferretti and P. Sozzani, J. Am. Chem. Soc., 2011, 133, 8982 CrossRef CAS PubMed; (d) P. A. Williams, C. E. Hughes, J. Martin, E. Courvoisier, A. B. M. Buanz, S. Gaisford and K. D. M. Harris, J. Phys. Chem. C, 2016, 120, 9385 CrossRef CAS.
- D. G. Gorenstein, J. Am. Chem. Soc., 1977, 99, 2254 CrossRef CAS.
- (a) A. Lesarri, A. Vega-Toribio, R. D. Suenram, D. J. Brugh, D. Nori-Shargh, J. E. Boggsd and J.-U. Grabowe, Phys. Chem. Chem. Phys., 2011, 13, 6610 RSC; (b) A. Pfeiffer, H.-G. Mack and H. Oberhammer, J. Am. Chem. Soc., 1998, 120, 6384 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental part, PXRD, 13C, 1H and 19F MAS NMR spectroscopy, conformational analysis. See DOI: 10.1039/c7cc06534e |
| This journal is © The Royal Society of Chemistry 2018 |