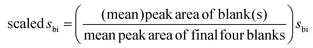Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Detection of opium alkaloids in a Cypriot base-ring juglet†
Rachel K.
Smith
 a,
Rebecca J.
Stacey
a,
Rebecca J.
Stacey
 *b,
Ed
Bergström
ac and
Jane
Thomas-Oates
*b,
Ed
Bergström
ac and
Jane
Thomas-Oates
 *ac
*ac
aDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: jane.thomas-oates@york.ac.uk
bDepartment of Scientific Research, The British Museum, London, WC1B 3DG, UK. E-mail: rstacey@britishmuseum.org
cCentre of Excellence in Mass Spectrometry, University of York, Heslington, York, YO10 5DD, UK.
First published on 3rd October 2018
Abstract
A method has been developed for extracting poppy alkaloids from oily matrices, specifically lipid residues associated with archaeological ceramics. The protocol has been applied to fresh and artificially aged poppyseed oil and to residue from a Late Bronze Age Cypriot juglet in the collections of the British Museum. The juglet is of a type that has been linked with ancient trade in opium due to its poppy-head shape and wide distribution; it is a rare example of an intact vessel with contents sealed inside. Bulk analysis of the residue by GC-EI-MS and pyGC-EI-MS indicated a degraded plant oil and possible presence of papaverine. Analysis of the alkaloid extracts by HPLC-ESI-MS using both triple quadrupole and FTICR mass spectrometers detected the five primary opium alkaloids in fresh poppyseed oil and papaverine in most of the aged samples. Papaverine and thebaine were detected in the juglet residue, providing the first rigorous chemical evidence to support a link between this vessel type and opium, or at least poppies. The association of opium with oil raises new questions about the ancient purpose of the commodities within these vessels, and the low levels (ng g−1) of opiates detected in this unusually well-preserved residue shed doubt on the scope for their detection in more fragmentary ceramic remains (potsherds). Papaverine was found to exhibit challenging carryover behaviour in all the analytical methods used in this study. The phenomenon has not been reported before and should be considered in future analyses of this analyte in all application areas.
Introduction
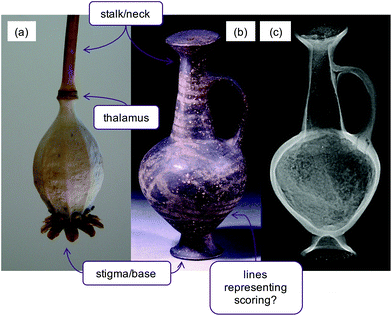
Base-ring juglets are a ceramic ware that was widely traded in the eastern Mediterranean in the Late Bronze Age (ca. 1650–1350 BC). They are characterised by ring-shaped bases, and thin walls usually with highly polished brown slip coatings.1 In 1962, Merrillees proposed a link between the vessels and opium because of their distinctive shape which, when inverted, resembles the capsule of the opium poppy (Papaver somniferum) (Fig. 1a).2 Opium is obtained from the latex which oozes from unripe poppy capsules when they are scored;3 striped decoration on some base-ring juglets has been proposed as further evidence to associate the vessels with opium.2Ever since Merrillees’ publication, base-ring juglets have attracted great interest for tracing opium trade in the Late Bronze Age Mediterranean. However, despite numerous attempts to detect opiates in base-ring juglet potsherds and vessels, little convincing chemical evidence has emerged to demonstrate that these vessels once contained opium. Indeed, the whole theory of the association has been questioned, and alternative theories posed that vessels were used for aromatic oils rather than opium.1,4,5
In the British Museum collection is a sealed base-ring juglet (BM reg. number 1981,1218.53, Fig. 1b), which radiography has revealed to be partially filled with residue (Fig. 1c). Such examples are extremely rare, providing an exceptional opportunity for chemical analysis of the unusually well-preserved contents.
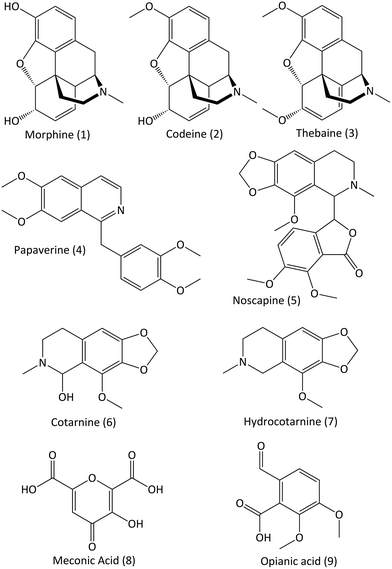
Over 40 alkaloids are produced by P. somniferum but the five primary alkaloids are morphine (1), codeine (2), thebaine (3), papaverine (4) and noscapine (5).6,7 Of these, morphine is the most abundant and is also the main source of opium's narcotic effects. Thus, most previous studies have attempted to detect morphine. However, artificial ageing studies of opium have shown that morphine does not survive well, and that papaverine, thebaine and the breakdown products of noscapine (cotarnine (6), hydrocotarnine (7), meconic acid (8) and opianic acid (9)) are the most resistant to degradation, making them much more promising targets for detection of opiates in ancient residues.8 Numbers in brackets refer to the chemical structures of the alkaloids shown in Fig. 2.
The only published positive result for opium alkaloids in a base-ring juglet dates to 1996. Morphine, codeine and noscapine were detected in residue from inside a base-ring juglet from Egypt. It was analysed using thin-layer chromatography (TLC) and gas chromatography-mass spectrometry (GC-MS),9 and immunoassay methods specific for morphine.10 The results are inconsistent with the more recent experimental study8 showing that morphine, codeine and noscapine are relatively unstable and unlikely to survive over long periods. In the 1996 analysis an authentic standard for thebaine was used; the paper reports its detection in a sample of crude opium using both GC-MS and TLC. Papaverine was also reported in the crude opium sample using TLC. It is surprising therefore that neither of these more stable alkaloids was reported in the juglet residue. This apparent disparity between the sole report of opium alkaloids and the experimentally determined stability of the alkaloids, plus the paucity of evidence from other studies, means that more evidence is needed to establish whether base-ring juglets can be linked with opium.
