Separation and detection of mutans streptococci by using magnetic nanoparticles stabilized with a cell wall binding domain-conjugated polymer†
Panida
Thanyasrisung‡
*a,
Aemvika
Vittayaprasit‡
b,
Oranart
Matangkasombut
ac,
Motoyuki
Sugai
de,
Piyaporn
Na Nongkai
f,
Suttawan
Saipia
b and
Voravee P.
Hoven
 *gh
*gh
aDepartment of Microbiology and Research Unit on Oral Microbiology and Immunology, Faculty of Dentistry, Chulalongkorn University, Henri-Dunant Road, Pathumwan, Bangkok 10330, Thailand. E-mail: panida.t@g.chula.edu
bProgram in Petrochemistry and Polymer Science, Faculty of Science, Chulalongkorn University, Phayathai Road, Pathumwan, Bangkok 10330, Thailand
cLaboratory of Biotechnology, Chulabhorn Research Institute, Vibhavadi Rangsit Road, Laksi, Bangkok 10210, Thailand
dDepartment of Bacteriology, Hiroshima University Graduate School of Biomedical and Health Sciences, Hiroshima 734–8551, Japan
eAMR Research Center, National Institute of Infectious Diseases, Tokyo 189-0002, Japan
fDepartment of Chemistry, Faculty of Science, Burapha University, Chon Buri 20131, Thailand
gDepartment of Chemistry, Faculty of Science, Chulalongkorn University, Phayathai Road, Pathumwan, Bangkok 10330, Thailand. E-mail: vipavee.p@chula.ac.th
hCenter of Excellence in Materials and Bio-interfaces, Chulalongkorn University, Phayathai Road, Pathumwan, Bangkok 10330, Thailand
First published on 19th June 2018
Abstract
A number of salivary mutans streptococci (MS: Streptococcus mutans and Streptococcus sobrinus) are used in dental caries risk assessment. In this study, a simple, yet effective assay was developed for MS detection. Magnetic nanoparticles (MNPs) were first grafted with poly(acrylic acid) that bears active carboxyl groups available for conjugation with the cell wall binding domain (CWBD) of automutanolysin which specifically binds to MS. The binding efficiency of CWBD-conjugated MNPs to MS was tested with pure cultures of streptococcal standard strains. After mixing CWBD-conjugated MNPs with culture, bacteria-bound particles were separated from unbound cells using a magnet and filtered through a cellulose acetate membrane (pore-size 0.8 μm). The color intensity of particles remaining on the membrane represents the number of bound bacteria. The CWBD-conjugated MNPs showed higher efficiency in binding to S. mutans and S. sobrinus than to non-mutans streptococci (S. sanguinis and S. salivarius) with capture efficiencies of 77 and 69% for MS and 38 and 15% for non-MS. Moreover, this method can quantify the number of MS in the range of 102 to 107 colony-forming units (CFU) mL−1, which covers the range of MS levels used in caries risk assessment. The calculated limit of detection of the assay was 16 and 72 CFU mL−1 for S. mutans and S. sobrinus, respectively. Furthermore, the CWBD-conjugated MNPs could be used to efficiently quantify the number of MS in human saliva samples containing highly complex mixtures of bacterial species. These results suggest that the assay could be applicable as a simple tool for MS determination in not only clinical settings but also community fields without clinical expert requirement.
Introduction
Dental caries is one of the most prevalent infectious diseases in mankind. Although it is not life-threatening, it can cause pain and lead to severe infection.1 Moreover, the disease has impact on quality of life, especially in children.2,3 While the direction of caries management has been gradually shifting from treatment by restoration to prevention in recent decades, the prevalence of dental caries remains high, especially in underprivileged populations worldwide. This situation is caused by the failure of treatments and ineffective prevention strategies. Caries risk assessment is a tool to help solve the problem since it gives information on individual risk of caries development and the dominant etiological factors in each patient. This information allows dentists to select an individualized treatment and a prevention program for the patient.2,4The number of mutans streptococci (MS; S. mutans and S. sobrinus) in saliva is one of the factors determining the risk of caries development since there is much evidence showing association between the amount of MS and caries status.5 A culture-based method is still widely used to determine the level of salivary MS, and is considered the conventional method.6 However, the method requires laboratory skill, equipment, and also time for bacterial growth, so it is impractical in clinical settings and for community field studies. To overcome these limitations, a number of commercial chair-side kits have been developed.7,8 However, despite their ease of use and their equivalent efficiency to the conventional culture-based method, these kits still require an incubator and time to grow bacteria. Recently, a commercial S. mutans rapid-detection kit based on an antigen–antibody reaction has become available. Gao and colleagues reported that this kit could determine the number of S. mutans in saliva samples more accurately than the culture-based method.9 Nevertheless, classification of caries risk is still based on the salivary MS levels obtained from the culture-based method. A level of MS > 105 colony forming units (CFU) mL−1 is considered as high-risk for caries, whereas that below 104 CFU mL−1 is considered low-risk.10,11
Here, to develop a specific detection system for MS, we exploit the binding specificity of the enzyme automutanolysin, a peptidoglycan hydrolase produced by S. mutans. The enzyme selectively digests MS.12 The substrate specificity of automutanolysin towards MS is conferred by the cell wall binding domain (CWBD) of the enzyme (unpublished observation). Thus, the CWBD could be a good probe for MS detection.
