A hand-held device for rapid single tube detection of hepatitis-C virus†
Abstract
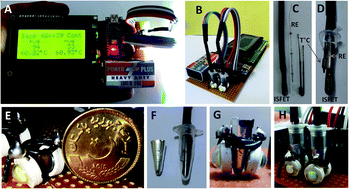
Hepatitis C virus (HCV) is a major threat to public health being the leading cause of chronic liver disease with 92 to 149 million people affected worldwide. A rapid and accurate diagnosis is a key to prevent viral transmission and the management of disease progression. Here we describe a hand-held device for rapid detection of HCV infection called as STALLION (Single Tube Analysis using LAMP, LED and ION-sensing). A custom detection probe was designed to dip into a PCR tube. The probe comprises an ion sensitive field effect transistor (ISFET), a micro-capillary based Ag/AgCl reference electrode and a temperature sensor. In vitro replication of HCV genomic RNA was performed by reverse transcription loop-mediated isothermal amplification (RT-LAMP) and detected in real-time by ISFET bio-sensing of released H+ ions. Incubation at 60 °C was provided by a novel low-cost concept based on heat dissipated from power LED(s). With this system, HCV positive samples with 101, 102, 103 copies per ml were detected in as less as 30, 18 and 13 minutes respectively compared to no-template control (NTC). The detection limit was comparable to those of available methods such as nested PCR. No significant false positive signal was observed for HCV negative samples. The results can be viewed on an accompanying LCD screen or alternatively can be transferred to a computer or smart phone. The STALLION system provides analysis in a conventional PCR tube format avoiding any complex fluidics or instrumentation requirements. Such a system will be particularly useful for rapid and reliable clinical diagnosis of HCV RNA by users with no prior expertise and provide the basis for point-of-care (POC) testing in limited resource settings especially for developing countries.
- This article is part of the themed collection: Coronavirus articles - free to access collection


 Please wait while we load your content...
Please wait while we load your content...