The aims of the work reported here were to exploit access to the rare, sealed British Museum base-ring juglet for organic residue analysis; having developed alkaloid extraction and state-of-the-art mass spectrometric analytical methods, we have sought to provide the first rigorous chemical evidence for a link between base-ring juglets and opium.
Experimental
Full details are supplied in the ESI.†Archaeological sample
Samples of the residue inside the juglet (BM reg. no.: 1981,1218.53) were removed using a dissection microprobe with bent needle, inserted through a hole drilled through the base. Portions (∼40–80 mg each) of the contents were removed and smeared onto the insides of six small glass vials.Oil samples
Cold pressed organic poppyseed oils (from Oshadhi or Fandler) and olive oil (from a local supermarket) were purchased. 1 mL aliquots of oil samples were spiked with papaverine for validating extraction procedures.Artificial ageing
Poppyseed oil samples (100 μL) were artificially aged by heating, sealed in glass Kilner® jars at 60 °C. Samples were aged: either at ambient or 100% relative humidity; in a thin layer on a glass slide or as a pool in a vial; mixed with or without approx. 40–50 mg of ceramic powder (to simulate the juglet surface). Samples were extracted and analysed after 17 days, 8.5 months, 10.5 months or 11 months in the oven (Table S-1†).Extraction and purification of lipids and alkaloids
Lipids were extracted into dichloromethane (DCM) with ultrasonication. After centrifugation (10 min, 3000 rpm) the supernatants were dried and trimethylsilylated (70 °C, 50 μL N,O-bis(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane) and analysed using GC-MS with electron ionisation (GC-EI-MS). A transesterified (methanolysed) sample was prepared using Meth-Prep II followed by GC-EI-MS analysis with high temperature injection. Pyrolysis-GC-EI-MS (pyGC-EI-MS) analysis was carried out directly on unextracted samples treated with excess trimethylphenylammonium hydroxide.For alkaloid extraction, DCM was used if needed to suspend the sample. Alkaloids were extracted into 4 mL HCl (0.1 M), ultrasonicated (15 min), and lipids removed using 1 mL hexane. The alkaloids were retrieved from the aqueous layer using C18 solid-phase extraction (SPE); after washing with water, the alkaloids were eluted in 1 mL methanol. The alkaloid-containing extract was dried and redissolved in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (9
acetonitrile (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for HPLC-MS analysis.
1, v/v) for HPLC-MS analysis.
GC-EI-MS analysis
DCM extracts were analysed using an Agilent 6890N gas chromatograph (GC) using two different chromatographic methods. In order to achieve optimal separation of fatty acids and their degradation products, samples were injected in splitless mode onto an Agilent HP5-MS column (30 m × 0.25 mm, film thickness 0.25 μm), with helium carrier gas (1.5 mL min−1), coupled to an Agilent 5973N mass spectrometer (MS). For detection of acylglycerols, samples were injected in on-column mode onto an SGE HT-5 column (12 m × 0.1 mm, film thickness 0.1 μm), with helium carrier gas (1.0 mL min−1), coupled to an Agilent 5975C MS.The methanolysed sample was analysed using the same equipment, but a different chromatographic programme, as that for optimal separation of the fatty acids in the DCM extract (helium flow rate 1.0 mL min−1).
Pyrolysis used a CDS Pyroprobe 1000 (probe temperature 350 °C (15 s), interface temperature 350 °C); products were introduced into the GC in split (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mode at 300 °C.
1) mode at 300 °C.
Mass spectral data were interpreted manually with the aid of the NIST/EPA/NIH Mass Spectral Library version 2.0 and comparison with published data.
HPLC-ESI-MS analysis
Analysis was carried out with a Dionex UltiMate 3000 high performance liquid chromatograph (HPLC) fitted with a Dionex Acclaim 120 C18 column (3 μm, 120 Å, 2.1 × 150 mm) and Phenomenex SecurityGuard system with a C18 (4 × 2.00 mm) cartridge, coupled to a Bruker HCTultra ETD II ion trap MS, a Bruker solariX XR 9.4 T Fourier transform ion cyclotron resonance (FTICR) MS (full scan modes) or an Applied Biosystems/MDS Sciex API 3000 triple quadrupole MS (selected reaction monitoring (SRM) mode), using electrospray ionisation (ESI, positive mode). Separation used a water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile gradient (10%–90% acetonitrile over 5 min). The ion trap instrument was used for method development experiments before moving onto the triple quadrupole in order to gain optimal sensitivity, while the FTICR was used to obtain full scan data with high mass accuracy in order to try to detect compounds for which standards could not be obtained.
acetonitrile gradient (10%–90% acetonitrile over 5 min). The ion trap instrument was used for method development experiments before moving onto the triple quadrupole in order to gain optimal sensitivity, while the FTICR was used to obtain full scan data with high mass accuracy in order to try to detect compounds for which standards could not be obtained.
