Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases
Ruptanu
Banerjee
 and
Amit
Jaiswal
and
Amit
Jaiswal
 *
*
School of Basic Sciences, Indian Institute of Technology Mandi, Kamand, Mandi-175005, Himachal Pradesh, India. E-mail: j.amit@iitmandi.ac.in
First published on 20th March 2018
Abstract
Lateral flow immunoassay (LFIA) technology is a paper-based, point-of-care strip biosensor designed to detect a specific analyte in a given sample. This type of assay is now of great interest to researchers for its cost-effectiveness, simplicity, portability and rapidness of detection of analytes, including but not limited to areas such as agriculture, food, biomedicine and pathogen detection. Various nanoparticles (such as metal nanoparticles, carbon-based nanoparticles, quantum dots, lanthanides and up-converting phosphor) functionalized by an antibody to detect an analyte protein or molecular marker present in the surface of an infectious pathogen are used for in LFIAs. Herein, we review the principle of the assay and recent advancements made in terms of the different approaches and designs of the assay towards the detection of infectious agents and diseases.
1. Introduction
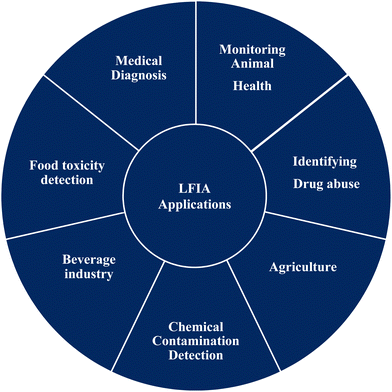
Lateral flow (immuno) assay (LFIA) is the technology behind many detection devices for the low-cost, fast and simple detection of an analyte of interest in a sample of a complex mixture, where the sample is placed on the test device and the results are displayed within few minutes. Originally, this technique was called the “sol particle immunoassay”, as it was first introduced by Leuvering et al. in 1980;1 however, nowadays it is more commonly known as LFIA. In recent years, LFIA-based diagnostic devices have been widely studied because of the low development cost and vast range of applications across multiple areas where rapid detection is a requisite. Another major advantage of LFIA-based tests is that they can be performed on a variety of biological samples, including plasma,2 sweat,3,4 saliva,5,6 serum,7,8 urine9,10 and whole blood,11,12 and the quantity of sample required for detection is much less than that needed with conventional assays.Since its development, LFIA has achieved penetration in a broad spectrum of markets. Fig. 1 depicts the application in various industries where LFIAs are already in production or are known to be in the development stages.13 Amongst the different applications of LFIA-based tools, one of the major areas is detecting infectious diseases. Over the year, various detection methods have been implemented for the fast, correct and low-cost detection of infection-causing pathogens or molecular markers associated with them, such as antigens or proteins.14 LFIA allows a wide range of qualitative, semi-quantitative and quantitative detection of infectious diseases caused by pathogens with high specificity and selectivity.15 In general, disease diagnosis and its subsequent treatment or prevention heavily depend on highly skilled professionals. LFIA devices can bridge the gap between the production of highly sensitive and selective data for the diagnosis of disease and use by minimally skilled or trained personal to produce results in a very short time.
In a global scenario, nearly 9 million people die annually due to various infectious diseases (tuberculosis, gonorrhoeae, HIV/AIDS, hepatitis, dengue, chikungunya to name a few), which accounted for about 15.8% of all deaths in 2015.16 The diagnosis of infectious diseases is mostly laboratory based, time consuming (several days to months) and requires well-equipped technical facilities and highly trained technicians. It is clear that for infectious diseases, diagnostics play a valuable and critical role in the care of patients with the disease and for those at risk of developing them. Early diagnosis reduces the chances of death or the severity of the disease as there are various treatments available ranging from vaccination to antibacterial drug supplementation. In this scenario, point-of-care (POC) tests, like LFIA, emerge as a key protagonist in fulfilling the necessities of an early test that has a comparable confidence with laboratory-based diagnostic methods and also the advantages of being rapid, simple, low-cost and can be performed irrespective of the need for a laboratory set-up or a trained person for its operation.
The global risk of infectious diseases is very high and in the upcoming years developing countries will face a major threat of pandemics (Fig. 2).17 Over the past few decades, nanoparticle-based lateral flow biosensors have rapidly been developed to meet the diagnostic needs for detecting infectious diseases. In the present review, we emphasize the detection of infectious diseases with nanoparticle-based LFIA devices. Nanoparticles are used as a probe molecule and serve as the detection module of the LFIA device. Nanoparticles have become a material of choice for probes due to their robustness, size- and shape-dependent tuneable opto-electronic properties, rapid synthesis and ease of functionalization, biocompatibility, persistent stability, capability for visual signal interpretation with a high signal-to-noise ratio and low developmental cost. There are several interesting reviews available covering the fabrication of LFIA devices using various labels and their application towards biosensing.14,15,18–26 However, only one review, published in 2010 by Ngom et al., covered the application of lateral flow test strip devices for the detection of infectious agents and chemical contaminants.27 After this, several new strategies as well as improvements were implemented with new-age technology for the detection of biomarkers of infectious diseases. The present review aims to provide a comprehensive collection of all the upgrades and recent trends in the detection of infectious diseases using nanoparticle-based LFIA devices.
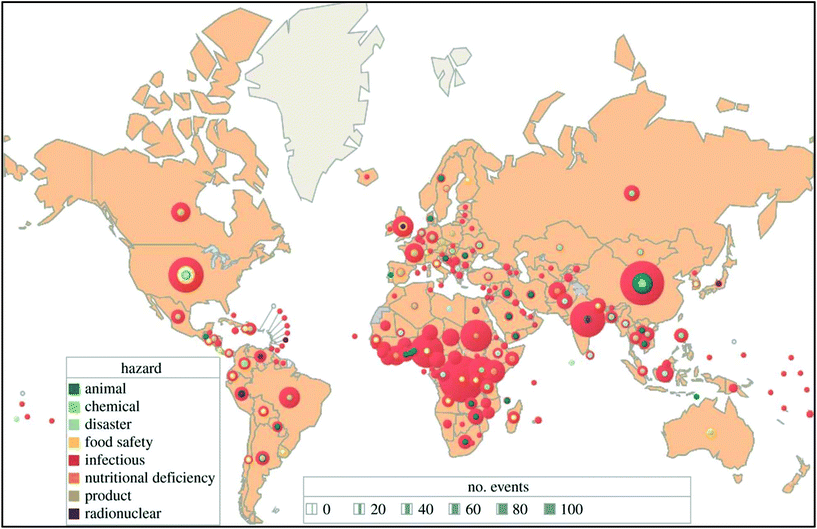 | ||
| Fig. 2 A total of 2797 international health hazards by type and country, January 2001–September 2013. Eighty-four percent were outbreaks of infectious diseases. Unpublished WHO data (2013). Reprinted (adapted) with permission from ref. 17. Copyright (2014) The Royal Society Publishing. | ||
2. Principle of the assay
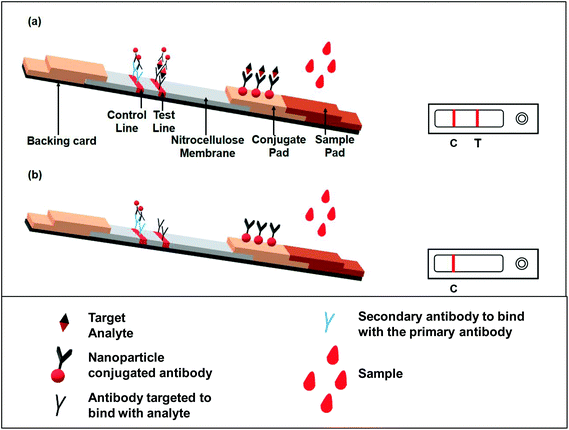
The principle behind the lateral flow assay is the movement of the liquid sample or the sample containing the analyte of interest along a strip (Fig. 3). The strip is made with polymeric materials and has specific zones or sites where molecules have been conjugated with a label. When the analyte-containing sample passes through these zones, it interacts with molecules designed to bind specifically to the analyte present in the sample.As shown in Fig. 3; a sample pad placed at one end of the strip helps to promote and evenly distribute the sample to its next component, the conjugate pad. The sample pad is generally made up of cellulose, cross-linked silica or glass fibres.15,26 The choice of material to produce a sample pad is based on the material's property to hold certain buffer solutions, proteins, surfactants, etc., while applying the test material to facilitate the flow upon addition of the buffer.28 The conjugate pad, which is generally made of cross-linked silica, is used to hold the probe particles and keeps them functionally stable and active. Additionally, the interaction between the nanoparticles and the sample pad should be weak in order to release the conjugated particle when encountering a moving fluid. This is accomplished by using a buffer or mixture of buffers typically containing carbohydrate molecules. These sugar molecules (like sucrose) stabilize the probe particles by forming a layer around them, thus shielding the particles from degradation.29 The probe particles are conjugated to an antibody or a molecule that specifically binds to the target analyte. In most cases, the probe used is a conjugated colloidal gold nanoparticle (AuNP). However, a few recent reports have also demonstrated the use of other nanoparticles, like silver,30 carbon,31 selenium32 or coloured latex particles,33 as a probe for LFIA devices. After crossing the conjugate pad, the flowing fluid, now also carrying the probe particles either free or bound to the target analyte (if present in the sample), comes into a membrane typically made of nitrocellulose. This membrane has two different zones or lines, namely the test and control zones or lines. Biological components, mainly antigens or antibodies, are immobilized to form these lines. The next step is the interaction between the target analyte and specific antibody coated in the test and control zones. The design of the assay is made in such a way that if the analyte of interest is present in the sample, it will be captured by the antibody present in the test zone. This antigen–antibody aggregation will result in developing a colour or band formation at the site of the test zone depending upon the type of label used (such as AuNPs).34 To conclude that the flow through the membrane has been properly established, the response in the control zone is observed. This is independent of the target analyte and it will always form the band even if there are no analyte molecules present in the given sample. This happens because of the interaction of the unloaded probe and the antibodies present in the control zone. A positive test is indicated by the development of bands at both the test and control regions, while band formation only in the control zone demonstrates a negative test (Fig. 3). A response either only in the test zone but not in the control zone or no response at all in any of the zones is indicative of an invalid result.21
There are two types of format exploited for the immunoassay: sandwich and competitive assays.18 The sandwich format is applicable for analytes with more than one epitope. In this case, one site of the target analyte binds with the antibody conjugated with the label molecule for e.g. AuNPs. This antibody–gold conjugated target analyte then flows through the membrane and comes into contact with another antibody specific to any other binding site of the target analyte in the test zone. The capture of the antibody–AuNP–antigen conjugate and antibody present in the test zone makes a pattern or line that can be visually verified or can be detected via optical/magnetic detectors based on the type of label used.
When the analyte is of low molecular weight and/or is a hapten, i.e. it has only one binding site or epitope, the competitive format is used. In this format, the signal is negatively correlated with the concentration of the analyte.35 The immobilized analyte conjugated with the antibody–AuNP complex is sprayed over the test line giving a strong response signal. When the analyte flows through the strip, it competitively binds with the antibody and thus releases the antibody–gold nanoparticle conjugate from the test line, and thus the signal gets reduced with increasing the concentration of the analyte.
Nanoparticles are widely used as label or probe molecules for detection in LFIA devices. Coloured or fluorescent nanoparticles in the range of 15–800 nm are ideal as their small size does not interfere with the flow or the path of the flow. As mentioned earlier, though AuNPs are a popular choice for the label, other nanoparticles have also been employed. Liposomes incorporated with bioluminescent dyes,36 quantum dots,37 up-converting phosphorus technology38 and surface-enhanced Raman scattering (SERS) reporters39–43 are some of the new approaches for achieving better sensitivity and quantitative analysis.
Surface functionalization is a key part of using nanoparticles as a label in LFIA systems. The major role of nanoparticles involved in the sensing of specific analytes in the LFIA platform is their ability to bind with biomolecules, such as antibodies. This conjugation is achieved by adsorption of the ligand moiety onto the surface of the nanoparticle. Though only weak interaction forces are involved in the conjugation process, this can degrade the antibody or the protein attached to it.44 Hence, the surface functionalization of nanoparticles is done prior to conjugation for stability of the conjugated system. Poly(ethylene glycol) (PEG) is the most commonly used polymer to coat the surface of nanoparticles. PEG modified with a thiol group (–SH) in one end, carboxyl group (–COOH) or amine group (–NH2) in another end is predominantly used as it can attach with the AuNPs surface via a thiol bridge and the antibody can attach at the other end via an aldehyde-carboxyl/amine interaction.44 Surface modification with carbohydrate molecules can also be done for potential biosensor and diagnosis applications.45 For a detailed overview on the surface functionalization of different nanoparticles, readers may refer to ref. 46–49, as this is beyond the scope of the present review.46–49
Another form of LFIA is the nucleic acid lateral flow immuno assay (NALFIA), which is used to detect the presence or absence of amplified ds-nucleic acid structure (amplicons) specific to the pathogenic organism. There are several formats reported for NALFIA: (1) ds-amplicon marked with two different tags at two strands, where one is biotin labelled and the other is a fluorescent tag. The avidin-coated nanoparticle binds to the biotin-labelled strand and the anti-tag antibody captures the other strand labelled with the tag and thus the signal produced is directly proportional to the amount of ds-amplicon present.50 (2) Nanoparticle-conjugated reporter probe and biotin-tagged capture probe for ss-amplicon detection. The capture probe is immobilized by avidin interaction and the amplicon binds with the probe to generate the signal.51 The capture probe can also be BSA tagged or can be simply immobilized in the strip through passive adsorption.
