Enhanced adsorptive removal of hazardous anionic dye “congo red” by a Ni/Cu mixed-component metal–organic porous material†
Abstract
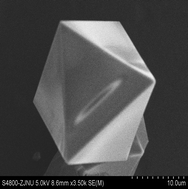
A new type of metal–organic porous material (Ni/Cu–BTC) has been successfully synthesized by a solvothermal approach for the first time. The as-synthesized materials were characterized using XRD, SEM, ICP, and TGA analyses and N2 adsorption at 77 K. The Ni/Cu–BTC was applied to the adsorption of anionic dye congo red (CR) from aqueous solution, and from the experimental data, adsorption kinetics and isotherms were obtained. The results showed that a pseudo-second-order kinetic model and the Langmuir adsorption isotherm matched well for the adsorption of CR onto Ni/Cu–BTC. Thermodynamic parameters including free energy, enthalpy, and entropy of adsorption were obtained, and all the results were favorable for the adsorption. Compared with other adsorbents reported in the literature, Ni/Cu–BTC demonstrated a superior CR dye adsorption capability. The results of the present study substantiate that Ni/Cu–BTC is a promising adsorbent for the removal of the dye from wastewater.

 Please wait while we load your content...
Please wait while we load your content...