Results and discussion
Archaeological sample appearance
The residue is a dark brown, thick, oily material, that appears to fill the main body of the juglet. This contrasts with the material analysed in the 1996 study, described as ‘a yellowish-brown amorphic resine like residue mixed with quartz sand grains’.9 While that description is consistent with dried poppy latex, no lipid analysis was carried out and therefore the presence or absence of lipids in that sample cannot be established. It is also unclear from the 1996 report whether the vessel was sealed, although the inclusion of sand might suggest that it was open. If the contents of the British Museum vessel, which was sealed, had been exposed to the air for a long period, or buried, then its texture would be expected to have changed and a dried oil residue might become hard and resin-like. The juglet has a narrow neck and pouring spout, consistent with the contents being a pourable liquid, which is inconsistent with the original contents being solid opium.1GC-EI-MS and pyGC-EI-MS
Lipid analysis was carried out due to the oily character of the residue (Fig. S-1†) and revealed an abundance of free fatty acids (FFAs) in two ranges: C7:0 to C10:0 with C9:0 the most abundant, and C14:0 to C24:0 with C16:0 the most abundant. C16:0 was the most abundant FFA overall. Low levels of mono- and diunsaturated FFAs were observed but mono- and diacylglyerols were detected only as traces and most could not be conclusively identified. The FFAs were accompanied by abundant dicarboxylic acids in the range C6:0 to C11:0 with C9:0 being most abundant, as well as 9,10-dihydroxy octadecanoic acids and a range of keto–dicarboxylic acids. The lipid profile is consistent with a highly degraded plant-derived oil.11 The predominance of C16:0 over C18:0 is generally typical of plant oils rather than animal fats.12 The short chain fatty acids, dicarboxylic and dihydroxy acids are degradation products of unsaturated fatty acid components.13,14 The specific plant origin of the oil cannot be securely determined due to the alterations to the molecular profile brought about by ageing and the possibility of mixed sources. Nevertheless, absence of distinctive characteristic biomarkers argues against certain oils, for example Brassicaceae oils which contain Z-11-eicosenoic acid and Z-13-docosenoic acid,15 and castor oil which contains 12-hydroxy-9-cis-octadecenoic and 9,12-dihydroxyoctadecanoic acid.14 The C16:0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C18:0 (P/S) ratio has long been used in characterisation of oil paint films to distinguish different plant oil sources, with a high (>3) ratio considered characteristic of poppyseed oil.16 This approach is less used for interpretation of archaeological lipids due to the greater variety of degradative processes that can alter lipid profiles17 and the wider range of lipid starting materials. However, the closed environment of the sealed juglet provides for exceptional preservation conditions and so the high P/S ratios obtained in these analyses (>3.5, see Fig. S-1†) are worthy of note and suggest that poppyseed could be a plausible candidate origin for the oil. Presence of poppyseed oil would present a new perspective on the theory linking the vessel shape to the origin of its contents, and would be consistent with the contents being a pourable liquid.
C18:0 (P/S) ratio has long been used in characterisation of oil paint films to distinguish different plant oil sources, with a high (>3) ratio considered characteristic of poppyseed oil.16 This approach is less used for interpretation of archaeological lipids due to the greater variety of degradative processes that can alter lipid profiles17 and the wider range of lipid starting materials. However, the closed environment of the sealed juglet provides for exceptional preservation conditions and so the high P/S ratios obtained in these analyses (>3.5, see Fig. S-1†) are worthy of note and suggest that poppyseed could be a plausible candidate origin for the oil. Presence of poppyseed oil would present a new perspective on the theory linking the vessel shape to the origin of its contents, and would be consistent with the contents being a pourable liquid.
No evidence for ingredients such as natural waxes or resins was noted, nor were any volatile terpenes that might indicate fragrance ingredients. However, although volatile monoterpenes have previously been detected in vessel residues,18 they are extremely vulnerable to loss in archaeological residues so fragrance ingredients cannot be ruled out on this basis.
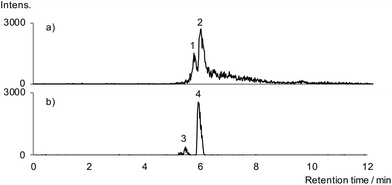
PyGC-EI-MS was performed to screen the residue for non-lipid organic constituents (polysaccharide, proteins, alkaloids). The resulting pyrogram was dominated by the bulk lipid composition and no pyrolysis products indicative of protein (e.g. diketopiperazines19) or saccharide (e.g. furan derivatives20) were detected; this is important, since polysaccharide and protein21 are known to be constituents of raw opium. Significantly, fragment ions characteristic of papaverine were detected (Fig. 3), with retention times that correlate with those from a papaverine standard. Consistent peak areas, however, could not be achieved due to incomplete transfer of papaverine from the pyrolyser interface and/or inlet to the column which caused inter-run carry-over and precluded meaningful quantitation.
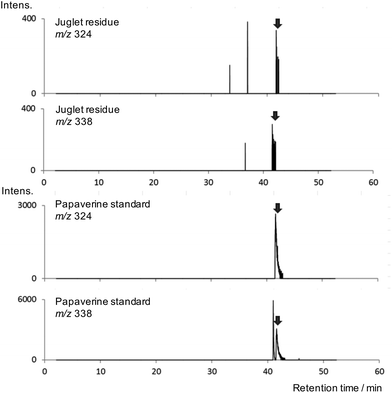 | ||
| Fig. 3 Extracted ion chromatograms for m/z 324 and m/z 338 obtained on pyGC-EI-MS analysis of residue from the juglet (top) and papaverine standard (bottom). | ||
Alkaloid extraction method development
The GC-EI-MS and pyGC-EI-MS analyses show the contents of the vessel to be primarily consistent with a highly degraded plant-based oil, with an indication of papaverine being present. To investigate the problem further it was necessary to develop a method for extraction of alkaloids from the oily matrix.The possibility that the juglet contained poppyseed oil was considered a crucial question and, in particular, whether the oil itself could prove to be a source of opiates, so poppyseed oil was analysed. The method used for extraction and purification of papaverine was modified from that published by Guo et al.22 for the extraction of poppy alkaloids from Chinese hot pot broth. The method was chosen because it was designed for extracting alkaloids from an oily matrix, as here.
Analysis of two different poppyseed oil samples using HPLC-ESI-SRM showed the presence of all four alkaloids for which standards were purchased (morphine, codeine, thebaine, papaverine) (Fig. S-2†). HPLC-FTICR-ESI-MS analysis allowed detection of opium-related compounds for which standards could not be obtained. Extracted ion chromatograms (EICs) were produced of the m/z values (to 3 decimal places) for noscapine and its breakdown products cotarnine, hydrocotarnine, meconic acid and opianic acid, and for the four alkaloids already detected using SRM (Table S-3†). As well as peaks in the EICs for codeine, thebaine and papaverine, noscapine was detected with good signal-to-noise (S/N) in both types of poppyseed oil. Morphine was not detected using EICs in either oil, probably due to the lower sensitivity of full scan analysis on FTICR-ESI-MS compared with ESI-SRM on a triple quadrupole MS. Meconic acid was also not detected in the oil extracts. Cotarnine, hydrocotarnine and opianic acid were, however, detected in the Fandler poppyseed oil, and cotarnine and hydrocotarnine were detected in the Oshadhi poppyseed oil (Fig. S-3†).