3. LFIA application strategies for diagnosing infectious diseases
In the following sections, we have divided the LFIA devices in to three broad categories based on the type of the disease: 1. pathogenic bacterial strains and toxins and 2. protozoal and 3. viral infections. AuNP is the most exploited nanoparticle reporter in the LFIA devices due to its stability, ease-of-synthesis, surface modification and functionalization for desired change in the physical and chemical properties, which leads to its optimum function as a reporter molecule as needed for the device. Besides AuNPs, carbon nanoparticles (CNPs), quantum dots (QDs), up-converting phosphor (UCP) and other nanomaterials are also being used for the detection of infectious agents through LFIA. A brief discussion on all the above categories is represented in Tables 1 and 2 with a focus on the reported works with promising results along with some enhancement or modification over the existing techniques for improved sensitivity and detection limits.| Bacterial analyte | Sample used for detection | Type of label | LFIA principle | Sensitivity or LOD | Ref. |
|---|---|---|---|---|---|
| Escherichia coli O157 | Faecal samples from bovine and swine, raw beef, pork | AuNPs | 40 nm AuNP-conjugated Murine monoclonal antibody (mAb) to E. coli O157:H7 lipopolysaccharide (LPS) | 1.8 × 105 CFU mL−1 without enrichment and 1.8 CFU mL−1 after enrichment | 52 |
| Specificity: pork (98.8%), bovine faeces (87.9%) and swine faeces (93.4%) | |||||
| Escherichia coli O157:H7 | Milk powder, flour, starch, coffee, biscuit, cake, jelly and juice | AuNPs | Colloidal-gold-conjugated double antibody sandwich assay | 1 × 105 CFU mL−1 with no cross-reaction with 30 strains of 24 species in Enterobacteriaceae (including non-O157 E. coli, Salmonella, Shigella, Proteus, Citrobacter, Enterobacter, Serratia and Yersinia), Staphylococus, Listeria, Aeromonas and Vibrios | 53 |
| Spiked milk, purified water and beef | AuNPs | Colloidal gold conjugated with E. coli O157:H7 mAbs. Immunomagnetic separation (IMS) is done by immunomagnetic particle (IMP) conjugated with E. coli O157:H7 polyclonal antibodies (pAb) | Without IMP 105 CFU ml−1 | 54 | |
| With IMP 103 CFU ml−1 | |||||
| Sensitivity: 95.5% | |||||
| Escherichia coli O157:H7 | Culture broth | Fluorescent microsphere | Fluorescein isothiocyanate fluorescent microspheres conjugated with E. coli O157:H7 mAbs | 1.6 × 103 CFU mL−1 | 55 |
| Escherichia coli O157 | Culture broth | AuNPs | AuNP conjugated with antibodies against E. coli O157 and S. typhimurium | E. coli O157: 105 CFU mL−1 | 56 |
| Salmonella typhimurium | S. typhimurium: 106 CFU mL−1 | ||||
| Escherichia coli | Standard buffer, bottled water and milk samples | Streptavidin-QD 655 (quenching by Graphene oxide (GO) in absence of pathogen (OFF state)) | Streptavidin-QD conjugated biotinylated antibody against E. coli followed by treatment with GO | 10 CFU mL−1 in standard buffer | 57 |
| 100 CFU mL−1 in bottled water and milk | |||||
| Salmonella enteritidis | Raw eggs | AuNPs | Anti-S. enteritidis antibodies conjugated to colloidal gold particles | 107 CFU mL−1 | 58 |
| No cross-reactivity with Salmonella typhimurium | |||||
| Bacteria spiked milk powder | AuNPs | 15 nm AuNP-conjugated DNA | 10 CFU mL−1 | 59 | |
| AuNPs | AuNP-conjugated 16s rDNA/rRNA | 5 fmol for synthetic DNA | 60 | ||
| Staphylococcal Enterotoxin B | Simulated samples of human urine, serum and cow milk powder | AuNPs | 25 nm AuNP conjugated with purified anti-SEB IgG | 1 ng mL−1 | 61 |
| With silver enrichment: 10 pg mL−1 | |||||
| Anthrax (Bacillus anthracis) | Milk, water samples | AuNPs | IMS of anthrax spores followed by lateral flow assay with AuNP as label | 5 × 105 CFU mL−1 | 62 |
| Bacillus anthracis | Culture media | Super-paramagnetic nanoparticles | mAb 12F6 conjugated to super-paramagnetic iron oxide particles | 400 pure B. anthracis spores | 63 |
| 4 × 103–106 CFU mL−1 | |||||
| Bacillus anthracis | Spiked samples of milk powder, starch and baking soda | Carboxylated super-paramagnetic iron oxide particles | mAbs 12F6 against B. anthracis conjugated with super-paramagnetic iron oxide particles | 500–700 pure B. anthracis spores | 64 |
| 6 × 104 spores per g in milk powder, 2 × 105 spores per g in starch and 5 × 105 spores per g in baking soda | |||||
| Enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC) | Supplemented stool sample | AuNPs | AuNP-conjugated anti-EspB IgG | 4 ng mL−1 | 65 |
| In stool sample: 0.06 μg mL−1 | |||||
| Sensitivity: 96.9% | |||||
| Campylobacter jejuni | Faecal extract | AuNPs | mAb (4B4) against a 15 kDa cell surface antigen-colloidal gold conjugate | 1.8 × 104 to 8.2 × 104 CFU mL−1 | 66 |
| Sensitivity – 84.8% | |||||
| Aflatoxin B1 (AFB1) | Culture media | GO or carboxylated GO (GO-COOH) | Anti-AFB1 mAb labelled with GO or GO-COOH | Visual LOD: 0.3 ng mL−1 | 67 |
| Aflatoxin B2 (AFB2) | Culture media | Magnetic nanogold microspheres with nano-Fe2O3 particles as core and AuNPs as shell | Monoclonal anti-AFB2 antibody (clone 3A7) conjugated Nano-Fe2O3 particles | 27 μg kg−1 | 68 |
| Aflatoxin M1 (AFM1) | Spiked raw milk | Immuno magnetic nanobeads (MNB) | MNBs conjugated with anti-AFM1 antibody | 0.1 ng ml−1 in raw milk | 69 |
| Aflatoxin M1 (AFM1) | Raw milk samples | Immuno MNB | Anti-AFM1 mAb conjugated MNBs | 0.02 μg L−1 | 70 |
| Fumonisin mycotoxins | Maize flour sample | CdSe/ZnS QDs | BSA tagged fumonisin antigen conjugated with QD (fluorescence quenching by AuNP-conjugated anti-fumonisin antibody) | Visual LOD: 1.56–6.25 ng ml−1 | 71 |
| Botulinum (Clostridium botulinum) | Spiked faecal sample | AuNPs | AuNP-conjugated monoclonal Botulinum Neurotoxin Type D (BoNT/D) antibody | 50 pg ml−1 | 72 |
| Spiked samples of serum, urine and cooked meat broth | AuNPs | AuNP-conjugated anti-BoNT/A heavy chain mAb (44.1) | 50 ng ml−1 | 73 | |
| With silver enhancement: 1 ng ml−1 | |||||
| Spiked milk sample, orange and apple juice | AuNPs | AuNP conjugated with two different mAbs: F1-51 (BoNT/A HC-specific) and BoB-92-32 (BoNT/B HC-specific) | In 2% and 1% milk | 74 | |
| BoNT/A: 5 ng ml−1 | |||||
| BoNT/B: 10 ng ml−1 | |||||
| In diluted apple juice BoNT/A: 25 ng ml−1 | |||||
| BoNT/B: 10 ng ml−1 | |||||
| No cross-reactivity between BoNT/A and BoNT/B | |||||
| Staphylococcus aureus | Pork, raw chicken, crab meat and inoculated beef, fried chicken, corn flake, cooked rice, noodle | AuNPs | Anti-protein A IgG was conjugated with AuNPs | Sensitivity: 100% with 28 S. aureus strains and 23 non-S. aureus strains | 75 |
| Naturally contaminated food samples | AuNPs | Anti-protein A IgG conjugated AuNP | Sensitivity: 100% | 76 | |
| Specificity: 96–100% with 44 non-S. aureus strains | |||||
| Respiratory samples | AuNPs | Monoclonal anti-S. aureus antibody conjugated 40 nm colloidal gold particles | 106 CFU ml−1 | 77 | |
| Staphylococcus enterotoxin B (SEB) | Spiked tap and surface water, apple juice, raw milk, ham extracts, cheese extracts | Sulforhodamine B (SRB)-encapsulating immunoliposomes | Dextran/BSA/rhodamine B isothiocyanate conjugated thiol derivatized anti-SEB antibody | 20 pg ml−1 (liner range: 0.02–5 ng ml−1) | 78 |
| Recombinant SEB | Hollow gold nanospheres (HGNs) with Malachite green isothiocyanate (MGITC) as Raman reporter (Raman-active probe) | Anti-SEB pAb | 0.001 ng ml−1 | 40 | |
| Stretococcus suis | Bacteria isolated from diseased piglets, healthy piglets and human patients | AuNPs | AuNP-conjugated antibody against SS2 (serotype-2) | 106 CFU ml−1 | 79 |
| Bacterial culture solution | AuNPs | Colloidal gold coated with pAbs against serotype 2 capsular polysaccharides (CPS) | 104 CFU ml−1 | 80 | |
| 0.05 μg of CPS | |||||
| Pneumolysin (Streptococcus pneumoniae) | Pneumolysin sample | Au@Ag with rhodamine as SERS reporter | Au@Ag with rhodamine as SERS reporter conjugated anti-pneumolysin antibody, PLY-7) | 1 pg mL−1 | 41 |
| Enterobacter cloacae | Simulated water sample | AuNPs | Colloidal AuNPs conjugated with monoclonal anti-E. cloacae antibody | 102 CFU ml−1 | 81 |
| Enterobacter sakazakii | Spiked infant formulae samples | CNP | Neutravidin labelled CNP to bind with biotin-tagged amplicon | <10 cells of Cronobacter sp. per 10 g | 82 |
| Leptospira sp. | Human sera | AuNPs | Leptospira specific antibody against broadly reactive leptospiral antigen and colloidal gold-labelled anti-human IgM antibody | Sensitivity: 85.8% | 83 |
| Specificity: 93.6% | |||||
| Culture media | AuNPs | Colloidal AuNPs conjugated mouse anti-fluorescein antibody to detect m-LAMP label based biotin/fluorescein labelled LipL32 gene amplicons | Specificity: 100% | 84 | |
| LOD: DNA concentration 3.95 × 10−1 genomic equivalent per ml | |||||
| Vibrio cholerae serogroup O1 and O139 | Culture media | AuNPs | Murine mAb against V. cholerae LPS-conjugated AuNPs | Sensitivity: 93–100% (O1), 100% (O139) | 85 |
| Specificity: 100% (O1), 98–100% (O139) | |||||
| 108 and 107 CFU ml−1 for O1 and O139, respectively | |||||
| Vibrio cholerae O139 | Seafood samples | AuNPs | VC-273 Mab conjugated to AuNPs | 104 CFU ml−1 | 86 |
| 1 CFU ml−1 with pre-incubation of sample with in alkaline peptone water for 12 hours | |||||
| Vibrio parahaemolyticus | Culture media grown bacterial antigen | CdTe QDs | pAb against V. parahaemolyticus conjugated with CdTe QDs; quenching by mAb labelled AuNPs | 5.03 × 104 cfu L−1 | 87 |
| Treponema pallidum (Syphilis) | Serum samples from 8 geographically diverse sites | AuNPs | 9 Commercially available rapid syphilis tests | Sensitivity: 84.5–97.7% | 88 |
| Selectivity: 84.5–98% | |||||
| Mycobacterium tuberculosis Tuberculosis (TB) | Whole blood and serum spiked with M. tuberculosis antigen | AuNPs | 40 nm AuNP conjugated with protein-A | 5 ng ml−1 | 89 |
| Listeria monocytogenes | Bacterial culture | AuNPs | AuNP-conjugated nucleotides to detect hlyA mRNA | 0.5 pg μl−1 of genomic RNA | 90 |
| 20 CFU ml−1 | |||||
| Listeria monocytogenes | Spiked milk samples and other food samples (including salad, frozen vegetables etc.) | CNP | CNP-neutravidin conjugate to bind to biotin modified aptamer | 0.1 ng of labelled amplicon 50 pg of L. monocytogenes template DNA (approximately 105 cells) | 91 |
| Helicobacter pylori | Human faecal sample | AuNPs | Mixture of DF2a and IH10b mAb conjugated AuNP | 104 CFU mL−1 | 92 |
| Cronobacter spp. | Pure culture and powdered infant formula (PIF) | AuNPs | Biotin-labelled 16s ribosomal DNA of Cronobacter sp. detected using streptavidin linked AuNPs | 107 CFU mL−1 | 93 |
| Salmonella typhimurium | Bacterial culture derived antigen | Liposome entrapped Methylene Blue (MB) | Thiol derivative of anti-Salmonella antibody conjugated with maleimide-derivatized liposomes containing MB | 1680 cells | 94 |
| Salmonella choleraesuis | Pure culture and spiked whole milk samples | Gold magnetic bifunctional nanobeads (GMBN) | Anti-S. choleraesuis mAbs (11D8-D4) conjugated GMBN | Sensitivity: 5 × 105 CFU mL−1 | 95 |
| Brucella sp. | Bacterial culture | UCP | pAb against B. melitensis M55009 covalently coupled with UCP particles | 2.0 × 103 to 3.9 × 105 CFU mg−1 | 96 |
| Pantoea stewartii subsp. stewartii | Spiked corn seed samples | Lanthanide (Europium chelate-coated silica nanoparticles) | Anti-Pss antibody conjugated Europium chelate-coated silica nanoparticles | 104 CFU mL−1 | 97 |
| Y. pestis and B. pseudomallei | Bacterial culture | UCP | mAb against Y. pestis and B. pseudomallei conjugated UCP | Sensitivity: 103 CFU per test | 98 |