Magnetic separation in combination with size-selective membrane filtration was recently introduced as a rapid, versatile, simple, low-cost and effective method for bacterial separation/detection.13,14 Magnetic nanoparticles (MNPs) usually appear in a magnetite form (Fe3O4) which responds to a magnetic field.15 Moreover, their small sizes (5 to 500 nm diameter) result in a large surface to volume ratio available for conjugation of probes (mostly antibodies) specific for bacteria, which enhances the bacterial capture efficiency.16–19 Based on these properties of MNPs and the binding specificity of the probe, target bacteria can be isolated from contaminants using an external magnet.13,20,21 After magnetic separation, the targeted bacterial suspension is filtered through a membrane with a specific pore size. If the MNPs conjugated with the probe capture target bacteria, their size increases, so they are retained on the membrane.14,22 Since MNPs are brown, the bacteria-bound MNPs remaining on the membrane can easily be detected by the naked eye.
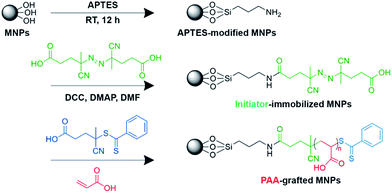
A number of polymers such as dextran,23 polystyrene (PS),24,25 and poly(acrylic acid) (PAA)26,27 have been coated onto MNPs to increase their stability and dispersibility. A polymeric stabilizer can increase the affinity of MNPs for a specific solvent and inhibit particle aggregation.28–31 Polymer-grafted MNP nanocomposites form very stable dispersions in organic solutions.32–34 Among the most effective methods for preparing polymer-coated MNPs is surface functionalization by grafting of polymer, particularly via the “grafting from” approach or surface-initiated polymerization. This strategy involves the formation of a covalently bound polymeric layer growing from a surface-attached initiator. This method provides high grafting density with controlled polymer molecular weight and the thickness of the coated polymer layer if a controlled polymerization process (i.e. atom transfer radical polymerization (ATRP), reversible addition fragmentation chain transfer (RAFT) polymerization) is employed.32,35–37
Based on these previous studies, this research aimed to develop CWBD-conjugated MNPs as a simple tool for MS detection based on magnetic separation and size-selective membrane filtration. MNPs were grafted with PAA via surface-initiated RAFT polymerization to yield PAA-grafted MNPs. The CWBD was then conjugated to the carboxyl groups of PAA via 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS) coupling chemistry. The CWBD-conjugated MNPs specifically bound to the target bacteria and then an external magnetic field was applied to separate and concentrate the MNPs-bound captured bacteria from contaminants. The bacteria-bound CWBD-conjugated MNPs were subsequently filtered through a cellulose acetate membrane. With an appropriate pore size of the membrane, the bacteria-bound CWBD-conjugated MNPs remained on the membrane and could be detected by the naked eye.
Experimental
Materials
Iron(III) chloride hexahydrate (FeCl3·6H2O) was purchased from Merck (Germany). MNPs were prepared via a solvothermal method following a published procedure.38 3-Aminopropyltriethoxysilane (APTES), 4,4-azobis(4-cyanovaleric acid) (ACVA), 4-cyano-4-(phenylcarbonothio) pentanoic acid, 4-(dimethylamino)pyridine (DMAP), N,N′-dicyclohexylcarbodiimide (DCC), dimethylformamide (DMF), EDC, NHS, acrylic acid (AA), sodium acetate, and ethylene glycol were purchased from Sigma-Aldrich (USA). Phosphate buffered saline (PBS, pH 7.4) and a cellulose acetate membrane (CA membrane with a 0.2, 0.45, 0.8 and 1.2 μm pore sizes) were purchased from Merck Millipore (USA). All culture media except Mitis Salivarius Bacitracin (MSB) agar were purchased from HiMedia (India). Mitis salivarius agar and bacitracin were bought from Becton, Dickinson and Company (USA) and Sigma-Aldrich respectively. Materials for protein expression and purification, isopropyl-β-D-thiogalactoside (IPTG), HIS-Select® Nickel Affinity Gel, imidazole, dialysis bags (MWCO 10 kDa) and the Bradford Protein Assay reagent were bought from Calbiochem (India), Sigma-Aldrich, Affymetrix (USA), Thermo Scientific (USA) and Bio-Rad (USA), respectively. All reagents and materials were of analytical grade and used without further purification. Ultrapure distilled water was obtained using a Millipore Milli-Q system (USA).Characterization of MNPs