No published information on the alkaloid content of poppyseed oil could be found, but detection of alkaloids in the oil is not surprising; although alkaloids are most concentrated in the poppy latex, they do also occur in other tissues, including the seeds from which the oil is pressed. Published analysis, by GC-EI-MS, has shown poppy seeds to contain morphine, codeine, noscapine, papaverine and thebaine.23
The detection of opium alkaloids in poppyseed oil using our extraction method is an encouraging finding, demonstrating that the method can be used to extract and detect opium alkaloids successfully from fresh poppyseed oil.
Artificial ageing experiments
Fandler poppyseed oil was artificially aged to identify degradation products and to assess whether opiates can still be detected after a period of ageing. A study by Chovanec et al. on the artificial ageing of opium alkaloids in raw opium (rather than poppyseed oil), using similar methods to those used here, has shown that papaverine and thebaine are the most stable opium alkaloids, while morphine degrades rapidly.8 They concluded that it would not be surprising if morphine were not to be identified in archaeological samples, since it does not preserve well, and point out that papaverine and thebaine, being more stable, should be the targets of future archaeological analyses.8 In addition, the pyGC-EI-MS analysis of the juglet contents provided tentative evidence for papaverine.Because the ageing process is likely to change the material properties of the oil, e.g. its viscosity, it was anticipated that the extraction method may need adapting for aged samples. To test this, ten aliquots of poppyseed oil were aged for 17 days. The samples became more viscous during the heating and could not easily be transferred for extraction. To allow the oil to mix with the extracting acid it was necessary first to solubilise or at least suspend the samples. Three solvents were tested: hexane, DCM and acetone. Although none of the solvents produced a completely clear solution, it was possible to transfer the oil into the extraction vessel using the solvent as a carrier. After addition of the acid, the two phases mixed on ultrasonication when using all three of the solvents. For the samples in DCM or acetone, hexane was then added (as in the method for fresh poppyseed oil) and the samples were centrifuged to separate the layers. The alkaloids were extracted from the aqueous layer using SPE and analysed by HPLC-ESI-SRM. The samples in hexane and acetone did not give peaks corresponding to any of the alkaloids. However, the samples dissolved in DCM gave peaks corresponding to papaverine and tentatively to thebaine (Fig. S-4†). The thebaine SRM chromatogram gave only a very low S/N peak at the retention time for thebaine (tR = 5.50 min), with a larger peak at an incorrect retention time (tR = 5.89 min).
Opiates other than papaverine and thebaine were not detected in these aged samples (nor were the breakdown products of noscapine). However, since the extraction method had been shown to be appropriate for all four of the alkaloids in fresh poppyseed oil samples, and it was known that morphine and codeine are less stable than papaverine and thebaine, it is proposed that morphine, codeine and thebaine had degraded to the extent that they were no longer detectable, rather than that they had not been extracted (despite thebaine's higher stability it will still be subject to some degradation). Therefore, while bearing in mind that the archaeological sample may behave differently than artificially aged poppyseed oil, DCM was considered a suitable carrier solvent, and used for handling all subsequent samples.
A further 16 poppyseed oil aliquots were artificially aged for 8.5 months, 10.5 months and 11 months (Table S-1†). During the ageing experiments, two of the thin layers of oil were lost by sliding off the glass slide on which they were being heated (Table S-1†). On heating, the oil darkened from a pale yellow colour to amber and its viscosity increased to the extent that it did not flow freely, while the samples with added ceramic set solid. There were consequently some difficulties in transferring these aged samples into the extraction vials. For the pools, DCM was added to the vials to loosen the oil for pipetting, while the thin layers were scraped off into extraction vials before DCM was added.
After heating, morphine and codeine were not detected in any of the samples. This is not surprising, since they have already been shown to degrade under similar conditions to those used here,8 and they were not detected in the samples which were aged for only 17 days.
Papaverine was detected in some of the samples, particularly those aged for the shorter time periods. In the 17 day samples papaverine was detected at concentrations ranging from 79–307 pg mg−1 of fresh poppyseed oil extracted. In the 8.5 month samples the concentrations had reduced to between 2.2 pg mg−1 and 3.2 pg mg−1 of fresh poppyseed oil, and after 10.5 months papaverine was no longer detected in most of the samples, although the ambient humidity pools did contain detectable amounts. Some of the 11 month samples, which were all heated at ambient humidity, also contained detectable amounts of papaverine. Overall, the levels of papaverine detected were variable, probably because of difficulties in transferring all samples reliably into the extraction vials after heating. However, there did seem to be a reduction in levels over time, as would be expected.
Thebaine was not detected in any of the aged samples, except for its tentative detection in the 17 day samples, but in each of the aged samples there was a peak (tR ≈ 5.9 min) in the SRM chromatogram at thebaine's m/z value but at the wrong retention time. This peak had very good S/N in some of the aged samples, and had the same retention time as papaverine. The peak was also observed in the archaeological sample, and is discussed in more detail below.
HPLC-FTICR-ESI-MS analysis did not give peaks in EICs for noscapine or its breakdown products in the aged samples.
Extraction efficiency and limits of detection
In order to test the extraction efficiency it was necessary to use a matrix containing no opium alkaloids and to spike the alkaloids into it. Olive oil was thus extracted and analysed and, as expected, was found to contain no detectable alkaloids, making it a suitable oily matrix for spiking experiments.Extraction efficiency
The efficiency of the extraction method was calculated using papaverine as the only alkaloid. Papaverine was spiked into olive oil, extracted using the developed method, and analysed on the ion trap MS alongside standard papaverine solution (5 μL injected). Papaverine spiked at 10 ng mL−1 (two replicates) and 5 ng mL−1 of olive oil (i.e. 50 pg and 25 pg injected onto the column) gave extraction efficiencies of 89% and 87%, respectively, using peak areas for quantification. This was deemed to be an acceptable extraction efficiency.The extraction method was developed using fresh olive oil, which was liquid enough to mix with the aqueous extraction solvent. However, when it was applied to the aged poppyseed oil and the archaeological sample, these were too viscous to allow mixing with the extraction solvent without using DCM for solubilisation/suspension of the very viscous samples. Extraction of aged poppyseed oil was tried without the addition of the extra solvent and no papaverine was detected. Because of the introduction of DCM into the extraction method, the extraction efficiency was recalculated. All four alkaloids were spiked into olive oil at concentrations of 5 ng mL−1 and 50 ng mL−1 each (i.e. 25 pg and 250 pg injected), in triplicate. DCM was added and the mixtures were extracted. On analysis, these gave widely varying extraction efficiencies, between almost as low as 0% and as high as 100%. Since the only difference between these extraction experiments and the first set of extraction experiments was the addition of DCM, the explanation must be that the DCM undermined the reproducibility of the extraction. This is unfortunate because it means that the alkaloids in the archaeological sample cannot be accurately quantified (because the extraction efficiency cannot be allowed for). However, it was the only way to analyse the viscous samples, and does not at all detract from the very clear qualitative detection of the alkaloids. It may also account for the variation in levels of papaverine detected in the different aged poppyseed oil samples.