| Pseudomonas aeruginosa and Staphylococcus aureus | Bacterial culture | AuNPs | AuNP-conjugated pAb against P. aeruginosa and S. aureus | 500–5000 CFU mL−1 | 99 |
| Specificity: 100% | |||||
| E. coli O157:H7 and Salmonella typhimurium | Recovered bacteria from contaminated lettuce | AuNP | AuNP-conjugated anti-S. aureus and anti-E. coli O157 antibody | E. coli O157:H7: 1.87 × 104 CFU | 100 |
| S. typhimurium: 1.47 × 104 CFU | |||||
| After enrichment | |||||
| E. coli O157:H7: 1 CFU | |||||
| S. typhimurium: 1 CFU |
| LFIA-based diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Disease condition | Causing agent | Laboratory diagnosis | Sensitivity or LOD/drawback | Type of label | Analyte | Label used | Sensitivity or LOD | Ref. |
| Protozoal | ||||||||
| 1. Malaria | Plasmodium falciparum (major) | Laboratory-based thin blood smear microscopy | Parasitemias of 50 parasites per μL | AuNP | Plasmodium lactate dehydrogenase (pLDH) | Colloidal AuNP-labelled pan-specific-anti-pLDH-mAbs | Parasitemias of ≥100 parasites per μL | 120 |
| Other Plasmodium species | ||||||||
| AuNP | Plasmodium falciparum histidine-rich protein 2 (pfHRP-II) | Thermally responsive magnetic nanoparticle (MNP) | 10 ng mL−1 of pfHRP-II | 122 | ||||
| AuNP | pLDH | AuNP-conjugated anti-pLDH with QR barcodes linked with Google Analytics | 10 ng mL−1 of recombinant pLDH | 123 | ||||
| AuNP | Micellar Aqueous Two-Phase System (ATPS) with anti-pLDH conjugated AuNP | 1 ng μL−1 | 124 | |||||
| CNP | Biotin-labelled 18s RNA of Plasmodium sp. | Neutravidin labelled CNP to bind with biotin-tagged amplicon | 0.3 to 3 parasites per μL | 31 | ||||
| Leishmaniasis | Leishmania infantum | Skin biopsies, ELISA, Immunofluorescence | Sensitive but time consuming and difficult to operate | AuNP | Leishmania infantum kinetoplast DNA | 20 nm AuNP-conjugated pAb to recognize anti-FITC antibodies. | 0.038 parasites (1 parasite per 100 μL of DNA sample) | 125 |
| Schistosomiasis | Schistosoma sp. | Eggs identification in stool, ELISA, microscopy | Time consuming and highly expertise technician and facilities are must | Up-converting phosphor (UCP) | Schistosoma Circulating Anodic Antigen (CAA) | Mouse monoclonal anti-CAA antibody 147 coupled to 400 nm UCP reporter particles | 0.5 pg mL−1 | 126 |
| S. haematobium | UCP | Schistosoma CAA | Mouse monoclonal anti-CAA antibody 147-3G4 coupled to 400 nm UCP reporter particles | 30 pg CAA per mL serum; (equivalent to about 10 worm pairs) | 127 | |||
| Viral | ||||||||
| Influenza | Influenza virus with different subtypes | HI assay, seroconversion detection between acute- and convalescent-stage samples | HI assay: 92% | AuNP | Hemagglutinin (HA) | AuNP-labelled Mab against HA | 0.25 HA units | 128 and 129 |
| AuNP | M gene, H1, H3. H5 gene | Streptavidin-coated AuNPs linked with the biotin moieties incorporated in the middle of the DNA strands | 102 copies | 130 | ||||
| AuNP | AuNPs to capture biotin-labelled reverse-transcription loop-mediated isothermal amplification (RT-LAMP) | 10 copies | 131 | |||||
| AuNP | Flu A antigen, Flu B antigen | Silver growth on AuNP-conjugated anti-Flu A | Sensitivity and specificity: Flu A – 91.2% and 95.8% | 132 | ||||
| Flu B –94.4% and 98%; respectively | ||||||||
| AuNP | Nucleoprotein and matrix protein | Anti-nucleoprotein (NP4) MAb and anti-matrix protein (M1) MAb conjugated AuNP (15 nm) | 47 TCID50 per mL | 133 | ||||
| AuNP | HA | Anti-HA (H3, H5, H7, H9) antibody conjugated AuNP to detect biotin-tagged aptamer captured specific virus | 2 × 106 virus particles | 134 | ||||
| Cy5-doped silica nanoparticles | Influenza A | mAb against influenza A nucleoprotein conjugated Silica nanoparticles | 250 ng mL−1 | 135 | ||||
| Hepatitis | Hepatitis virus type-A, B, C, E (HAV, HBV, HCV, HEV) | Liver biopsy | Time consuming with need of skilled technician and well-equipped laboratory | AuNP | HBsAg, HBeAg | Colloidal-gold-labelled antibody against HBsAg | HBsAg – 95% | 136 |
| HBeAg – 80% | ||||||||
| AuNP | HEV IgM | Anti-human-IgM MAb, colloidal-gold-labelled antibody to HEV preincubated with HEV antigen | 93% | 137 | ||||
| AuNP | IgM antibody specific to HEV | Colloidal gold-labelled anti-HEV mAb 4B2 and recombinant protein EP2.1 | 96.7% | 138 | ||||
| Fluorescent lanthanide | HCV | Eu3+-chelate conjugated recombinant Multiepitope protein (MEP) | Sensitivity: Secondary antibody assay – 95.6% | 139 | ||||
| Double-antigen assay – 91.4% | ||||||||
| UCP | Hepatitis B surface antigen (HBsAg) | HBsAg conjugated UCP | 60 mIU mL−1 | 140 | ||||
| ZnSe/CdSe/CdS/CdxZn1−xS/ZnS QDs | HBsAg | HBsAg conjugated QDs | 0.05 ng mL−1 | 141 | ||||
| Carboxyl-modified CdSe/ZnS QDs | HBsAg | Anti-HBsAg mAb-labelled QB | 75 pg mL−1 | 142 | ||||
| AIDS | HIV | HIV ELISA, HIV viral load test, quantitative PCR | 95–100% (∼1 ng mL−1) | AuNP | HIV-1 p24 antigen | Anti-HIV-1 p24 antibody-selenium colloid based sandwich format with monoclonal biotinylated anti-HIV-1 p24 antibody | 25 pg mL−1 | 143 |
| AuNP | HIV-1 p24 antigen | Mouse mAb conjugated to super-paramagnetic particles (200–300 nm) | 30 pg mL−1 | 144 | ||||
| AuNP | HIV-1 RNA | AuNP-labelled probe (partially complementary strand) | 10.5–13log10 copies per mL (50 copies of gag RNA) | 145 and 146 | ||||
| CNP | p24 antigen | Mouse monoclonal anti-p24 mAb108 coupled to CNP | LOD: 50 pg mL−1 Sensitivity: 90% Specificity: 100% | 147 | ||||
| Platinum core–shell nanocatalyst (PtNC) | p24 antigen | Anti-HIV-1/2 conjugated PtNCs | 0.8 pg mL−1 | 148 | ||||
| AuNP | HIV-1 nucleic acid sequence | AuNP-conjugated amplification probe and AuNP-conjugated complementary and detector probe | 0.1 nM | 149 | ||||
| Surface-modified AuNP by MGITC as a Raman reporter (Raman-active probe) | HIV-1 nucleic acid sequence | Raman-active probe-conjugated detection DNA sequence | 0.24 pg mL−1 | 150 | ||||
| Adenoviral diseases | Adenovirus | Virus isolation from tissue culture | 100% (takes 3–4 weeks) | AuNP | Adenovirus capsid hexon (signal antibody) | Gold colloid-conjugated mouse mAb to adenovirus capsid hexon | 72.6% (within 10 minutes) | 151 |
| AuNP | Recombinant HA of H5N1 | mAb 25/2 conjugated AuNP | 250 ng ml−1 without amplification | 152 | ||||
| 0.5 ng ml−1 with silver amplification | ||||||||
| AuNP (later dissolved, collected and GSH capped CdTe QDs added for fluorescent detection) | Avian influenza virus (AIV) | AuNP-conjugated AIV mAb | 0.09 ng ml−1 | 153 | ||||
| Sensitivity: 100% | ||||||||
| Specificity: 88.2% | ||||||||
| WNV infection (mostly asymptomatic) | Western Nile virus (Flaviviridae family) | WNV IgM/IgG capture (MAC) ELISA | 100% (takes 2–3 days) | AuNP | WNV antigen (protein E) | Colloidal gold particles conjugated flavivirus specific mAb (6B6C-1) | 95–100% agreement with results from CDC WNV IgM/IgG ELISA (within 15 minutes) | 154 and 155 |
| Dengue | Dengue virus (DENV) | Virus isolation, ELISA, RT-PCR | 1–10 infectious virus particles, 1–100 PFU ml−1 (PCR) (takes days to weeks) | AuNP | NS1 dengue antigen, anti-dengue IgM and IgG | Colloidal gold-labelled specific mAbs against dengue NS1 antigen, IgM, IgG, or IgA | 90–100% | 156–163 |
| AuNP | Dengue specific IgG | Streptavidin conjugated 40 nm AuNPs | — | 164 | ||||
| Chikungunya | Chikungunya virus (CHIKV) | Virus isolation, RT-PCR | Relative sensitivity 100% with PCR | AuNP | Chikungunya envelope proteins (E1, E2, E3), CHIKV specific IgM | Colloidal gold-conjugated anti-IgM antibody against CHIKV specific IgM and CHIKV recombinant envelope protein in test line | 90–100% (arguable as per multiple studies) | 165–169 |
| Herpes Simplex Virus (HSV) infection | HSV-2 | Virus isolation, RT-PCR, ELISA | Relative sensitivity 95–100% with ELISA | AuNP | HSV-2 specific IgG | Colloidal gold-conjugated anti-IgG antibody with gG1 and gG2 in pre-absorption line and test line respectively | Sensitivity: 100% | 170 |
| Specificity: 97.3% | ||||||||
| Newcastle disease | Newcastle disease Virus (NDV) | Hemagglutination inhibition (HI) assay, agar gel immunodiffusion (AGID), ELISA, RT-PCR | Highly sensitive and selective | AuNP | Purified NDV | 40 nm AuNP conjugated with anti-NDV-6C4 mAb | 2 × 10−12 M | 171 |
| Ebola | Ebola Virus (EBOV) | Whole antigen ELISA, virus isolation in cell culture | Sensitive with prolonged time consuming | AuNP | EBOV Glycoprotein (EBOV-GP1–649) | Anti-human IgG conjugated AuNP | Sensitivity: 100% | 172 |
| Selectivity: 98% | ||||||||
| Fe3O4 MNPs | EBOV glycoprotein (EBOV-GP) | Anti-EBOV-GP (4G7) conjugated MNPs | 1 ng ml−1 | 173 | ||||
| Norovirus | Norovirus | Microscopy, ELISA, RT-PCR | Reduced clinical specificity, expensive | Biotin-labelled anti-Norovirus antibodies linked with streptavidin–biotin conjugated AviTag M13 phage with HRP/anti-M13 antibody | Virus-like particles (VLPs) from GI.1 (first recognized Norovirus) | Biotin-labelled anti-Norovirus antibodies linked with streptavidin–biotin conjugated AviTag M13 phage with HRP/anti-M13 antibody | 107 VLPs per ml | 174 |
| Dengue, Zika | DENV and Zika virus (ZIKV) | PCR amplification of specific genes, virus isolation culture, ELISA | Hard to distinguish from each other based on isolation and ELISA, expensive, cross-reactivity with other viruses of Flaviviridae family | AuNS with 1,2-bis(4-pyridyl)ethylene (BPE) and 4-mercaptobenzoic acid (MBA) as Raman reporter | DENV-NS1 | Anti-DENV NS1 and anti-ZIKV NS1 conjugated gold nanostars with different Raman reporters | DENV – 7.67 ng ml−1 | 43 |
| ZIKV-NS1 | ZIKV – 0.72 ng ml−1 | |||||||
| AuNP | DENV-NS1 | Serotype-specific DENV nanoparticle-mAb conjugates and ZIKV nanoparticle-mAb conjugates | Sensitivity/specificity: 0.76 to 1.00 for DENV 1–4 and the pan-DENV; 0.81/0.86 for ZIKV respectively | 175 | ||||
| ZIKV-NS1 | ||||||||
| AIDS, Hepatitis C, Hepatitis A | HIV, HCV, HAV | ELISA, Viral Load Test, quantitative PCR, liver biopsy | Co-infection of HIV and HCV | AuNP | Proteinticles (P) PHIV-gp41, p24 | Protein-A conjugated AuNP | Sensitivity: 100% | 176 |
| PHCV-511p, c100p, c22p, and c33c | ||||||||
| PHAV- E1, E2, E3 | ||||||||
| Dengue, yellow fever, Zika | DENV, yellow fever virus (YFV), ZIKV | AgNP | DENV NS1 protein, yellow fever virus NS1 protein, and EBOV, Zaire strain glycoprotein GP | AgNP conjugated anti-dengue NS1, anti-YFV NS1, anti-EBOV-GP mAb | 150 ng ml−1 | 177 | ||
| Kaposi's sarcoma-associated herpesvirus (KSHV) and Bacillary Angiomatosis (BA) | HSV, Bartonella sp. | AuNP modified with MGITC as Raman reporter (Raman-active probe) | DNA sequences associated with KSHV and BA | Thiol-modified DNA conjugated SERS active AuNPs | KSHV: 0.043 pM and BA: 0.074 pM | 178 | ||
3.1. LFIA for detecting bacteria and bacterial infections
There are various bacterial toxins and food-borne infectious diseases where LFIA devices are used for rapid detection. Staphylococcal enterotoxin B (SEB) has been reported to be detected using 25 nm colloidal gold as a label with anti-SEB IgG, with a detection limit of 1 ng mL−1 in less than 5 minutes.61 An improvement over conventional SEB detection was reported by using fluorescent immunoliposomes, which allowed the detection of SEB close to 20 pg mL−1.78 SERS-based enhancement for the detection of SEB has shown a detection limit of 0.001 ng mL−1. Here, MGITC, a SERS reporter conjugated with gold nanospheres, was used as a label in the assay.40 Anthrax, a deadly infectious disease caused by Bacillus anthracis, has also been investigated through LFIA. Anthrax incidents in 2001 and its potential to be used as a weapon in bioterrorism led researchers to develop a method for the fast and sensitive detection of spores causing anthrax in food and feeds.101,102 For this, an additional step of IMS was performed prior to subjecting the sample to a LFIA device. This proved successful in sensing the spores of Bacillus anthracis in water and dairy products with a spore recovery of 95% from IMS62 with 105–107 CFU mL−1 of spores in the milk or water sample. IMS was achieved by using anti-spore antibodies conjugated with COOH-magnetic beads. A magnet was used to separate the complex, and formamide and EDTA were used to elute the spores, which were then used in the LFIA device (Fig. 4).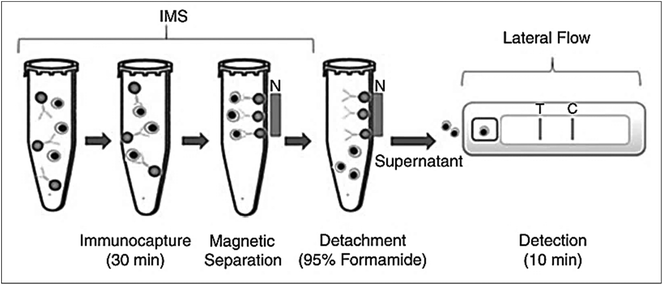 | ||