The crystal structure of MNPs was characterized by X-ray diffraction (XRD). The XRD pattern was obtained using a Rigaku RINT-2000 diffractometer (Japan) using CuKα radiation (λ = 1.5418 Å). Infrared spectra of surface-modified MNPs prepared as KBr disks were obtained using a Fourier Transform-Infrared (FTIR) spectrometer (Nicolet, USA), model Impact 410, with 32 scans at resolution 4 cm−1, in the frequency range 400–4000 cm−1, using a TGS detector. The morphology of MNPs before and after modification was examined by transmission electron microscopy (TEM) using a Philips Tecnai 12, FM 208 instrument (USA) at 100 kV acceleration. Negative staining was performed by dropping 1% (w/v) aqueous solution of phosphotungstic acid on the MNPs deposited on the carbon-coated copper grid. The excess solution was blot-dried using filter paper. The sample was then air-dried in the dark overnight before being subjected to TEM analysis. Thermogravimetric analysis (TGA) was performed on a SDTA 851 (Mettler-Toledo, USA) in the range of 32–900 °C at a heating rate of 20 °C min−1 under nitrogen. The size and zeta potential of the MNPs were measured by dynamic light scattering using a Malvern Nano ZS90 instrument (UK) at room temperature at a scattering angle of 90°.Preparation of the CWBD of automutanolysin
Escherichia coli BL-21 carrying a recombinant CWBD-expressing plasmid were grown in Luria–Bertani broth at 37 °C until an OD600 nm of 0.4–0.6 was reached. The expression of 6× His-tagged CWBD protein was induced by addition of 1 mM IPTG and incubation of cells at 15 °C for 24 h. The proteins were then purified using a HIS-Select® Nickel Affinity Gel in native conditions. The active fractions were pooled and dialyzed against 0.1 M phosphate buffer, pH 6.8. The protein concentration was measured by the Bradford protein assay. Sequences of the CWBD together with schematic plasmid map, including the CWBD and the expressed CWBD protein on SDS-PAGE (Fig. S1) are shown in the ESI†.Preparation of PAA-grafted MNPs
PAA was grafted onto the surface of MNPs via a “grafting from” method. MNPs were first immobilized with APTES. Briefly, 0.1 g of MNPs was dispersed ultrasonically in 100 mL of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ethanol–water mixture with 1 mL of added ammonia solution (38%) for 30 min. To the above dispersed solution, 1 mL of APTES was added dropwise, and the resulting mixture was stirred at room temperature for 12 h. Thereafter, the APTES-modified MNPs obtained were magnetically concentrated, washed thoroughly with ethanol, and vacuum dried. The APTES-modified MNPs were immersed in 20 mL DMF containing ACVA (37.5 mmol), DCC (47.0 mmol) and DMAP (3.74 mmol) at room temperature for 20 h under a nitrogen atmosphere. After the reaction was complete, the product was rinsed thoroughly with DMF and ethanol four times each. The obtained initiator-immobilized MNPs were then placed in a vial containing ACVA (0.5 mmol), CTA (5 mmol), and AA (0.8 mol) in 10 mL of Milli-Q water. The vial was then placed in a preheated oil bath at 70 °C for 20 h under a nitrogen atmosphere. The resulting PAA-grafted MNPs were removed from the vial and washed with ethanol and Milli-Q water, respectively. The synthetic scheme for the preparation of PAA-grafted MNPs via surface-initiated RAFT polymerization is shown in Scheme 1.
1 (v/v) ethanol–water mixture with 1 mL of added ammonia solution (38%) for 30 min. To the above dispersed solution, 1 mL of APTES was added dropwise, and the resulting mixture was stirred at room temperature for 12 h. Thereafter, the APTES-modified MNPs obtained were magnetically concentrated, washed thoroughly with ethanol, and vacuum dried. The APTES-modified MNPs were immersed in 20 mL DMF containing ACVA (37.5 mmol), DCC (47.0 mmol) and DMAP (3.74 mmol) at room temperature for 20 h under a nitrogen atmosphere. After the reaction was complete, the product was rinsed thoroughly with DMF and ethanol four times each. The obtained initiator-immobilized MNPs were then placed in a vial containing ACVA (0.5 mmol), CTA (5 mmol), and AA (0.8 mol) in 10 mL of Milli-Q water. The vial was then placed in a preheated oil bath at 70 °C for 20 h under a nitrogen atmosphere. The resulting PAA-grafted MNPs were removed from the vial and washed with ethanol and Milli-Q water, respectively. The synthetic scheme for the preparation of PAA-grafted MNPs via surface-initiated RAFT polymerization is shown in Scheme 1.