Limits of detection and quantitation
Because of problems with carryover in the papaverine analysis (discussed below), the limits of detection and quantitation (LOD and LOQ) were determined by comparison with solvent injection blanks, as defined in the IUPAC Gold Book:24xL = ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) bi + ksbi bi + ksbi |
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) bi is the mean of blank measures, k is the confidence level (i.e. 3 for LOD and 10 for LOQ) and sbi is the standard deviation of blank measures.
bi is the mean of blank measures, k is the confidence level (i.e. 3 for LOD and 10 for LOQ) and sbi is the standard deviation of blank measures.
Carryover meant that, when standards were analysed to construct a calibration plot for quantitation of papaverine, the size of the peak for papaverine in the blanks became progressively greater. The standards were injected in triplicate sets, each set starting from the lowest concentration to the highest. One or two solvent blank injections were interspersed between each standard injection and after each sequence from low to high concentration there were four injections of solvent blank. The solvent reservoir from which the blank was sampled was replaced at the start of each sequence. Because of the increase in size of the papaverine peak in the blanks over the course of injecting each set of calibration standards, the standard deviation would be artificially large if all of the blanks were used in determining the LOD and LOQ. Therefore, ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) bi and sbi for each set of calibration standards were calculated based on the peak areas of the four blank injections at the end of each set. For each blank injection (or pair) earlier in the run, a scaled sbi was produced, based on a comparison of the peak area (or mean of a pair of peak areas) of the blank(s) with the mean of the four blanks from which the original sbi was calculated, as follows:
bi and sbi for each set of calibration standards were calculated based on the peak areas of the four blank injections at the end of each set. For each blank injection (or pair) earlier in the run, a scaled sbi was produced, based on a comparison of the peak area (or mean of a pair of peak areas) of the blank(s) with the mean of the four blanks from which the original sbi was calculated, as follows:
LODs and LOQs were calculated based on each blank's peak area (or mean of a pair of peak areas) and its associated scaled sbi value; whether a standard peak area was above the limits was assessed based on the calculated values for the blank(s) immediately following it. For each of the three sequences of calibration standards, standards from 15 pg injected onto the column were all above the LOD. One of the 5 pg standards was just above the LOD and two were just below. All of the 50 pg standards were above the LOQ and two of the 15 pg standards were above the LOQ, whilst the third 15 pg standard was very slightly below the LOQ.
For thebaine, the same process was carried out. There were not the problems of carryover for thebaine that there were for papaverine, so there were fewer blank injections and one value of ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) bi and one of sbi were calculated for each sequence to give LOD and LOQ values. The lowest injected amount of thebaine that was above the LOD in all three standard runs was 25 pg and for LOQ it was 100 pg.
bi and one of sbi were calculated for each sequence to give LOD and LOQ values. The lowest injected amount of thebaine that was above the LOD in all three standard runs was 25 pg and for LOQ it was 100 pg.
As can be seen from the analysis of a mixture of 25 pg each of papaverine, thebaine, morphine and codeine standards (Fig. S-5†), the signal was different for each of the analytes, with papaverine giving by far the best signal and morphine the worst. Therefore the LODs of each alkaloid will be different. However, because morphine and codeine were not quantified, their LODs have not been calculated.
For samples, the LOD and LOQ were calculated in a similar way to those for the quantitation standards, with peak areas of blank injections carried out during the sequence used as the blank values. There was not the same problem of the blank peak area increasing in size because increasing concentrations of papaverine were not used, and the levels analysed were typically fairly low, and therefore one value of ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) bi and one of sbi were calculated for each sequence to give LOD and LOQ values. All of the juglet extracts gave peak areas for papaverine above the LOD and LOQ. For thebaine, all of the juglet extracts gave peak areas above the LOD, and all but one gave peak areas above the LOQ.
bi and one of sbi were calculated for each sequence to give LOD and LOQ values. All of the juglet extracts gave peak areas for papaverine above the LOD and LOQ. For thebaine, all of the juglet extracts gave peak areas above the LOD, and all but one gave peak areas above the LOQ.
Archaeological sample
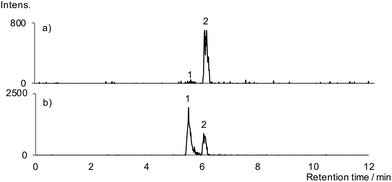
Portions (six replicates) of the vessel contents were extracted using the method developed on artificially aged poppyseed oil, using DCM to transfer the residue to the extraction vessel, followed by extraction and SPE. The extracts were analysed using HPLC-ESI-SRM and full scan HPLC-FTICR-ESI-MS.Papaverine and thebaine were both detected in each of the portions of extract (SRM chromatograms in Fig. 4). No morphine, codeine or noscapine breakdown products were detected. Papaverine concentrations in the extracts varied from 0.4–2.6 pg mg−1 of residue (though, because of the problems with extraction efficiency, these values cannot be converted into concentrations in the juglet contents). As can be seen in Fig. 4, there are two peaks in the papaverine transition chromatogram that are not fully resolved. The larger, later-eluting, peak (tR = 5.91 min) has a retention time corresponding to that for papaverine. It is proposed that the other peak (tR = 5.67 min) may be due to a breakdown product of one of the alkaloids, and since it has the same transition as papaverine is perhaps a papaverine isomer. Thebaine concentrations varied from 2–12 pg mg−1 of residue. In addition to a peak at tR = 5.51 min for thebaine, there is a significantly larger peak at tR ≈ 6 min observed with the same transition (Fig. 4). This was observed for each juglet extract and at the same retention time as the additional peak in the thebaine chromatogram obtained from the artificially aged samples. Like the additional peak in the papaverine chromatogram, this is proposed to be due to a breakdown product of one of the alkaloids. However, in order to check that it was not due to thebaine, standard thebaine was spiked into a portion of one of the extracts (Fig. 5). This caused the peak with tR = 5.51 to increase in size but caused no change in the size of the other peak (tR ≈ 6 min), showing that the tR ≈ 6 min peak is due to an analyte other than thebaine. Since the peak with tR ≈ 6 min has the same retention time as papaverine, it could be due to an in-source fragment of papaverine that has the same m/z value as thebaine. However, the product ion scan of papaverine does not give fragments with the m/z values used for the thebaine transition so this seems unlikely.