| Fig. 4 Principle of IMS and LFIA for the detection of Bacillus anthrax spores. Anti-spore antibodies coupled with COOH-magnetic beads are used for immunocapture. Magnetic separation is achieved by using magnetic field. The spores are recovered using formamide and EDTA and are used directly in the LFIA device. Reprinted (adapted) with permission from ref. 62. Copyright (2009) John Wiley and Sons. | ||
Human salmonellosis diagnosis has also been established by LFIA, which was designed to detect Salmonella enteritidis with a detection limit of 107 CFU mL−1 without any cross-reactivity with Salmonella typhimurium.58 As low as 10 CFU mL−1 of Salmonella enteritidis was detected by using aptamer-based strand-displacement amplification (SDA).59 In this process, the bacteria were first mixed with an excess amount of aptamers of two types that specifically bind to the bacteria: one of which is biotin labelled, while the other carries the sequence for SDA. Then, the streptavidin-labelled magnetic beads were used for magnetically separating the S. enteritidis bacterium from other species. These magnetic-bead–aptamer–bacteria complexes were then directly amplified by SDA, and the amplified product containing ssDNA was applied on a LFIA test strip and subsequently detected, as shown in Fig. 5. A Gram negative bacterial strain, Escherichia coli O157:H7, causes haemorrhagic colitis and hemolytic uremic syndrome in humans.103 A LFIA strip for the rapid detection of E. coli O157:H7 was developed by Jung et al. in 2005. This assay was based on murine mAb to the said antigen (E. coli O157:H7 LPS) conjugated with 40 nm colloidal AuNPs with a lowest detection limit of 1.8 × 105 CFU mL−1 without any enrichment.52 A later study reproduced a similar detection limit, namely 1 × 105 CFU mL−1, in various food samples, namely milk powder, flour, etc.53 The immunomagnetic enrichment technique further increased the sensitivity for the detection of E. coli O157:H7 with a detection limit of 103 CFU mL−1 with 95.5% sensitivity and 100% specificity.54 Another study reported aptamer-mediated magnetic enrichment as well as a strand-displacement amplification method for the detection of E. coli 0157:H7 with a detection limit of 10 CFU mL−1.104 In this study, they used two different aptamers that selectively bind to two different outer membrane proteins of E. coli. One of the aptamers were biotin labelled and were magnetically enriched by streptavidin-labelled magnetic beads separation. The other aptamer was used for amplification and subsequently targeted for detection via NALFIA. In this context, carbon-based nanoparticles and nanomaterials have also been exploited as labels in LFIA devices. Carbon-based nanoparticles are preferred for their very low production cost, high contrast signal and ease of scale-up reactions.19 The fluorescence quenching of QDs by GO 2D nanosheets as a mechanism for detection has been used to detect as low as 100 CFU mL−1 of E. coli in bottled water and milk samples.57 The detection mechanism is described in Fig. 6. When the analyte is not present in the sample, GO can effectively quench the fluorescence of the QDs (OFF mode). In the presence of the target analyte, this quenching is hindered and the fluorescence signal is detected (ON mode). The detection of EPEC and EHEC by using AuNP-based LFIA was also reported with a specificity and sensitivity of 100% and 96.9%, respectively.65 In-solution hybridization for the detection of specific sequences of 16S ribosomal RNA allowed the detection of 5 × 104 CFU mL−1 of E. coli within 25 minutes (Fig. 7).105Campylobacter species are reported as the causative agent for human bacterial gastroenteritis in many industrialized countries.106 A 15 kDa cell surface protein of Campylobacter jejuni has been targeted for the rapid detection by LFIA from human faecal extract. Within 15 minutes, a specificity of 100% and a sensitivity of 84.8% was achieved with the designed LFIA device.66
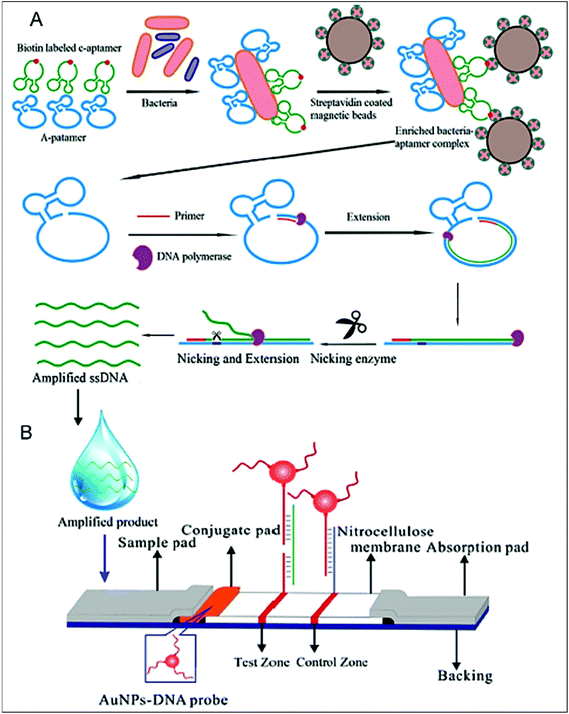 | ||
| Fig. 5 Schematic design of the aptamer-based detection of Salmonella enteritidis, A. magnetic separation using a streptavidin-coated magnetic bead. The biotin-labelled aptamer bound bacteria is detected by streptavidin interaction and magnetically separated, followed by strand-displacement amplification leading to the production of an amplified sample containing ss DNA, B. the LFIA strip containing the ss DNA-AuNP probe for detection. Reprinted (adapted) with permission from ref. 59. Copyright (2014) Elsevier. | ||
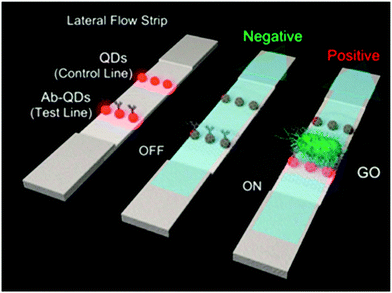 | ||
| Fig. 6 Schematic representation of the working mechanism of GO quenching of fluorescence in the absence (OFF mode) and presence (ON mode) of bacteria. Reprinted (adapted) with permission from ref. 57. Copyright (2015) American Chemical Society. | ||
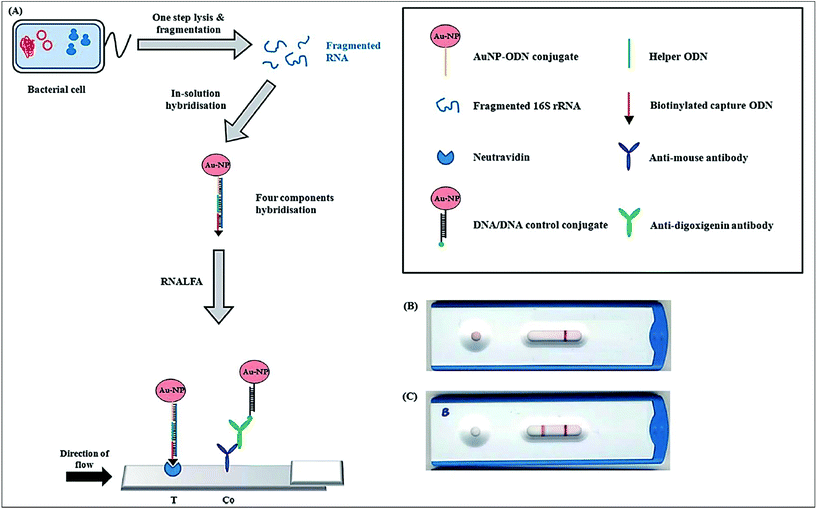 | ||
| Fig. 7 Principle of the ribonucleicacid lateral flow assay (RNALFIA); A. lysis and subsequent fragmentation of E. coli cells produces fragmented RNA, which is required for the four component hybridization with the AuNP-oligodinucleotide (ODN) conjugate, biotinylated capture ODN and a helper ODN. This hybridized assembly is then applied on the lateral flow strip with neutravidin immobilized at the test line and anti-mouse antibody at the control line. Reprinted (adapted) with permission from ref. 105. Copyright (2014) Royal Society of Chemistry. | ||
There are various enterobacteriaceae family members (like Shigella sp. and Aeromonas sp.), for which their detection carried out by LFIA devices. The detailed description of these LFIA device can be found in ref. 107. Enterobacteriaceae family members are known for causing various water-borne diseases. They produce various endotoxins and exotoxins that cause the pathogenesis of the infection by interacting with the digestive juices, resulting in enormous water loss from the body. Botulism is a fatal infectious disease caused by a neurotoxin botulinum (BoNT) produced by Clostridium botulinum and is capable of causing paralytic illness in human when found even in a very small amount in food.108 The toxin is classified in 7 antigenic types (A–G) based on their absence of cross-neutralization, e.g. anti-G antitoxin does not neutralize toxin types A–F.109 The detection of BoNT/D by sandwich format LFIA has been established using AuNPs as a label with a detection limit of 50 pg mL−1.72 A similar strategy was used for BoNT/A detection, where mAb against BoNT/A conjugated with AuNP was used. This detection showed no cross-reactivity with BoNT/B or BoNT/E when a silver enhancement reagent was used to amplify the signal generated from colloidal gold. The detection limit for this LFIA strip was found to be 1 ng mL−1.73 The simultaneous detection of BoNT/A and BoNT/B was reported by using separate mAbs with a high affinity and specificity for the two mentioned serotypes.74 Another bacterium, Staphylococcus aureus, is the leading cause of food poisoning, especially in processed and canned foods.110 LFIA based on a sandwich format was developed for detecting S. aureus in processed food (pork, beef, fried chicken).75 The sensitivity of the immunochromatography test was found to be 100% when tested with 28 S. aureus and 23 non-S. aureus strains. Stretococcus suis has been identified as a causative agent of meningitis and endocarditis in humans.111,112 A LFIA strip based on colloidal AuNPs has been developed against serotype 2, which is the most common and pathogenic both to humans and pigs.79 The sensitivity of the immunochromatography strip was found to be 106 CFU mL−1. A recent breakthrough in detecting Enterobacter cloacae was achieved by using an IMS of E. cloacae from a sample and then detecting it with monoclonal anti-E. cloacae antibody conjugated with colloidal AuNPs.81E. cloacae can induce various pathogenic conditions including respiratory tract infection, urinary system infection, skin and soft tissue infection and septicaemia.113,114 The LFIA test strip showed 102 CFU mL−1 of E. cloacae detection with IMS, which showed a 10 times lower LOD than that of the direct detection. mAb against Vibrio cholerae subtype O1 and O139 was used with colloidal gold for the multiplexed detection of the subtypes of V. cholerae. The detection limit of the device was found to be 108–107 CFU mL−1.85 There are several toxins (like mycotoxins and aflatoxins) produced by various bacteria or fungi, and some specific types of bacterial infection (like Listeria sp. infection) are extensively described in other reviews.23,115–119
3.2 LFIA for detecting protozoal infection
The nanoparticle-labelled simple lateral flow-based tool can identify labels of a whole pathogen. For example, commercially available rapid test strips for malaria detection are capable of detecting P. falciparum and other Plasmodium species at levels of ≤500 parasites per μL and ≤5000 parasites per μL, respectively. Also, as per WHO guidelines, some rapid test strips are available for use in the market (CareStart™ Malaria test, OptiMAL-IT™) that can detect Plasmodium very efficiently with parasitemias of ≥100 parasites per μL.120 In other standard rapid diagnostic tests, P. falciparum dehydrogenase and Histidine-rich Protein 2 (pfHRP II) are used as species-specific markers for P. falciparum.180 OptiMAL-IT's™ design is based on the detection of pLDH (plasmodium lactate dehydrogenase) using pan-specific-anti-pLDH-mAbs labelled with AuNP.181 NTA-functionalized gold and AgNP has recently been exploited for the detection of pfHRP II mimics, specifically without any cross-reactivity with human serum proteins. NTA is a chelating agent that has a high affinity towards histidine (the dissociation constant (Kd) value is in the micromolar range182). At a low pH, NTA-NPs do not show aggregation with human serum proteins, which enables further development to detect pfHRP-II in human serum or saliva.121 On the other hand, nucleic-acid-based lateral flow assay for detecting malaria is also widely studied.180 A recombinase polymerase amplification (RPA) strategy has also been used to design a LFIA device. The RPA amplicon was labelled for the detection by a tag-specific antibody in the control and test lines. The conjugate pad contained AuNPs for visualizing colour formation (Fig. 8). This gave a high species-specific result with high sensitivity and specificity (100%). The detection limit was found to be 100 fg of genomic P. falciparum DNA (equivalent to a sensitivity of 4 parasites per reaction).179 Thermally responsive MNP is also used in studies to concentrate and aggregate a greater amount of pfHRP II as a pre-step before putting the sample on the strip (Fig. 9). Here, the sample was treated with a biotinylated antibody, which formed aggregation when treated with heat after the addition of streptavidin-pNIPAm-AuNP, pNIPAm MNP and free pNIPAm polymer. The sample was then subjected to a magnetic field for separating out the mixed AuNP/MNP aggregates and was then re-dissolved in a cold buffer for enrichment.122 10 ng mL−1 of pfHRP-II detection was achieved using this method of enrichment. A very recent report on pLDH detection using a LFIA platform was successfully implemented on the Google Analytics platform by using a QR barcode for positive, negative and invalid results from the device (Fig. 10). The cross-platform data acquisition could store a very large amount of data from the device and was readable by a smartphone.123 The QR codes were initially set as “Blank” with both the QR codes incomplete (deactivated). Whether the test result is positive, negative or invalid, it can produce three different types of QR code, which can be scanned using a smartphone camera to obtain data about the test.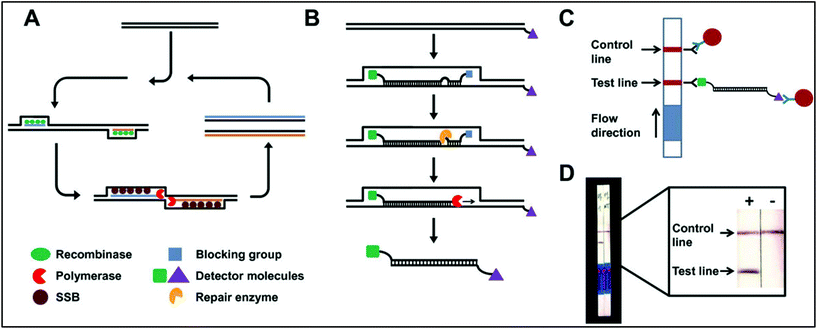 | ||