Immobilization of the CWBD on PAA-grafted MNPs
PAA-grafted MNPs were conjugated with the CWBD of automutanolysin via the EDC/NHS coupling method, as follows. PAA-grafted MNPs (5 mg) were dispersed in 1 mL 0.01 M PBS, pH 7.4. To this, 0.1 mL of EDC (2 M) and 0.1 mL of NHS (0.5 M) were added, and then the mixture was incubated for 30 min at room temperature at 240 rpm on a rotary shaker. The CWBD (0.1 mL, 1 mg mL−1) was added to the mixture and was incubated for 24 h at 4 °C. Finally, the CWBD-conjugated MNPs were isolated using a magnet and rinsed with 0.01 M PBS, pH 7.4 and Milli-Q water, respectively. The product was stored at 4 °C until use.Bacterial preparation
S. mutans UA159, S. sobrinus OMZ176, S. sanguinis ATCC10556 and S. salivarius DMST18781 were cultured in a brain heart infusion broth at 37 °C in a 5% CO2 atmosphere by shaking at 180 rpm for 24 h. The optical density of 24 h culture was measured at 600 nm (OD600nm). The cultures were then diluted to the OD600nm = 0.1 and incubated for 2.5 h to obtain an OD600nm of 0.5 (∼1010 CFU mL−1). After that, the bacterial cultures were 10-fold serially diluted from 107 to 102 CFU mL−1 with 0.01 M PBS, pH 7.4. Colony counting was performed to verify the number of bacteria.Determination of bacteria captured by CWBD-conjugated MNPs
The efficiency of CWBD-conjugated MNPs in capturing S. mutans UA159, S. sobrinus OMZ176, S. sanguinis ATCC10556, and S. salivarius DMST18781 was determined. Briefly, 0.1 mL bacterial suspensions in the range 102 to 107 CFU mL−1 were transferred to a 1.5 mL tube containing 0.2 mL of CWBD-conjugated MNP suspension (0.15 mg mL−1 in 0.01 M PBS, pH 7.4) and then incubated for 30 min at 4 °C. After incubation, the tube was placed on a magnet for 10 min to isolate and concentrate the bacteria-bound CWBD-conjugated MNPs. The supernatant was removed and bacteria-bound CWBD-conjugated MNPs were resuspended in 0.1 mL of 0.01 M PBS, pH 7.4. A piece of filter paper (Whatman #1, i.d. = 125 mm) was screen-printed with PS in a pattern that leaves circular areas of 5 mm diameter uncovered with PS, using a previously published method.39 For selective filtration, a CA membrane with selected pore size (0.2, 0.45, 0.8, or 1.2 μm) was placed on top of the PS-screen-printed paper in a Büchner funnel equipped with an Erlenmeyer flask and an aspirator. The membrane was wetted with 0.01 M PBS, pH 7.4. Fifty microliters of the bacteria-bound CWBD-conjugated MNP suspension was then pipetted onto the membrane in a position that exactly matched the circular area of the PS-screen-printed paper underneath under vacuum. The overall concept for bacterial detection is illustrated in Scheme 2.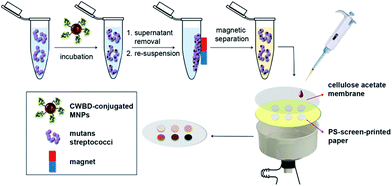 | ||
| Scheme 2 Schematic representation of the stepwise procedure for colorimetric detection of mutans streptococci by CWBD-conjugated MNPs based on magnetic separation and selective filtration. | ||
The color intensity on the membrane, which reflected the amount of bacteria in the sample, was quantified and averaged from three independent samples using scanned images recorded on an Epson Perfection V33 scanner in 24 bit Professional Mode. The brightness/contrast/resolution was set to 128/128/300. The images were saved as TIFF files. The intensity of each spot was determined using Scion image software by first converting to grayscale at 300 dpi. Intensity measurements were carried out using the line tool to select an area for analysis to obtain profile images. The amount of bacteria was determined from a standard curve generated from the intensity as a function of known bacterial concentration.
Study population and saliva collection
The study protocol was approved by the Ethics Committee at the Faculty of Dentistry, Chulalongkorn University (HREC-DCU-P 2016-013). Sixteen healthy adult volunteers were recruited from among dental students at the Faculty of Dentistry, Chulalongkorn University and workers at a private company. Saliva samples were collected following the procedures of Wennerholm and Emilson with some modifications.40 Briefly, all volunteers were informed not to brush their teeth and to refrain from having breakfast before collection of the saliva. Stimulated saliva was collected while chewing a piece of paraffin film for 10 min. The collected saliva was immediately placed on ice, delivered to the laboratory directly, kept at 4 °C, and used the same day.Determination of the number of salivary mutans streptococci
Results and discussion
Preparation and characterization of PAA-grafted MNPs and CWBD-conjugated MNPs
MNPs were synthesized by a solvothermal method. As displayed in Fig. S2 (ESI†), an XRD pattern of the MNPs showed diffraction peaks at 2θ = 30.12, 35.48, 43.12, 57.02 and 62.62°, which can be assigned to (311), (440), (220), (511), and (400) planes of Fe3O4, respectively. This characteristic coincides with that of standard magnetite (JCPDS card no. 19-0629). No other diffraction peaks corresponding to other iron oxides, such as α-Fe2O3 and γ-Fe2O3, could be detected, suggesting that the MNPs obtained are in the form of pure Fe3O4.PAA-grafted MNPs were prepared by surface-initiated RAFT polymerization. The success of stepwise surface modification of MNPs was confirmed by FTIR as shown in Fig. S3 (ESI†). A characteristic Fe–O vibration of the unmodified MNPs (Fig. S3a†) was observed at 584 cm−1. The spectrum of PAA-grafted MNPs (Fig. S3b†) shows a band at 1740 cm−1, which is assignable to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of carboxyl groups of PAA. This signal disappeared upon CWBD conjugation (Fig. S3c†), indicating that an amide linkage was formed between the carboxyl group of PAA and the amino group of the enzyme.