The failure to detect morphine and codeine in the juglet contents extract is not unexpected, since these have been shown to be more liable to degradation than papaverine and thebaine8 and the experiments on the artificial ageing of poppyseed presented here have shown the same results. Although the analysis of the four alkaloid standards (Fig. S-5†) shows that they each have different LODs, analysis of fresh poppyseed oil (Fig. S-2†) demonstrates that the method is capable of detecting morphine and codeine if they are present at sufficient levels. Consequently, the detection of papaverine and thebaine in this base-ring juglet is extremely significant as this is the first rigorous demonstration of chemical evidence to support the proposed link between base-ring juglets and poppies. In contrast with the single previous study to yield positive evidence,9 these results are fully consistent with the known ageing behaviour of opium alkaloids; consequently the findings of the earlier study9 ought now to be reconsidered.
Moreover, our results raise significant questions about the use of these vessels and their relationship with opium. For use as a narcotic, opium would not be expected to be prepared or stored in an oil, as the usual methods for taking opium as a drug are either to smoke it or to ingest it, usually dissolved in alcohol. The detection of opiates in an oil medium therefore demands an alternative explanation. The results could indicate that the vessel was used for poppyseed oil rather than opium. This interpretation would chime with the conclusions of the most recent critique of Merrillees's theory, which proposes that base-ring juglets were containers for scented oils and have no association with opium.4,5 Similar observations are made by Bushnell in her comprehensive study of the archaeological distribution of base-ring juglets and she notes that poppyseed oil could offer an intriguing alternative version of the theory linking the base-ring juglets with the poppy via its seed oil instead of opium.1 Nevertheless, the interpretation of the juglets as containers for perfumed oils does not preclude the possibility of opium as one of the ingredients, perhaps the only surviving evidence of a complex mixture of minor components added to the oil, which could have been included as much for symbolic as for psychoactive properties. An alternative explanation could be reuse of the vessel in ancient times, so that the oil was a second use after the opium had been removed. This theory has already been proposed by Merrillees25 to explain the presence of fats and waxes in base-ring juglets. Chovanec et al.5 have pointed out that the argument can be made in both directions, with opium residue representing secondary use of an oil vessel, a fair point for vessels or sherds with no extant surface residues but not valid in this instance where the bulk oil contents of the vessel are preserved in situ.
Ideally, in order to draw broader conclusions about juglet use, many juglets would be analysed and the recent critiques of Merrillees’ theory4,5 have drawn on the larger corpus of negative results arising from residue analysis of base-ring juglet sherds. However, although they were traded widely at the time of their production, surviving sealed juglets complete with contents are very rare, which is why the specimen considered here is so valuable to the debate about the use of such vessels. Juglet sherds, freshly excavated from archaeological sites, offer the best opportunity for production of larger data sets with which to interrogate the veracity of Merrillees’ theory, so it is understandable that they have formed the basis of the unsuccessful attempts in recent years to detect opium alkaloids as absorbed residues, analogous to lipid residues absorbed into cooking pots.26 Preservation of lipids in archaeological potsherds is promoted in part by their hydrophobicity, which means they resist leaching by water when the potsherds are buried.27 However, since alkaloids are water soluble they are unlikely to be preserved to the same extent as lipids, which are typically detected at levels of μg per g of potsherd powder.26 Given the findings reported here, where only ng of alkaloid per g of extracted juglet contents were detected, it seems unlikely that alkaloids can be preserved as absorbed residues to any appreciable extent in potsherds. This may account for the lack of opiates found in the most recent published study of residues from base-ring juglets,5 where the lipids (interpreted by the authors as oil-derived) are also very poorly preserved in the surface scrapings of ceramic that were analysed. However, in that study heat was used in the extraction protocol, which, given the vulnerability of opiates to thermal degradation, risks the generation of false negatives. A strength of the cold extraction method developed here is that it eliminates the opportunity for such potentially misleading protocol-derived losses. The analytical approach is significant as well. Chovanec et al.5 used GC-EI-MS; in this study the alkaloids were not detected when the juglet residue was analysed by GC-EI-MS but the presence of papaverine was indicated by pyGC-EI-MS and validated, along with thebaine, by HPLC-ESI-MS. Thus the statement by Bunimovitz and Lederman4 that the data produced by Chovanec et al.5 indicate ‘categorically that the vessels examined did not contain opium’ cannot be supported. On the basis of our results, a more promising approach for detection of opiates in sherds should be either pyGC-EI-MS of unprepared ceramic powder with a derivatising reagent or HPLC-ESI-MS after extraction following the protocol reported here. Nevertheless, the low yield of opiate alkaloids from this well-preserved residue implies that, even with the application of robust and proven methodologies, positive results from juglet sherds will be rare and caution is vital for appropriate interpretation of both negative and positive results.
Papaverine analysis
The published work on artificial ageing of opium reveals that papaverine is one of the most stable opium alkaloids,8 so it was one of the primary analytical targets for this work. There are many publications which describe HPLC-MS analysis of papaverine,22,28–30 including the use of papaverine as an internal standard.31,32 However, in the work presented here, many problems with carryover were encountered, with papaverine eluting in blanks run after samples/standards in both pyGC-EI-MS and HPLC-ESI-MS analyses.PyGC-ESI-MS was the first analytical method to be applied to the contents of the vessel in an attempt to detect papaverine, and the analyte was tentatively detected in the juglet contents using this method. However, papaverine was also observed in blank runs following sample injections. For this reason, a different analytical method was sought and HPLC-ESI-MS was chosen, since it is well-reported to be an appropriately sensitive method for detecting the relevant alkaloids.