| Fig. 8 Schematic representation of an RPA-mediated LFIA device. A. Reaction principle, B. modified-probe labelling, C. detection mechanism, D. observation of positive and negative results. Reprinted (adapted) with permission from ref. 179. Copyright (2014) BioMed Central Limited. | ||
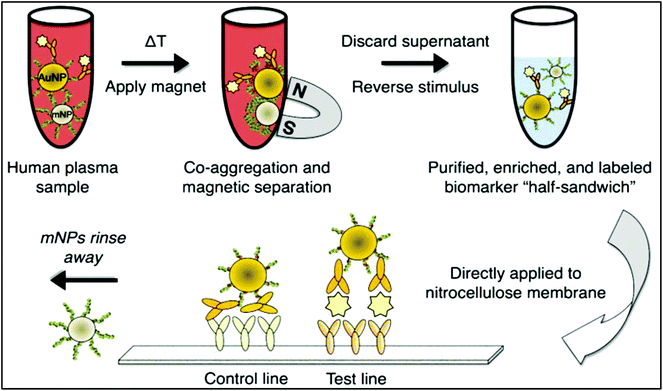 | ||
| Fig. 9 Schematic diagram of magnetic-enrichment LFIA. Reprinted (adapted) with permission from ref. 122. Copyright (2012) American Chemical Society. | ||
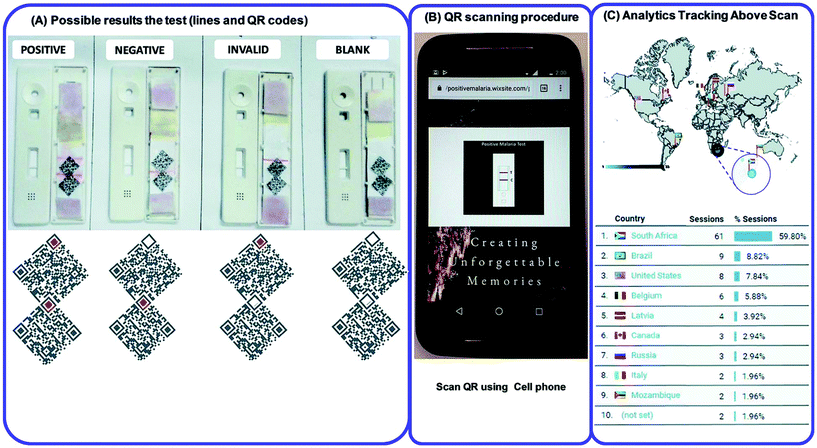 | ||
| Fig. 10 (A) QR code generation for positive, negative, blank and invalid tests. (B) QR scanning procedure by using a cell phone. (C) Statistical Google analytics real-time data with details like number of users, location, duration and results (positive, negative or invalid). Reprinted (adapted) with permission from ref. 123. Copyright (2017) Royal Society of Chemistry. | ||
Schistosomiasis is a parasite disease caused by Schistosoma, a blood-dwelling fluke worm.183 UCP has been employed for the detection of circulating anodic antigen (CAA), a biomarker of Schistosoma. The detection limit achieved by using UCP was 0.5 pg mL−1 of CAA126 and the robustness of the same method was improved by using dry (lyophilized) reagents with a detection limit of 30 pg mL−1.127
3.3 LFIA for detecting viruses and viral infections
Some other remarkable studies demonstrated the detection of virus and viral protein using LFIA devices. The H9 subtype of AIV was successfully detected using a LFIA strip developed using two different mAbs for test line and control line separately with a sensitivity of 0.25 hemagglutinin units in less than 10 minutes. The antibody 4C4 against HA was labelled with AuNP and the antibody 4D4 against nucleoprotein and applied on the test line.128 A similar type of approach was used to detect the H5 subtype (H5N1) without any cross-reactivity with the H1–H4 or H6–H14 subtypes.184 In comparison with the hemagglutination (HI) assay and agar gel immunodiffusion assay (AGID), a gold-immunochromatographic (GICA) test strip showed high sensitivity and specificity.185 The rapid detection of HBV and HEV138 was largely achieved through the immunochromatography assay principle. There are various commercial kits available for HBV and HEV rapid detection (like ACON, Atlas Medical, Dima, Cortez, Blue, Intec, HEPACARD, Assure).136,137,186,187 They provide excellent screening with comparable sensitivity and specificity with PCR.188 A sensitivity and specificity both ranging between 97.5% and 98.3% have been observed with a 240 patient serum sample. The use of fluorescent QDs as labels are advantageous as they have a high quantum yield, tuneable fluorescent emission spectra and a greater lifetime. Carboxyl-modified CdSe/ZnS quantum beads (QBs) as labels have shown a detection limit of hepatitis B surface antigen (HBsAg) in the picograms per mL range.142 The detection platform comprised anti-HBsAg mAbs conjugated with QBs. The detection limit achieved by this method was 75 pg mL−1.Several LFIA devices have been designed to detect DENV in blood or saliva samples. Commercial kits for detecting DENV are designed: (a) to detect the dengue antigen NS1 at the early stages of infection (Panbio Dengue Early Rapid NS1, SD Bioline Dengue NS1 Ag, BIORAD Strip), (b) to detect anti-dengue IgM and IgG (Panbio Dengue Duo Cassette and SD Dengue IgG/IgM, Merlin IgM, Biosynex IgM, Merlin Dengue Fever IgG and IgM combo device) or (c) to detect both antigen and IgG/IgM (BIOLINE Dengue Duo NS1antigen and IgG and IgM Combo Device).156–163 For improving the specificity and sensitivity of the test, several other approaches have been implemented. The detection of dengue specific IgG in salivary fluid using a stacking flow platform enabled the detection of DENV in a completely non-invasive sample collection platform (Fig. 11).164 As shown in Fig. 11, the device was fabricated using two flow paths, one called the sample pad and the other one the reagent pad. The sample pad was introduced with spiked salivary samples and the reagent pad was supplied with protein-G conjugated 40 nm AuNPs. On the other hand, the specific detection of anti-dengue IgM from a blood sample was achieved by using a protein-G coated membrane, which selectively eliminated IgG from the sample.189 Chikungunya is another viral disease that has been predominantly seen around the world over the years.190–192 SD Bioline Chikungunya IgM and OnSite Chikungunya IgM Combo Rapid Test are two commercially available immunochromatography-based lateral flow devices that have been approved by the European Commission. Both of these LFIA devices are designed to detect chikungunya-specific IgM present in blood, where recombinant structural proteins from the chikungunya envelope are conjugated with colloidal gold particles for the detection. SD Bioline Chikungunya IgM was tested with 407 samples with a sensitivity and specificity of 97.1% and 98.9%, respectively,165 while the OnSite Chikungunya IgM Combo Rapid Test showed a 90.3% sensitivity and 100% specificity (both are relative to MAC-ELISA results) with 93 samples.166 Surprisingly, multiple field studies have been conducted based on these two LFIA devices but have shown very poor performances.167–169,193 The first EBOV outbreak took place in West Africa in 2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 194 with a fatality rate of 70.8%.195 The rapid diagnosis of EBOV by using LFIA as a POC tool showed a 100% sensitive detection of IgG antibodies against EBOV Glycoprotein (EBOV-GP1–649).172 A detection limit of 1 ng ml−1 of EBOV-GP was achieved by using Fe3O4 MNPs conjugated anti-EBOV-GP (4G7).173Recently, a multiplexed disease diagnostic strip was designed to detect three different types of viruses causing Dengue, Yellow fever and Ebola.177 The label used in this study comprised AgNPs with three different sizes and having different colours. These nanoparticles were conjugated to antibodies specific for a particular disease and used for three different test lines to easily identify the specific virus present in the sample with a detection limit of 150 ng ml−1 (Fig. 12). Multiplexed as well as the SERS-based enhanced detection of Dengue NS1 antigen and Zika NS1 antigen was also investigated. Very low detection limits of 7.67 ng mL−1 and 0.72 ng mL−1 for DENV NS1 and ZIKV NS1, respectively, were achieved by using two different Raman reporters conjugated gold nanostars.43 The detection of rabies virus in rabies endemic countries is often challenging as they often lack the infrastructure and funds to employ the gold standard for definitive diagnosis of rabies infection, i.e. the fluorescent antibody test (FAT).196 Instead, for the low-cost and rapid detection of rabies infection, two types of immunochromatography test kits were developed by using mAbs, which can recognize epitope II and III of the nucleoprotein of the virus.197 The specificity and sensitivity for type 1 (a single mAb was used) and type 2 (two different mAbs were used) were found out to be 88.9%, 95.5% and 100%, 93.2% respectively. Furthermore, the assay showed no cross-reactivity with other common canine-pathogenic viruses. The commercially available immunochromatography strip for rabies virus detection was evaluated and the specificity and sensitivity were found to be 100% and 91.66%, respectively, with brain samples from different rabies suspected animals.198–201
194 with a fatality rate of 70.8%.195 The rapid diagnosis of EBOV by using LFIA as a POC tool showed a 100% sensitive detection of IgG antibodies against EBOV Glycoprotein (EBOV-GP1–649).172 A detection limit of 1 ng ml−1 of EBOV-GP was achieved by using Fe3O4 MNPs conjugated anti-EBOV-GP (4G7).173Recently, a multiplexed disease diagnostic strip was designed to detect three different types of viruses causing Dengue, Yellow fever and Ebola.177 The label used in this study comprised AgNPs with three different sizes and having different colours. These nanoparticles were conjugated to antibodies specific for a particular disease and used for three different test lines to easily identify the specific virus present in the sample with a detection limit of 150 ng ml−1 (Fig. 12). Multiplexed as well as the SERS-based enhanced detection of Dengue NS1 antigen and Zika NS1 antigen was also investigated. Very low detection limits of 7.67 ng mL−1 and 0.72 ng mL−1 for DENV NS1 and ZIKV NS1, respectively, were achieved by using two different Raman reporters conjugated gold nanostars.43 The detection of rabies virus in rabies endemic countries is often challenging as they often lack the infrastructure and funds to employ the gold standard for definitive diagnosis of rabies infection, i.e. the fluorescent antibody test (FAT).196 Instead, for the low-cost and rapid detection of rabies infection, two types of immunochromatography test kits were developed by using mAbs, which can recognize epitope II and III of the nucleoprotein of the virus.197 The specificity and sensitivity for type 1 (a single mAb was used) and type 2 (two different mAbs were used) were found out to be 88.9%, 95.5% and 100%, 93.2% respectively. Furthermore, the assay showed no cross-reactivity with other common canine-pathogenic viruses. The commercially available immunochromatography strip for rabies virus detection was evaluated and the specificity and sensitivity were found to be 100% and 91.66%, respectively, with brain samples from different rabies suspected animals.198–201
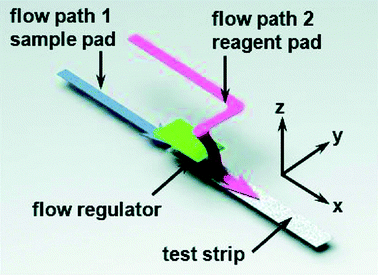 | ||
| Fig. 11 Schematic illustration of the stacking flow immunoassay device. Reprinted (adapted) with permission from ref. 164. Copyright (2015) Royal Society of Chemistry. | ||
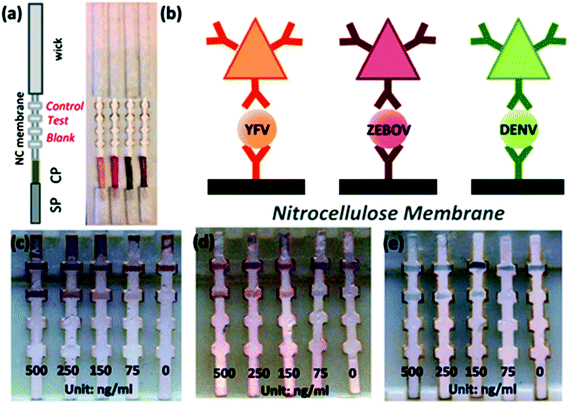 | ||
| Fig. 12 a. Lateral flow strip, b. schematic of the sandwich assay showing different coloured antibodies conjugated with mAbs specific to YFV NS1, ZEBOV GP, DENV NS1 (from left to right), c–e. Limit of detection of the LFIA strip. Reprinted (adapted) with permission from ref. 177. Copyright (2015) Royal Society of Chemistry. | ||