O stretching of carboxyl groups of PAA. This signal disappeared upon CWBD conjugation (Fig. S3c†), indicating that an amide linkage was formed between the carboxyl group of PAA and the amino group of the enzyme.
Fig. 1 illustrates the morphology of the MNPs before and after modification as examined by TEM. The unmodified MNPs (Fig. 1a) were spherical with an average diameter of 328.2 ± 22.7 nm. The average diameter of the MNPs increased upon surface modification, to 357.4 ± 19.2 and 745.5 ± 29.9 nm for PAA-grafted MNPs (Fig. 1b) and CWBD-conjugated MNPs (Fig. 1c), respectively. A higher degree of aggregation was also realized in the case of CWBD-conjugated MNPs. This may be ascribed to the conjugation with a relatively large molecule of the CWBD (MW = 80 kDa). Negative-stained TEM images of both PAA-grafted MNPs and CWBD-conjugated MNPs demonstrating the organic shell layer of grafted PAA and the conjugated CWBD, respectively, are shown in Fig. 1e–f.
The particle size and zeta potential of the MNPs were also evaluated by DLS Table S1 (ESI†). In good agreement with the TEM data, the average size of the MNPs increased after PAA grafting and again after CWBD conjugation. The pKa of PAA is about 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 and the isoelectric point of the CWBD determined by calculation is approximately 5.17, which should result in both PAA and the CWBD being negatively charged in PBS solution at pH 7.4. For this reason, the PAA-grafted MNPs exhibited a negative zeta potential (−31.43 mV), verifying the presence of ionizable carboxyl groups of PAA surrounding the MNPs. After CWBD conjugation, the zeta potential of the MNPs became less negative (−14.40 mV), which was due to the CWBD being less negatively charged than PAA. This set of data also indicated the successful immobilization of the CWBD on the PAA-grafted MNPs.
41 and the isoelectric point of the CWBD determined by calculation is approximately 5.17, which should result in both PAA and the CWBD being negatively charged in PBS solution at pH 7.4. For this reason, the PAA-grafted MNPs exhibited a negative zeta potential (−31.43 mV), verifying the presence of ionizable carboxyl groups of PAA surrounding the MNPs. After CWBD conjugation, the zeta potential of the MNPs became less negative (−14.40 mV), which was due to the CWBD being less negatively charged than PAA. This set of data also indicated the successful immobilization of the CWBD on the PAA-grafted MNPs.
The presence of organic components of PAA and the CWBD on the PAA-grafted MNPs and CWBD-conjugated MNPs, respectively, was further confirmed by TGA analysis. As shown in Fig. S4 (ESI†), the first weight loss of all MNPs took place below 200 °C, which should originate from evaporation of water. CWBD-conjugated MNPs showed the highest weight loss (about 3.44%), implying that they contained the greatest bound water content. The second weight loss, occurring in the temperature range 300–600 °C, could be ascribed to the decomposition of organic content bonded to MNPs. The greater weight loss of 9.1 and 9.8% from PAA-grafted MNPs and CWBD-conjugated MNPs, respectively, compared with 5.5% from MNPs, confirmed the presence of PAA and the CWBD on the modified MNP forms. The third weight loss, appearing at the highest temperatures (>730 °C) in both PAA-grafted MNPs and CWBD-conjugated MNPs, is assigned to char of macromolecular organic content (PAA and CWBD) on the MNPs, because such weight loss was absent in the unmodified MNPs curve. As determined by the Bicinchoninic acid protein assay, the quantity of immobilized CWBD was found to be 6.66 mg g−1 for PAA-grafted MNPs. The calculated immobilization efficiency was 32.8%.