However, when using HPLC-ESI-MS similar problems with carryover were observed. To rule out accidental contamination of the mobile phase, the glass reservoirs were washed with 5% nitric acid solution, the lines were washed with 1% formic acid solution and the injection system rinsed with 1% formic acid. This acidic washing regime was used with the intent to protonate contaminating papaverine and increase its solubility in water to try to help wash it away. The mobile phase reservoirs were then rinsed three times with HPLC-MS grade solvent (each bottle being rinsed with the solvent it was to hold) and refilled with HPLC-MS grade solvent. These measures ensured that on injection of a solvent blank no papaverine peak was observed.
When the first portion of juglet contents was extracted and analysed, the extraction blank prepared alongside it also gave a peak for papaverine. The extract of the juglet contents gave a papaverine peak that had an area 18 times the size of the extraction blank peak area, so it was persuasive evidence that the juglet did contain papaverine. However, the peak area of the extraction blank was not negligible and so before further portions of juglet contents were extracted it was necessary to understand and to eradicate the problem. Great care has therefore been taken to ensure that all glassware was clean and solvent was fresh from the bottles to rule out contamination of the solvents or glassware used for the extractions.
Importantly, there were also problems with carryover when a high concentration standard was analysed, when the blanks run afterwards had significant papaverine peaks which would reduce in intensity with the injection of subsequent blanks but only to a point, after which it would not reduce any further. The problem started to be noticeable with 1.5 ng injected onto the column and became significant with 5 ng injected, resulting in levels of papaverine in the blanks equivalent to approximately 5 pg of papaverine being injected. The high papaverine level standards were required to construct a calibration curve of the appropriate range, to allow quantitation for example of the fresh poppyseed oil samples. It was supposed that the papaverine may have contaminated the injection system. However, carrying out extra washes of the injection system using 1% formic acid did not solve the problem. Only after washing the HPLC glassware with 5% nitric acid and replacing the solvent was the problem solved. It remains unclear how it might be possible to contaminate the solvent reservoirs during an automated sample injection series, so carryover in the analytical system seems the most credible explanation for the observations.
In spite of the clearly demonstrated carryover of papaverine, no reports of contamination by and carryover of papaverine could be found. However, since during the analysis of the vessel reported in this work the problem was encountered with two different analytical methods in three different labs (work was carried out at the University of York and the British Museum as well as at a third site, the National Gallery, in labs where no prior papaverine handling had taken place), it is a real concern for handling and measuring this analyte. It appears that papaverine sticks to the HPLC system and is not rinsed out fully, even at high organic mobile phase compositions. The problem is more marked after running the HPLC system at low organic mobile phase compositions, as might be expected, and high organic compositions do remove some of the papaverine. However, it seems that when a relatively large amount of papaverine is injected it contaminates the whole system and the only way to remove the contamination is to clean all of the lines and glassware and replace the solvents.
Having encountered these problems, in order to carry out the extraction and analysis effectively and produce convincing results, it was necessary to take the utmost care to exclude cross-contamination. This involved using HPLC-MS grade solvents rather than lower HPLC grade solvents, and making sure the solvents used for extraction and purification were taken fresh from the bottle daily, as well as replacing the solvent blank reservoirs daily. With these measures it was possible to produce extraction blanks that were almost completely blank, giving confidence in the results obtained from the replicate aliquots of the archaeological sample that were extracted and analysed.
The difficulties with papaverine were not encountered with any of the other alkaloids analysed. We believe it is important that this issue is reported, so that others working with the analyte are aware of the difficulties, and in particular, papaverine's use as an internal standard can be reconsidered.
Conclusions
A method for extracting opium alkaloids from oily matrices has been developed and applied to poppy-related samples including fresh and artificially aged poppyseed oil and the contents of a Bronze Age Cypriot base-ring juglet. The extracts have been analysed by HPLC-ESI-SRM and HPLC-FTICR-ESI-MS to obtain high mass resolution full scan data to screen for alkaloids for which standards could not be obtained. The five primary opium alkaloids were detected in fresh poppyseed oil, and papaverine was detected in most of the aged samples. The archaeological sample extracts were found to contain papaverine and thebaine, at concentrations of 0.4–2.6 pg mg−1 of residue for papaverine and 2–12 pg mg−1 for thebaine. These cannot be converted to quantities in the juglet contents due to uncertainty over the extraction efficiency. Table 1 shows the qualitative and quantitative findings of the study.| Sample | Papaverine | Thebaine | Other alkaloids |
|---|---|---|---|
| Aged for 17 days (ambient humidity, n = 10) | 79–307 pg mg−1 | Tentative detection | N/D |
| Aged for 8.5 months (high humidity, n = 1) | 2.2–3.2 pg mg−1 | N/D | |
| Aged for 8.5 months (ambient humidity, n = 2) | |||
| Aged for 10.5 months (high humidity, n = 3) | N/D | ||
| Aged for 10.5 months (ambient humidity, n = 4) | Below LOQ | ||
| Aged for 11 months (ambient humidity, n = 4) | Below LOQ | ||
| Juglet extracts (n = 6) | 0.4–2.6 pg mg−1 | 2–12 pg mg−1 |
Problems with carryover were encountered when analysing papaverine, using both HPLC-MS and pyGC-MS. No other reports of this problem have been found, but since during the course of this work it was encountered with two different techniques, with two different operators in three different labs it seems to be a fundamental problem for this analyte and should be borne in mind in future analyses in all application areas.