As per WHO, around 30% of the HIV affected people are unaware of their infectious status. Critical gaps exist in many developing countries in HIV prevention, diagnosis and treatment.202 For that, in low resource settings, LFIA devices as a POC tool can serve this gap by providing faster and simpler detection platforms. WHO criteria for using such POC must meet the ASSURED (Affordable, Sensitivity and Specificity, User-friendly, Rapid and robust, Equipment-free, Deliverable) requirement.203 Determine HIV-1/2 (Abbott Laboratories) is a commercially available selenium colloid based immunochromatographic rapid test strip that uses a sandwich format to detect anti-HIV-1 or anti-HIV-2 antibodies present in the patient's serum or plasma or from the whole blood sample.204 The test was evaluated by various groups. Samples from various locations around the world were tested and the test correctly predicted the results from the samples with a 100% sensitivity.205–207 Alere Determine HIV-1/2 Ag/Ab Combo is an FDA-approved rapid LFIA strip that is used commercially for HIV diagnosis.208 The principle is the same as that of the previously described kit (Determine HIV-1/2 by Abbott Laboratories) with the only difference being the analyte; here the analyte is the p24 antigen as well as antibodies to HIV-1 and HIV-2.143 Some of the other commercially available HIV detection kits have also been evaluated and in most cases the sensitivity was found to be nearly 100% with serum or plasma samples.145,206,207,209–211 HIV-1 p24 detection by the magnetic immuno chromatographic test (MICT) by using super-paramagnetic nanoparticles212 has also been reported.144,213 The magnetic moment of the super-paramagnetic particles that serve as a label for the assay has been detected using a cost-effective instrument involving multiple coil sensors arranged as a gradiometer to form a dedicated magnetic assay reader system.144 A recent report on the detection of p24 showed the use of PtNCs, which serve as a catalyst in the LFIA platform. The signal amplification by using this method showed a 100-fold catalytic enhancement and it was thus able to detect 0.8 pg ml−1 of p24.148 Amplified HIV-1 RNA detection145 by NALFIA using AuNPs showed a resolution of 10.5–13log10 copies per mL over a linear range and 50 copies of HIV gag RNA when coupled with nucleic acid sequence-based amplification (NASBA).146 The assay was designed to detect an amplified 142 bp RNA sequence by an AuNP-labelled partly complementary nucleotide strand. When a sample containing the RNA sequence is present, it binds with the AuNP-labelled probe as well as with nucleotide sequence present in the nitrocellulose membrane of the strip. After washing the strip, gold enhancement is done for signal amplification and for improving the LOD (Fig. 13). SERS-based enhancement has been done for HIV nucleic acid detection. A detection limit as low as 0.24 pg mL−1 for HIV-1 DNA was achieved by using MGITC-modified AuNSs as the probe.150 Some opportunistic infections of HIV, like cryptococcosis caused by Cryptococcus sp., were also diagnosed and evaluated using a LFIA.8,214
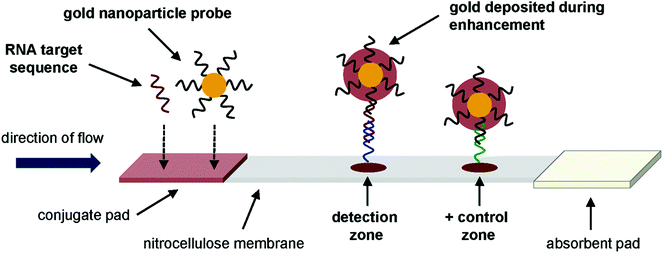 | ||
| Fig. 13 Schematic design of a lateral flow assay to detect amplified HIV-1 RNA. Reprinted (adapted) with permission from ref. 146. Copyright (2012) Public Library of Sciences (PLOS). | ||
A significant cause of diseases in respiratory tract and eye (Pharyngoconjunctival fever) is the human adenovirus. The adenovirus antigen immunochromatographic test is being developed that can detect the virus within 10 minutes from pharyngeal swap specimen.151 The IC test showed a sensitivity of 72.6% and a specificity of 100% with serotypes 1, 2, 3, 5 and 7. Other studies have also shown comparable sensitivity with the previous results.215,216 Another mosquito-borne virus, Western Nile virus is a human neuropathogen commonly found in Africa, Asia, Europe and the Middle East.217,218 Though there are many immunofluorescence assays and as MAC-ELISA-based techniques are available for laboratory screening of WNV, they show 4% to 10% cross-reactivity with other flaviviruses.219–221 A solid-phase immunochromatographic strip with colloidal gold particles as the label RapidWN™ was developed in 2007.154 The LFIA showed a high sensitivity and selectivity of 98.8% and 95.3%, respectively, which is comparable with the gold standard IgM-based EIA.155 NDV belongs to the family Paramyxoviridae. A LFIA device for the detection of NDV was achieved by using 40 nm AuNPs conjugated with anti-NDV-6C4 mAb.171 Gold enhancement on the immobilized AuNP-antibody conjugate showed an increased detection limit by a factor of 10–100 fold. An unconventional method to detect the norovirus used M13 bacteriophage nanoparticles as reporters instead of coloured latex or AuNPs. Here, the M13 bacteriophage was used to selectively bind to biotin-labelled anti-norovirus antibodies by biotin–streptavidin–biotin conjugation. Then, the horseradish peroxidase (HRP) conjugated anti-M13 antibody was bound to the bacteriophage. TMB was used then as a chromogenic substance to develop colour.174 The detection limit achieved by using this system was 107 VLPs per ml.
Over the years, LFIA devices have been improved by various means, such as by using a two-dimensional paper flow network,222,223 installing an organic light emitting diode (OLED) with filters,224 multiplexing the assay with two or more analytes,225 as hand-held devices (including smartphone apps) for the quantitative detection of analytes,100,123 enzyme-based signal amplification148 and gold- or silver-based signal amplification.132,152,226 The two-dimensional paper flow system has shown controlled release from patterned, dried reagents, which allows the fabrication and implementation of complex and multiplexed flow patterns (like the flow of reagents for gold enhancement) that must not mix with each other before flowing through the strip by capillary action.222 These amplification strategies were applied to improve the sensitivity of the detection device. Further, the sensitivity for the detection of fluorescence coming from QD labels was improved through the use of an OLED. This enabled certain advantages over conventional LEDs, such as flexibility and a 7 times improvement over the conventional LFIA device in the detection of influenza as a proof of concept.224
4. Conclusion and outlook
This review summarizes the analysis and diagnosis of infectious diseases by LFIA with nanoparticles used as a label. We have seen various pandemics and epidemics around the globe caused by various infectious diseases; so, it is of utmost importance to diagnose infectious diseases and their causative agents as early as possible. This can not only lead to the deployment of early preventive measures but can also help to contain the infection from spreading. Different methods can detect either these infection causing pathogens or their products, in which the lateral flow assay approach provides several advantages. This method of diagnosis is simple, rapid, cost-effective and does not need any specialized persons or training to be operated, thus it can serve as a point-of-care (POC) testing device. Different labels are used, of which colloidal AuNP is the primary label for visualizing and/or quantifying the infection load. AuNP is comparatively easy to prepare, stable and can be readily tuned depending on the type of application. Another advantage of the lateral flow assay is that different analytes can be tested at the same time, which makes LFIA devices multiparametric. Several LFIA-based devices have been commercialized for the diagnosis of infectious disease and further extensive research is underway to enhance the signal produced from the device with several techniques that can be applied, such as silver enhancement, SERS and magnetic enrichment.Although LFIA devices enjoy great application already, there are still some limitations associated with LFIA-based devices. The selectivity and signal generation of these devices often hugely depends on the type of sample, its condition and preparation, the volume of the sample, pH, amount of analyte present and the weak interaction forces between the analyte and recognition element. Some of the strategies described here are experimental only so far with the target analyte under controlled conditions. This may affect the outcome of the device's performance in real samples from a patient. To overcome this, present research is focussed more on making LFIA devices more feasible, less dependent on the sample preparation processes, with a shorter incubation time and quantitative result generation. Further, for analytes present in a very low concentration in the sample of interest, a pre-concentration or enrichment step is generally performed. This makes LFIA not suitable for POC applications. To overcome this hurdle, methods such as catalytic enhancement and SERS-based detection are being employed to achieve better sensitivity.
Abbreviations
| AgNP(s) | Silver nanoparticles(s) |
| AIDS | Acquired immunodeficiency syndrome |
| AIV | Avian influenza virus |
| AuNP(s) | Gold nanoparticle(s) |
| BoNT (A/B/C/D/E) | Botulinum neurotoxin (A/B/C/D/E) |
| CAA | Circulating anodic antigen |
| CFU | Colony forming unit |
| CNP(s) | Carbon nanoparticles(s) |
| CPS | Capsular polysaccharide |
| DENV | Dengue virus |
| ds | Double stranded |
| EBOV | Ebola virus |
| EHEC | Enterohemorrhagic E. coli |
| EIA | Enzyme immunoassay |
| ELISA | Enzyme-linked immunosorbent assay |
| EPEC | Enteropathogenic E. coli |
| FAT | Fluorescent antibody test |
| GO | Graphene oxide |
| H(A/B/C/E)V | Hepatitis (A/B/C/E) virus |
| HA | Hemagglutinin |
| HBeAg | Hepatitis B envelope antigen |
| HBsAg | Hepatitis B surface antigen |
| HI | Hemagglutinin inhibition |
| HIV | Human immunodeficiency virus |
| HRP | Horseradish peroxidase |
| HSV | Herpes simplex virus |
| IMP | Immunomagnetic particle |
| IMS | Immunomagnetic separation |
| LFIA | Lateral flow immuno assay |
| LPS | Lipo-polysaccharide |
| mAb(s) | Monoclonal antibody(ies) |
| MAC | IgM antibody capture |
| MB | Methylene blue |
| MNB | Magnetic nanobeads |
| MNP(s) | Magnetic nanoparticle(s) |
| mRNA | Messenger RNA |
| NALFIA | Nucleic acid lateral flow immuno assay |
| NASBA | Nucleic acid sequence-based amplification |
| NDV | Newcastle disease virus |
| NTA | Ni(II)-nitrilotriacetic acid |
| ODN | Oligodinucleotide |
| pAb | Polyclonal antibody |
| PCR | Polymerase chain reaction |
| PEG | Poly(ethylene glycol) |
| pf HRP | Plasmodium falciparum histidine-rich protein |
| PFU | Plaque-forming unit |
| PIF | Powdered infant formulae |
| pLDH | Plasmodium lactate dehydrogenase |
| pNIPAm | Poly(N-isopropylacrylamide) |
| POC | Point of care |
| QD(s) | Quantum dot(s) |
| QR | Quick response |
| r-DNA/RNA | Recombinant DNA/RNA |
| RNALFIA | Ribonucleic acid LFIA |
| RT-LAMP | Reverse-transcription loop-mediated isothermal amplification |
| SEB | Staphylococcus enterotoxin B |
| SERS | Surface-enhanced raman scattering |
| SRB | Sulforhodamine B |
| ss | Single stranded |
| ss DNA | Single stranded DNA |
| TCID | Tissue culture infectious dose |
| UCP | Up-converting phosphor |
| VLP(s) | Virus-like particle(s) |
| WHO | World Health Organization |
| YFV | Yellow fever virus |
| ZIKV | Zika virus |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support from BioX centre, Indian Institute of Technology Mandi for research and infrastructure facility. RB acknowledges the fellowship from MHRD, Govt. of India.References
- J. H. Leuvering, P. J. Thal, M. van der Waart and A. H. Schuurs, J. Immunoassay, 1980, 1, 77–91 CrossRef CAS PubMed.