Selection of the filter membrane suitable for the selective filtration assay
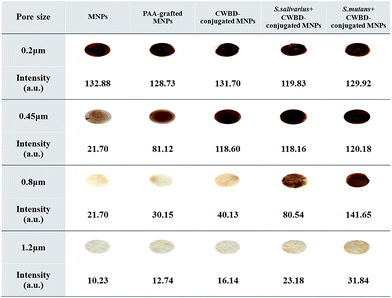
To determine an appropriate pore size of the filter membrane that would selectively retain bacteria-bound CWBD-conjugated MNPs, CA membranes with pore sizes in the range 0.2–1.2 μm were tested. The CWBD-conjugated MNPs would become larger in size once bound with bacteria and therefore retain on the membrane upon filtration if their size is larger than the pore diameter of the membrane. Since MNPs are brown, the bacteria-bound MNPs would appear as a brown spot on the membrane. The higher bacterial concentration leads to a higher level of MNPs aggregation and thus yielded the brown spot with greater intensity. As Fig. 2 shows, even the unmodified MNPs (the smallest particles) could not go through the 0.2 μm pore-size filter membrane. This outcome corresponded to the results of TEM and DLS analyses, which indicated that the approximate diameter of the unmodified MNPs was 0.32 μm. When membranes with pore-size 0.45 μm were used, they could not separate bacteria-bound CWBD-conjugated MNPs from the unbound, CWBD-conjugated MNPs. This finding was consistent with TEM and DLS analyses showing that the diameter of CWBD-conjugated MNPs was approximately 0.75 ± 0.03 and 0.63 ± 0.02 μm, respectively. The 0.8 μm pore-size filter membrane worked effectively; a difference in brown color intensity was apparent between bacteria-bound and unbound CWBD-conjugated MNPs. Moreover, higher color intensity of retained CWBD-conjugated MNPs when bound to S. mutans (MS bacteria) implied greater binding efficiency of the CWBD toward S. mutans compared with S. salivarius (non-MS bacteria). All tested particles could pass through the 1.2 μm pore-size filter membrane. Based on these findings, the 0.8 μm pore-size filter membrane was selected for further investigation.Determination of bacterial binding efficiency of CWBD-conjugated MNPs
Bacterial binding efficiency of the CWBD-conjugated MNPs was determined with S. mutans and S. sobrinus (MS bacteria) in the range 102 to 107 CFU mL−1. Fig. 3 shows the intensity of the brown spots produced by bacteria-bound CWBD-conjugated MNPs on 0.8 μm pore-size filter membranes. The intensity was converted to numeric values to generate a standard curve. The intensity of the colored spots increased linearly as a function of log(CFU mL−1) for both bacteria (Fig. 3a and b). The limit of detection (LOD) is the lowest detectable bacterial concentration, which is calculated using eqn (3) (ESI†). The background intensity (0 CFU mL−1) was 23.82 ± 5.13, which resulted in LOD values of 16 and 72 CFU mL−1 for S. mutans and S. sobrinus, respectively. | ||
| Fig. 3 Linear relationship between log(CFU mL−1) and signal intensity (a.u.) for (a) S. mutans and (b) S. sobrinus. | ||
Determination of bacteria capturing efficiency of CWBD-conjugated MNPs for various streptococcal species
Capture efficiencies of MS bacteria (S. mutans and S. sobrinus) and non-MS bacteria (S. sanguinis and S. salivarius) by the CWBD-conjugated MNPs were determined. First, a colony counting assay was performed before (Cbefore) and after (Cafter) binding of bacteria to the CWBD-conjugated MNPs. As shown in Fig. S5 (ESI†), the number of S. mutans and S. sobrinus before binding to the CWBD-conjugated MNPs was approximately 105 CFU mL−1, whereas after binding the numbers decreased to 133 and 147 CFU mL−1, respectively. In contrast, the numbers of S. sanguinis and S. salivarius after binding to the CWBD-conjugated MNPs were only slightly decreased from the values before binding. The capture efficiency (CE) was calculated using eqn (4) (ESI†). The capture efficiency of the CWBD-conjugated MNPs for S. mutans and S. sobrinus was 77 and 69%, respectively, whereas that for S. sanguinis and S. salivarius was 38 and 15%, respectively. These values indicated the specificity of the CWBD-conjugated MNPs for MS bacteria (S. mutans and S. sobrinus), rather than non-MS bacteria (S. sanguinis and S. salivarius). This specificity towards MS bacteria was further confirmed by the selective filtration assay. As shown in Fig. 4, the signal intensities obtained from the interaction with non-MS species (S. sanguinis and S. salivarius) were much lower than those detected with the MS species (S. mutans and S. sobrinus). | ||
| Fig. 4 Determination of binding specificity of the CWBD-conjugated MNPs to MS (S. mutans and S. sobrinus) and non-MS (S. sanguinis and S. salivarius) species. | ||
Comparative investigation of bacterial detection by the magnetic separation/selective filtration method using CWBD-conjugated MNPs and the culture method in saliva samples