The detection of opium alkaloids in the contents of a base-ring juglet is a hugely important finding, bringing new reliable evidence to the long-standing controversy among archaeologists over the use and role of these vessels in the Late Bronze Age. The implications of the detection of opium alkaloids in an oily matrix from this rare sealed juglet will undoubtedly prompt further debate on the function of these vessels and why they were traded so widely.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
RKS gratefully acknowledges studentship support from the Natural Environment Research Council and the Analytical Chemistry Trust Fund (NE/I01959X/1). The York Centre of Excellence in Mass Spectrometry was created thanks to a major capital investment through Science City York, supported by Yorkshire Forward with funds from the Northern Way Initiative, and subsequent support from EPSRC (EP/K039660/1; EP/M028127/1). Thanks are owed to the National Gallery Scientific Department (Trafalgar Square, London WC2N 5DN) for allowing duplication of analysis on their pyGC-MS system and to Dr David Peggie for his assistance. We are indebted to Judith Swaddling of the Department of Greece and Rome at the British Museum for drawing the sealed juglet to our attention, thus instigating this study and grateful also to her colleagues Thomas Kieley and Lesley Bushnell for many helpful discussions regarding the ancient function of base-ring juglets and their contents. Radiographic analysis was carried out at the British Museum (Department of Scientific Research) by Janet Ambers.References
- L. Bushnell, Precious Commodities: The Socio-economic Implications of the Distribution of Juglets in the Eastern Mediterranean During the Middle and Late Bronze Age, British Archaeological Reports, 2016 Search PubMed.
- R. Merrillees, Antiquity, 1962, 36, 287–292 CrossRef.
- M. Julyan and M. Dircksen, Akroterion, 2011, 56, 75–90 Search PubMed.
- S. Bunimovitz and Z. Lederman, Antiquity, 2016, 90, 1552–1561 CrossRef.
- Z. Chovanec, S. Bunimovitz and Z. Lederman, Mediterr. Archaeol. Archaeom., 2015, 15, 175–189 Search PubMed.
- A. Dittbrenner, H. Mock, A. Borner and U. Lohwasser, J. Appl. Bot. Food Qual., 2009, 82, 103–107 CAS.
- T. Reisine and G. Pasternak, in Goodman & Gilman's The Pharmacological Basis of of Therapeutics, ed. L. S. Goodman, A. Gilman and J. G. Hardman, McGraw Hill, 9th edn, 1995, pp. 521–555 Search PubMed.
- Z. Chovanec, S. Rafferty and S. Swiny, Ethnoarchaeology, 2012, 4, 5–36 CrossRef.
- K. Koschel, Egypt and the Levant, 1996, 6, 159–166 Search PubMed.
- N. G. Bisset, J. G. Bruhn and M. H. Zenk, Egypt and the Levant, 1996, 6, 203–204 Search PubMed.
- M. Serpico and R. White, in Ancient Egyptian Materials and Technology, ed. P. T. Nicholson and I. Shaw, Cambridge University Press, 2000, pp. 390–429 Search PubMed.
- M. P. Columbini, F. Modugno and E. Ribechini, in Organic Mass Spectrometry in Art and Archaeology, ed. M. P. Columbini and F. Modugno, John Wiley & Sons Ltd., 2009, pp. 191–215 Search PubMed.
- M. Regert, H. A. Bland, S. N. Dudd, P. F. van Bergen and R. P. Evershed, Proc. R. Soc. B, 1998, 265, 2027–2032 CrossRef CAS.
- M. S. Copley, H. A. Bland, P. Rose, M. Horton and R. P. Evershed, Analyst, 2005, 130, 860–871 RSC.
- K. Romanus, W. Van Neer, E. Marinova, K. Verbeke, A. Luypaerts, S. Accardo, I. Hermans, P. Jacobs, D. De Vos and M. Waelkens, Anal. Bioanal. Chem., 2008, 390, 783–793 CrossRef CAS PubMed.
- J. S. Mills, Stud. Conserv., 1966, 11, 92–107 CAS.
- R. P. Evershed, S. N. Dudd, M. S. Copley, R. Berstan, A. W. Stott, H. R. Mottram, S. A. Buckley and Z. Crossman, Acc. Chem. Res., 2002, 35, 660–668 CrossRef CAS PubMed.
- J. Bleton and A. Tchapla, in Organic Mass Spectrometry in Art and Archaeology, ed. M. P. Columbini and F. Modugno, John Wiley & Sons Ltd., 2009, pp. 261–302 Search PubMed.
- N. Gallois, J. Templier and S. Derenne, J. Anal. Appl. Pyrolysis, 2007, 80, 216–230 CrossRef CAS.
- Y. Keheyan and L. Giulianelli, e-Preserv. Sci., 2006, 3, 5–10 CAS.
- G. Decker, G. Wanner, M. H. Zenk and F. Lottspeich, Electrophoresis, 2000, 21, 3500–3516 CrossRef CAS PubMed.
- Q. Guo, J. Zhang, S. Zhao and B. Shao, Food Anal. Method., 2012, 6, 698–704 CrossRef.
- B. Paul, C. Dreka, E. Knight and M. Smith, Planta Med., 1996, 62, 544–547 CrossRef CAS PubMed.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the ‘Gold Book’), Blackwell Scientific Publications, Oxford, 1997. XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. DOI: 10.1351/goldbook Search PubMed.
- R. S. Merrillees, in Studies in Mediterranean Archaeology, ed. P. Åström, Lund, 1968, vol. 18 Search PubMed.
- R. P. Evershed, World Archaeol., 2008, 40, 26–47 CrossRef.
- R. P. Evershed, World Archaeol., 1993, 25, 74–93 CrossRef CAS PubMed.
- Z. Peng, W. Song, F. Han, H. Chen, M. Zhu and Y. Chen, Int. J. Mass Spectrom., 2007, 266, 114–121 CrossRef CAS.
- K. Taylor and S. Elliott, Forensic Sci. Int., 2009, 187, 34–41 CrossRef CAS PubMed.
- R. Kikura-Hanajiri, N. Kaniwa, M. Ishibashi, Y. Makino and S. Kojima, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2003, 789, 139–150 CrossRef CAS.
- P. Gu, Y. Ding, D. Sun, T. Hang, W. Liu and L. Ding, Biomed. Chromatogr., 2010, 24, 420–425 CAS.
- C. R. Mallet, J. R. Mazzeo and U. Neue, Rapid Commun. Mass Spectrom., 2001, 15, 1075–1083 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and additional experimental data.pdf. See DOI: 10.1039/c8an01040d. Raw data available. See DOI: 10.15124/80e04790-32d8-4dab-be3b-fa85ae5d7181 |
| This journal is © The Royal Society of Chemistry 2018 |