- E. C. Schramm, N. R. Staten, Z. Zhang, S. S. Bruce, C. Kellner, J. P. Atkinson, V. C. Kyttaris, G. C. Tsokos, M. Petri, E. Sander Connolly and P. K. Olson, Anal. Biochem., 2015, 477, 78–85 CrossRef CAS PubMed.
- N. De Giovanni and N. Fucci, Curr. Med. Chem., 2013, 20, 545–561 CAS.
- R. Pacifici, M. Farre, S. Pichini, J. Ortuno, P. N. Roset, P. Zuccaro, J. Segura and R. de la Torre, J. Anal. Toxicol., 2001, 25, 144–146 CrossRef CAS PubMed.
- A. Carrio, C. Sampedro, J. L. Sanchez-Lopez, M. Pimienta and P. Campoy, Sensors, 2015, 15, 29569–29593 CrossRef PubMed.
- R. Kwizera, J. Nguna, A. Kiragga, J. Nakavuma, R. Rajasingham, D. R. Boulware and D. B. Meya, PLoS One, 2014, 9, e103156 Search PubMed.
- S. Buhrer-Sekula, H. L. Smits, G. C. Gussenhoven, J. van Leeuwen, S. Amador, T. Fujiwara, P. R. Klatser and L. Oskam, J. Clin. Microbiol., 2003, 41, 1991–1995 CrossRef CAS PubMed.
- J. N. Jarvis, A. Percival, S. Bauman, J. Pelfrey, G. Meintjes, G. N. Williams, N. Longley, T. S. Harrison and T. R. Kozel, Clin. Infect. Dis, 2011, 53, 1019–1023 CrossRef CAS PubMed.
- M. L. Moreno, A. Cebolla, A. Munoz-Suano, C. Carrillo-Carrion, I. Comino, A. Pizarro, F. Leon, A. Rodriguez-Herrera and C. Sousa, Gut, 2017, 66, 250–257 CrossRef CAS PubMed.
- V. S. Vaidya, G. M. Ford, S. S. Waikar, Y. Wang, M. B. Clement, V. Ramirez, W. E. Glaab, S. P. Troth, F. D. Sistare, W. C. Prozialeck, J. R. Edwards, N. A. Bobadilla, S. C. Mefferd and J. V. Bonventre, Kidney Int., 2009, 76, 108–114 CrossRef CAS PubMed.
- S. H. Ang, M. Rambeli, T. M. Thevarajah, Y. B. Alias and S. M. Khor, Biosens. Bioelectron., 2016, 78, 187–193 CrossRef CAS PubMed.
- R. E. Biagini, D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. Striley, J. E. Snawder, S. A. Robertson and C. P. Quinn, Clin. Vaccine Immunol., 2006, 13, 541–546 CrossRef CAS PubMed.
- S. Rosen, in Lateral Flow Immunoassay, ed. R. C. Wong and H. Y. Tse, Springer, New York, 2009, ch. 2, pp. 35–50 Search PubMed.
- X. Huang, Z. P. Aguilar, H. Xu, W. Lai and Y. Xiong, Biosens. Bioelectron., 2016, 75, 166–180 CrossRef CAS PubMed.
- K. M. Koczula and A. Gallotta, Essays Biochem., 2016, 60, 111–120 CrossRef PubMed.
- World Health Organization, 2015, https://books.google.co.in/books?hl=en&lr=&id=Kl00DgAAQBAJ&oi=fnd&pg=PP1&dq=World+Health+Organization.+2015.+Mortality+and+global+health+estimates.+Geneva,+Switzerland:+World+Health+Organization&ots=8MrFnAPOwc&sig=2XZQEy7SlofM3888phKwJSe_dgc#v=onepage&q&f=false, Mortality and global health estimates. Geneva, Switzerland: World Health Organization, (accessed January 2018).
- C. Dye, Philos. Trans. R. Soc., B, 2014, 369, 20130426 CrossRef PubMed.
- G. A. Posthuma-Trumpie, J. Korf and A. van Amerongen, Anal. Bioanal. Chem., 2009, 393, 569–582 CrossRef CAS PubMed.
- G. A. Posthuma-Trumpie, J. H. Wichers, M. Koets, L. B. Berendsen and A. van Amerongen, Anal. Bioanal. Chem., 2012, 402, 593–600 CrossRef CAS PubMed.
- Y. Xu, Y. Liu, Y. Wu, X. Xia, Y. Liao and Q. Li, Anal. Chem., 2014, 86, 5611–5614 CrossRef CAS PubMed.
- D. Quesada-Gonzalez and A. Merkoci, Biosens. Bioelectron., 2015, 73, 47–63 CrossRef CAS PubMed.
- E. B. Bahadır and M. K. Sezgintürk, TrAC, Trends Anal. Chem., 2016, 82, 286–306 CrossRef.
- M. J. Raeisossadati, N. M. Danesh, F. Borna, M. Gholamzad, M. Ramezani, K. Abnous and S. M. Taghdisi, Biosens. Bioelectron., 2016, 86, 235–246 CrossRef CAS PubMed.
- K. Wang, W. Qin, Y. Hou, K. Xiao and W. Yan, Nano Biomed. Eng., 2016, 8, 172–183 Search PubMed.
- X. Gong, J. Cai, B. Zhang, Q. Zhao, J. Piao, W. Peng, W. Gao, D. Zhou, M. Zhao and J. Chang, J. Mater. Chem. B, 2017, 5, 5079–5091 RSC.
- M. Sajid, A.-N. Kawde and M. Daud, J. Saudi Chem. Soc., 2015, 19, 689–705 CrossRef.
- B. Ngom, Y. Guo, X. Wang and D. Bi, Anal. Bioanal. Chem., 2016, 408, 3923 CrossRef CAS PubMed.
- J. Y. Lee, Y. A. Kim, M. Y. Kim, Y. T. Lee, B. D. Hammock and H. S. Lee, Anal. Chim. Acta, 2012, 757, 69–74 CrossRef CAS PubMed.
- Rapid Lateral Flow Test Strips: Considerations for Product Development, Merck Millipore, Billerica https://www.emdmillipore.com/Web-US-Site/en_CA/-/USD/ShowDocument-Pronet?id=201306.12550 (accessed March, 2018).
- I. H. Cho, S. M. Seo, E. H. Paek and S. H. Paek, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 271–277 CrossRef CAS PubMed.
- P. F. Mens, A. van Amerongen, P. Sawa, P. A. Kager and H. D. Schallig, Diagn. Microbiol. Infect. Dis., 2008, 61, 421–427 CrossRef CAS PubMed.
- Z. Wang, D. Zhi, Y. Zhao, H. Zhang, X. Wang, Y. Ru and H. Li, Int. J. Nanomed., 2014, 9, 1699–1707 CrossRef PubMed.
- S. Takanashi, M. Okame, T. Shiota, M. Takagi, F. Yagyu, P. G. Tung, S. Nishimura, N. Katsumata, T. Igarashi, S. Okitsu and H. Ushijima, J. Virol. Methods, 2008, 148, 1–8 CrossRef CAS PubMed.
- J. S. Ponti, in Lateral Flow Immunoassay, ed. R. C. Wong and H. Y. Tse, Springer, New York, 2009, ch. 2, pp. 35–50 Search PubMed.
- A. van Amerongen, D. van Loon, L. B. Berendsen and J. H. Wichers, Clin. Chim. Acta, 1994, 229, 67–75 CrossRef CAS.
- J. A. Ho and M. R. Huang, Anal. Chem., 2005, 77, 3431–3436 CrossRef CAS PubMed.
- E. R. Goldman, A. R. Clapp, G. P. Anderson, H. T. Uyeda, J. M. Mauro, I. L. Medintz and H. Mattoussi, Anal. Chem., 2004, 76, 684–688 CrossRef CAS PubMed.
- P. Corstjens, M. Zuiderwijk, A. Brink, S. Li, H. Feindt, R. S. Niedbala and H. Tanke, Clin. Chem., 2001, 47, 1885–1893 CAS.
- Y. C. Cao, R. Jin and C. A. Mirkin, Science, 2002, 297, 1536–1540 CrossRef CAS PubMed.
- J. Hwang, S. Lee and J. Choo, Nanoscale, 2016, 8, 11418–11425 RSC.
- L. Blanco-Covian, V. Montes-Garcia, A. Girard, M. T. Fernandez-Abedul, J. Perez-Juste, I. Pastoriza-Santos, K. Faulds, D. Graham and M. C. Blanco-Lopez, Nanoscale, 2017, 9, 2051–2058 RSC.
- O. J. Clarke, B. L. Goodall, H. P. Hui, N. Vats and C. L. Brosseau, Anal. Chem., 2017, 89, 1405–1410 CrossRef CAS PubMed.
- M. Sanchez-Purra, M. Carre-Camps, H. de Puig, I. Bosch, L. Gehrke and K. Hamad-Schifferli, ACS Infect. Dis., 2017, 3, 767–776 CrossRef CAS PubMed.
- P. Sandström, M. Boncheva and B. Åkerman, Langmuir, 2003, 19, 7537–7543 CrossRef.
- J. M. de la Fuente and S. Penades, Biochim. Biophys. Acta, 2006, 1760, 636–651 CrossRef CAS PubMed.
- S. Zeng, K.-T. Yong, I. Roy, X.-Q. Dinh, X. Yu and F. Luan, Plasmonics, 2011, 6, 491 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- B. Pelaz, P. del Pino, P. Maffre, R. Hartmann, M. Gallego, S. Rivera-Fernandez, J. M. de la Fuente, G. U. Nienhaus and W. J. Parak, ACS Nano, 2015, 9, 6996–7008 CrossRef CAS PubMed.
- A. K. Gupta, R. R. Naregalkar, V. D. Vaidya and M. Gupta, Nanomedicine, 2007, 2, 23–39 CrossRef CAS PubMed.
- P. C. Soo, Y. T. Horng, P. R. Hsueh, B. J. Shen, J. Y. Wang, H. H. Tu, J. R. Wei, S. C. Hsieh, C. C. Huang and H. C. Lai, J. Microbiol. Methods, 2006, 66, 440–448 CrossRef CAS PubMed.
- K. A. Edwards and A. J. Baeumner, Anal. Bioanal. Chem., 2006, 386, 1335–1343 CrossRef CAS PubMed.
- B. Y. Jung, S. C. Jung and C. H. Kweon, J. Food Prot., 2005, 68, 2140–2143 CrossRef CAS PubMed.
- J. Wang, W. N. Chen, K. X. Hu and W. Li, Weisheng Yanjiu, 2006, 35, 439–441 CAS.
- H. Qi, Z. Zhong, H. X. Zhou, C. Y. Deng, H. Zhu, J. F. Li, X. L. Wang and F. R. Li, Int. J. Nanomed., 2011, 6, 3033–3039 CAS.
- Q. Y. Xie, Y. H. Wu, Q. R. Xiong, H. Y. Xu, Y. H. Xiong, K. Liu, Y. Jin and W. H. Lai, Biosens. Bioelectron., 2014, 54, 262–265 CrossRef CAS PubMed.
- J. Park, J. H. Shin and J. K. Park, Anal. Chem., 2016, 88, 3781–3788 CrossRef CAS PubMed.
- E. Morales-Narvaez, T. Naghdi, E. Zor and A. Merkoci, Anal. Chem., 2015, 87, 8573–8577 CrossRef CAS PubMed.
- K. H. Seo, P. S. Holt, H. D. Stone and R. K. Gast, Int. J. Food Microbiol., 2003, 87, 139–144 CrossRef PubMed.
- Z. Fang, W. Wu, X. Lu and L. Zeng, Biosens. Bioelectron., 2014, 56, 192–197 CrossRef CAS PubMed.
- C.-C. Liu, C.-Y. Yeung, P.-H. Chen, M.-K. Yeh and S.-Y. Hou, Food Chem., 2013, 141, 2526–2532 CrossRef CAS PubMed.
- S. Rong-Hwa, T. Shiao-Shek, C. Der-Jiang and H. Yao-Wen, Food Chem., 2010, 118, 462–466 CrossRef CAS.
- M. Fisher, Y. Atiya-Nasagi, I. Simon, M. Gordin, A. Mechaly and S. Yitzhaki, Lett. Appl. Microbiol., 2009, 48, 413–418 CrossRef CAS PubMed.
- D. B. Wang, B. Tian, Z. P. Zhang, J. Y. Deng, Z. Q. Cui, R. F. Yang, X. Y. Wang, H. P. Wei and X. E. Zhang, Biosens. Bioelectron., 2013, 42, 661–667 CrossRef CAS PubMed.
- D. B. Wang, B. Tian, Z. P. Zhang, X. Y. Wang, J. Fleming, L. J. Bi, R. F. Yang and X. E. Zhang, Biosens. Bioelectron., 2015, 67, 608–614 CrossRef CAS PubMed.
- N. Nakasone, C. Toma, Y. Lu and M. Iwanaga, Diagn. Microbiol. Infect. Dis., 2007, 57, 21–25 CrossRef CAS PubMed.