To develop the CWBD-conjugated MNPs for clinical application, we demonstrated that CWBD-conjugated MNPs can detect MS in human saliva, which contains a complex mixture of many bacterial species. Saliva samples were collected and then incubated with CWBD-conjugated MNPs. The number of MS was determined by comparing the detection intensity to the standard curve for S. mutans. The culture-based determination method was performed in parallel by inoculating the same saliva samples on MSB agar to determine the number of MS. As Table 1 shows, the number of MS determined by the CWBD-conjugated MNPs method was similar to that obtained by the culture-based method. This similarity is important and useful, since the level of caries risk is classified based on the saliva MS level obtained from the culture-based method. Together, our results indicated that the CWBD-conjugated MNPs have potential to be a simple colorimetric method to quantify the number of salivary mutans streptococci.| Number of colonies (CFU mL−1) | ||
|---|---|---|
| Saliva sample | Magnetic separation/selective filtration method | Culture-based method |
| a N/A = out of range of the standard curve (102 to 107 CFU mL−1). | ||
| 1 | 7.2 × 104 | 3.7 × 104 |
| 2 | 1.3 × 104 | 1.6 × 104 |
| 3 | 4.8 × 102 | 5.2 × 103 |
| 4 | N/A | <102 |
| 5 | 5.7 × 102 | <102 |
| 6 | N/A | <102 |
| 7 | N/A | <102 |
| 8 | N/A | <102 |
| 9 | 1.5 × 104 | 3.3 × 103 |
| 10 | 3.6 × 102 | 2.9 × 102 |
| 11 | 7.4 × 103 | 1.3 × 103 |
| 12 | N/A | <102 |
| 13 | N/A | <102 |
| 14 | N/A | <102 |
| 15 | N/A | <102 |
| 16 | N/A | <102 |
Conclusions
The present study demonstrated that PAA-grafted MNPs could be prepared by surface-initiated RAFT polymerization. They were successfully conjugated to the CWBD of automutanolysin by EDC/NHS coupling chemistry. The properties of CWBD-conjugated MNPs were characterized by XRD, FTIR, TEM, TGA and DLS. By combining bacterial binding to CWBD-conjugated MNPs with magnetic separation and selective filtration, we developed a colorimetric assay for quantitative MS detection in both pure cultures and human saliva samples. Most importantly, the assay could differentiate levels of salivary MS relevant for dental caries risk assessment.Without requiring for bacteria culturing or clinical experts, this effective assay is a promising tool for MS detection that can be easily implemented in not only clinical settings but also community fields. The simplicity of the assay would make dental caries risk assessment more accessible and efficient. The success of this work also suggests that the magnetic-based immunoassay may be applicable for detection of other bacteria in saliva, another biologically relevant matrix that has not been much explored.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Financial support for this work was provided by the Thailand Research Fund (RSA5980071 and DBG5580003 granted to VPH and TRG5780043 granted to PT) and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (to R. U., on Oral Microbiology and Immunology).References
- Z. Ling and H. Tao, in Dental Caries: Principles and Management, ed. Z. Xuedong, Springer, Heidelberg, Berlin, 2016, pp. 129–155 Search PubMed.
- M. Bönecker, J. Abanto, G. Tello and L. B. Oliveira, Braz. Oral. Res., 2012, 26, 103–107 CrossRef.
- A. Sheiham, Br. Dent. J., 2006, 201, 625–626 CrossRef PubMed.
- M. Hurlbutt and D. A. Young, J. Evid. Based Dent. Pract., 2012, 14, 77–86 CrossRef PubMed.
- E. Phattarataratip, B. Olson, B. Broffitt, F. Qian, K. A. Brogden, D. R. Drake, S. M. Levy and J. A. Banas, Mol. Oral Microbiol., 2011, 26, 187–199 CrossRef PubMed.
- E. S. Davenport, S. Day, J. M. Hardie and J. M. Smith, Community Dent. Health, 1992, 9, 261–271 Search PubMed.
- H. V. Jordan, R. Laraway, R. Snirch and M. Marmel, J. Dent. Res., 1987, 66, 57–61 CrossRef PubMed.
- B. Jensen and D. Bratthall, J. Dent. Res., 1989, 68, 468–471 CrossRef PubMed.
- X.-L. Gao, C. J. Seneviratne, E. C. M. Lo, C. H. Chu and L. P. Samaranayake, Int. J. Paediatr. Dent., 2012, 22, 363–368 CrossRef PubMed.
- B. Krasse, Int. Dent. J., 1988, 38, 219–225 Search PubMed.
- B. Krasse and S. Fure, Periodontology 2000, 1994, 4, 139–147 CrossRef PubMed.
- G. Yoshimura, H. Komatsuzawa, I. Hayashi, T. Fujiwara, S. Yamada, Y. Nakano, Y. Tomita, K. Kozai and M. Sugai, Microbiol. Immunol., 2006, 50, 729–742 CrossRef PubMed.
- D. J. Wright, P. A. Chapman and C. A. Siddons, Epidemiol. Infect., 1994, 113, 31–39 CrossRef PubMed.
- W. B. Shim, J. E. Song, H. Mun, D. H. Chung and M. G. Kim, Anal. Bioanal. Chem., 2014, 406, 859–866 CrossRef PubMed.
- H. Gu, K. Xu, C. Xu and B. Xu, Chem. Commun., 2006, 941–949 RSC.
- Z. Mei, A. Dhanale, A. Gangaharan, D. K. Sardar and L. Tang, Talanta, 2016, 151, 23–29 CrossRef PubMed.
- J. Gao, H. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097–1107 CrossRef PubMed.
- Y. Cheng, Y. Liu, J. Huang, K. Li, W. Zhang, Y. Xian and L. Jin, Talanta, 2009, 77, 1332–1336 CrossRef PubMed.
- H. Tumturk, F. Sahin and E. Turan, Analyst, 2014, 139, 1093–1100 RSC.
- K. S. Cudjoe, T. Hagtvedt and R. Dainty, Int. J. Food Microbiol., 1995, 27, 11–25 CrossRef PubMed.
- Y. Mine, J. Agric. Food Chem., 1997, 45, 3723–3727 CrossRef.
- Y. J. Sung, H. J. Suk, H. Y. Sung, T. Li, H. Poo and M. G. Kim, Biosens. Bioelectron., 2013, 43, 432–439 CrossRef PubMed.
- M. Khalkhali, S. Sadighian, K. Rostamizadeh, F. Khoeini, M. Naghibi, N. Bayat, M. Habibizadeh and M. Hamidi, BioImpacts, 2015, 5, 141–150 CrossRef PubMed.
- W.-C. Wang, K.-G. Neoh and E.-T. Kang, Macromol. Rapid Commun., 2006, 27, 1665–1669 CrossRef.
- M. Ramasamy, Y. Zhu, U. Paik and D. K. Yi, Mater. Lett., 2014, 137, 479–482 CrossRef.
- M. Rutnakornpituk, N. Puangsin, P. Theamdee, B. Rutnakornpituk and U. Wichai, Polymer, 2011, 52, 987–995 CrossRef.
- P. Padwal, R. Bandyopadhyaya and S. Mehra, Langmuir, 2014, 30, 15266–15276 CrossRef PubMed.
- H. Li, K. Yan, Y. Shang, L. Shrestha, R. Liao, F. Liu, P. Li, H. Xu, Z. Xu and P. K. Chu, Acta Biomater., 2015, 15, 117–126 CrossRef PubMed.
- E. Kadar, I. L. Batalha, A. Fisher and A. C. Roque, Sci. Total Environ., 2014, 487, 771–777 CrossRef PubMed.
- G. Chen, Z. Wu and Y. Ma, J. Biotechnol., 2015, 196–197, 52–57 CrossRef PubMed.
- H. Eskandari and A. Naderi-Darehshori, Anal. Chim. Acta, 2012, 743, 137–144 CrossRef PubMed.
- S. Qin, L. Wang, X. Zhang and G. Su, Appl. Surf. Sci., 2010, 257, 731–735 CrossRef.
- M. Khoobi, T. M. Delshad, M. Vosooghi, M. Alipour, H. Hamadi, E. Alipour, M. P. Hamedani, S. E. Sadat ebrahimi, Z. Safaei, A. Foroumadi and A. Shafiee, J. Magn. Magn. Mater., 2015, 375, 217–226 CrossRef.
- A. Akbarzadeh, N. Zarghami, H. Mikaeili, D. Asgari, A. M. Goganian, H. K. Khiabani, M. Samiei and S. Davaran, Nanotechnol., Sci. Appl., 2012, 5, 13–25 Search PubMed.
- R. Falatach, C. McGlone, M. S. Al-Abdul-Wahid, S. Averick, R. C. Page, J. A. Berberich and D. Konkolewicz, Chem. Commun., 2015, 51, 5343–5346 RSC.
- X. Fan, L. Lin and P. B. Messersmith, Compos. Sci. Technol., 2006, 66, 1198–1204 CrossRef.
- B. Mu, T. Wang, Z. Wu, H. Shi, D. Xue and P. Liu, Colloids Surf., A, 2011, 375, 163–168 CrossRef.
- Q. Yang, Z. Dai, K. Yang and Y. Li, Proceedings of the AASRI International Conference on Industrial Electronics and Applications, Atlantis Press, London, UK, 2015, pp. 47–51 Search PubMed.
- Y. Sameenoi, P. N. Nongkai, S. Nouanthavong, C. S. Henry and D. Nacapricha, Analyst, 2014, 139, 6580–6588 RSC.
- K. Wennerholm and C.-G. Emilson, Eur. J. Oral Sci., 2013, 121, 389–393 CrossRef PubMed.
- R. Dong, M. Lindau and C. K. Ober, Langmuir, 2009, 25, 4774–4779 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Characteristics of CWBD, the XRD pattern of bare MNPs, FTIR spectra, average size, zeta potential and TGA data of MNPs both before and after stepwise chemical modification, explanation on how to determine the limit of detection and capture efficiency, and photographs of oral streptococci colonies before and after binding to CWBD-conjugated MNPs. See DOI: 10.1039/c8ay00114f |
| ‡ These two authors contributed equally and are considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2018 |