- K. Kawatsu, Y. Kumeda, M. Taguchi, W. Yamazaki-Matsune, M. Kanki and K. Inoue, J. Clin. Microbiol., 2008, 46, 1226–1231 CrossRef CAS PubMed.
- L. Yu, P. Li, X. Ding and Q. Zhang, Talanta, 2017, 165, 167–175 CrossRef CAS PubMed.
- D. Tang, J. C. Sauceda, Z. Lin, S. Ott, E. Basova, I. Goryacheva, S. Biselli, J. Lin, R. Niessner and D. Knopp, Biosens. Bioelectron., 2009, 25, 514–518 CrossRef CAS PubMed.
- Y.-M. Huang, D.-F. Liu, W.-H. Lai, Y.-H. Xiong, W.-C. Yang, K. Liu and S.-Y. Wang, Chin. J. Anal. Chem., 2014, 42, 654–659 CAS.
- D. Liu, Y. Huang, S. Wang, K. Liu, M. Chen, Y. Xiong, W. Yang and W. Lai, Food Control, 2015, 51, 218–224 CrossRef CAS.
- L. Anfossi, F. Di Nardo, S. Cavalera, C. Giovannoli, G. Spano, E. S. Speranskaya, I. Y. Goryacheva and C. Baggiani, Microchim. Acta, 2018, 185, 94 CrossRef PubMed.
- T. Klewitz, F. Gessler, H. Beer, K. Pflanz and T. Scheper, Sens. Actuators, B, 2006, 113, 582–589 CrossRef CAS.
- D. J. Chiao, J. J. Wey, R. H. Shyu and S. S. Tang, Hybridoma, 2008, 27, 31–35 CrossRef CAS PubMed.
- K. H. Ching, A. Lin, J. A. McGarvey, L. H. Stanker and R. Hnasko, J. Immunol. Methods, 2012, 380, 23–29 CrossRef CAS PubMed.
- S.-H. Huang, H.-C. Wei and Y.-C. Lee, Food Control, 2007, 18, 893–897 CrossRef CAS.
- S.-H. Huang, Sens. Actuators, B, 2007, 127, 335–340 CrossRef CAS.
- S. Wiriyachaiporn, P. H. Howarth, K. D. Bruce and L. A. Dailey, Diagn. Microbiol. Infect. Dis., 2013, 75, 28–36 CrossRef CAS PubMed.
- N. Khreich, P. Lamourette, H. Boutal, K. Devilliers, C. Creminon and H. Volland, Anal. Biochem., 2008, 377, 182–188 CrossRef CAS PubMed.
- Y. Ju, H. J. Hao, G. H. Xiong, H. R. Geng, Y. L. Zheng, J. Wang, Y. Cao, Y. H. Yang, X. H. Cai and Y. Q. Jiang, Vet. Immunol. Immunopathol., 2010, 133, 207–211 CrossRef CAS PubMed.
- T. Nakayama, J. Zhao, D. Takeuchi, A. Kerdsin, P. Chiranairadul, P. Areeratana, P. Loetthong, A. Pienpringam, Y. Akeda and K. Oishi, Biosens. Bioelectron., 2014, 60, 175–179 CrossRef CAS PubMed.
- X. Zhang, J. Zhou, C. Zhang, D. Zhang and X. Su, RSC Adv., 2016, 6, 1279–1287 RSC.
- M. Blazkova, B. Javurkova, L. Fukal and P. Rauch, Biosens. Bioelectron., 2011, 26, 2828–2834 CrossRef CAS PubMed.
- H. L. Smits, C. K. Eapen, S. Sugathan, M. Kuriakose, M. H. Gasem, C. Yersin, D. Sasaki, B. Pujianto, M. Vestering, T. H. Abdoel and G. C. Gussenhoven, Clin. Diagn. Lab. Immunol., 2001, 8, 166–169 CAS.
- A. B. Nurul Najian, E. A. Engku Nur Syafirah, N. Ismail, M. Mohamed and C. Y. Yean, Anal. Chim. Acta, 2016, 903, 142–148 CrossRef CAS PubMed.
- C. Y. Yu, G. Y. Ang, A. L. Chua, E. H. Tan, S. Y. Lee, G. Falero-Diaz, O. Otero, I. Rodriguez, F. Reyes, A. Acosta, M. E. Sarmiento, S. Ghosh, T. Ramamurthy, C. Yean Yean, P. Lalitha and M. Ravichandran, J. Microbiol. Methods, 2011, 86, 277–282 CrossRef CAS PubMed.
- C. Pengsuk, P. Chaivisuthangkura, S. Longyant and P. Sithigorngul, Biosens. Bioelectron., 2013, 42, 229–235 CrossRef CAS PubMed.
- L. Wang, J. Zhang, H. Bai, X. Li, P. Lv and A. Guo, Appl. Biochem. Biotechnol., 2014, 173, 1073–1082 CrossRef CAS PubMed.
- A. J. Herring, R. C. Ballard, V. Pope, R. A. Adegbola, J. Changalucha, D. W. Fitzgerald, E. W. Hook, A. Kubanova, S. Mananwatte, J. W. Pape, A. W. Sturm, B. West, Y. P. Yin and R. W. Peeling, Sex. Transm. Infect., 2006, 82, v7–v12 CrossRef PubMed.
- P. Mdluli, P. Tetyana, N. Sosibo, H. van der Walt, M. Mlambo, A. Skepu and R. Tshikhudo, Biosens. Bioelectron., 2014, 54, 1–6 CrossRef CAS PubMed.
- H. Liu, F. Zhan, F. Liu, M. Zhu, X. Zhou and D. Xing, Biosens. Bioelectron., 2014, 62, 38–46 CrossRef CAS PubMed.
- M. Blažková, M. Koets, P. Rauch and A. van Amerongen, Eur. Food Res. Technol., 2009, 229, 867 CrossRef.
- Z. Lin, S. Cheng, Q. Yan, X. Liu, W. Zheng, X. Hu, J. Li, J. Zhang, T. Xiang, J. Zheng and J. Zhang, Clin. Biochem., 2015, 48, 1298–1303 CrossRef CAS PubMed.
- F. Chen, X. Ming, X. Chen, M. Gan, B. Wang, F. Xu and H. Wei, Biosens. Bioelectron., 2014, 61, 306–313 CrossRef CAS PubMed.
- J. A. Ho, S. C. Zeng, W. H. Tseng, Y. J. Lin and C. H. Chen, Anal. Bioanal. Chem., 2008, 391, 479–485 CrossRef CAS PubMed.
- S. Xia, Z. Yu, D. Liu, C. Xu and W. Lai, Food Control, 2016, 59, 507–512 CrossRef CAS.
- Q. Qu, Z. Zhu, Y. Wang, Z. Zhong, J. Zhao, F. Qiao, X. Du, Z. Wang, R. Yang, L. Huang, Y. Yu, L. Zhou and Z. Chen, J. Microbiol. Methods, 2009, 79, 121–123 CrossRef CAS PubMed.
- F. Zhang, M. Zou, Y. Chen, J. Li, Y. Wang, X. Qi and Q. Xue, Biosens. Bioelectron., 2014, 51, 29–35 CrossRef CAS PubMed.
- Z. Liang, X. Wang, W. Zhu, P. Zhang, Y. Yang, C. Sun, J. Zhang, X. Wang, Z. Xu, Y. Zhao, R. Yang, S. Zhao and L. Zhou, ACS Appl. Mater. Interfaces, 2017, 9, 3497–3504 CAS.
- C. Z. Li, K. Vandenberg, S. Prabhulkar, X. Zhu, L. Schneper, K. Methee, C. J. Rosser and E. Almeide, Biosens. Bioelectron., 2011, 26, 4342–4348 CrossRef CAS PubMed.
- J. H. Shin, J. Hong, H. Go, J. Park, M. Kong, S. Ryu, K. P. Kim, E. Roh and J. K. Park, J. Agric. Food Chem., 2018, 66, 290–297 CrossRef CAS PubMed.
- S. Shafazand, R. Doyle, S. Ruoss, A. Weinacker and T. A. Raffin, Chest, 1999, 116, 1369–1376 CrossRef CAS PubMed.
- Z. A. Kanafani, A. Ghossain, A. I. Sharara, J. M. Hatem and S. S. Kanj, Emerging Infect. Dis., 2003, 9, 520–525 CrossRef PubMed.
- L. W. Riley, R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake and M. L. Cohen, N. Engl. J. Med., 1983, 308, 681–685 CrossRef CAS PubMed.
- W. Wu, S. Zhao, Y. Mao, Z. Fang, X. Lu and L. Zeng, Anal. Chim. Acta, 2015, 861, 62–68 CrossRef CAS PubMed.
- C. Pohlmann, I. Dieser and M. Sprinzl, Analyst, 2014, 139, 1063–1071 RSC.
- R. V. Tauxe, C. M. Patton, P. Edmonds, T. J. Barrett, D. J. Brenner and P. A. Blake, J. Clin. Microbiol., 1985, 21, 222–225 CAS.
- J. Singh, S. Sharma and S. Nara, Food Chem., 2015, 170, 470–483 CrossRef CAS PubMed.
- H. D. Shukla and S. K. Sharma, Crit. Rev. Microbiol., 2005, 31, 11–18 CrossRef CAS PubMed.
- S. S. Arnon, R. Schechter, T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, J. Hauer, M. Layton, S. Lillibridge, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, D. L. Swerdlow, K. Tonat and B. Working Group on Civilian, J. Am. Med. Assoc., 2001, 285, 1059–1070 CrossRef CAS PubMed.
- J. Kadariya, T. C. Smith and D. Thapaliya, BioMed Res. Int., 2014, 2014, 827965 Search PubMed.
- M. C. Heidt, W. Mohamed, T. Hain, P. R. Vogt, T. Chakraborty and E. Domann, J. Clin. Microbiol., 2005, 43, 4898–4901 CrossRef PubMed.
- S. Sriskandan and J. D. Slater, PLoS Med., 2006, 3, e187 Search PubMed.
- X. Jiang, Y. Ni, Y. Jiang, F. Yuan, L. Han, M. Li, H. Liu, L. Yang and Y. Lu, J. Clin. Microbiol., 2005, 43, 826–831 CrossRef CAS PubMed.
- M. L. Mezzatesta, F. Gona and S. Stefani, Future Microbiol., 2012, 7, 887–902 CrossRef CAS PubMed.
- R. Chauhan, J. Singh, T. Sachdev, T. Basu and B. D. Malhotra, Biosens. Bioelectron., 2016, 81, 532–545 CrossRef CAS PubMed.
- S. Shan, W. Lai, Y. Xiong, H. Wei and H. Xu, J. Agric. Food Chem., 2015, 63, 745–753 CrossRef CAS PubMed.
- A.-L. Välimaa, A. Tilsala-Timisjärvi and E. Virtanen, Food Control, 2015, 55, 103–114 CrossRef.
- X. Wang, R. Niessner, D. Tang and D. Knopp, Anal. Chim. Acta, 2016, 912, 10–23 CrossRef CAS PubMed.
- T. Yang, H. Huang, F. Zhu, Q. Lin, L. Zhang and J. Liu, Sensors, 2016, 16, 1118 CrossRef PubMed.
- A. Ratsimbasoa, A. Randriamanantena, R. Raherinjafy, N. Rasoarilalao and D. Menard, Am. J. Trop. Med. Hyg., 2007, 76, 481–485 Search PubMed.
- J. D. Swartz, C. P. Gulka, F. R. Haselton and D. W. Wright, Langmuir, 2011, 27, 15330–15339 CrossRef CAS PubMed.
- M. A. Nash, J. N. Waitumbi, A. S. Hoffman, P. Yager and P. S. Stayton, ACS Nano, 2012, 6, 6776–6785 CrossRef CAS PubMed.
- C. L. Mthembu, M. I. Sabela, M. Mlambo, L. M. Madikizela, S. Kanchi, H. Gumede and P. S. Mdluli, Anal. Methods, 2017, 9, 5943–5951 RSC.
- D. Y. Pereira, R. Y. Chiu, S. C. Zhang, B. M. Wu and D. T. Kamei, Anal. Chim. Acta, 2015, 882, 83–89 CrossRef CAS PubMed.
- L. Rivas, A. de la Escosura-Muñiz, L. Serrano, L. Altet, O. Francino, A. Sánchez and A. Merkoçi, Nano Res., 2015, 8, 3704–3714 CrossRef CAS.
- P. L. Corstjens, L. van Lieshout, M. Zuiderwijk, D. Kornelis, H. J. Tanke, A. M. Deelder and G. J. van Dam, J. Clin. Microbiol., 2008, 46, 171–176 CrossRef CAS PubMed.
- G. J. van Dam, C. J. de Dood, M. Lewis, A. M. Deelder, L. van Lieshout, H. J. Tanke, L. H. van Rooyen and P. L. Corstjens, Exp. Parasitol., 2013, 135, 274–282 CrossRef CAS PubMed.
- F. Peng, Z. Wang, S. Zhang, R. Wu, S. Hu, Z. Li, X. Wang and D. Bi, Clin. Vaccine Immunol., 2008, 15, 569–574 CrossRef CAS PubMed.
- V. Veguilla, K. Hancock, J. Schiffer, P. Gargiullo, X. Lu, D. Aranio, A. Branch, L. Dong, C. Holiday, F. Liu, E. Steward-Clark, H. Sun, B. Tsang, D. Wang, M. Whaley, Y. Bai, L. Cronin, P. Browning, H. Dababneh, H. Noland, L. Thomas, L. Foster, C. P. Quinn, S. D. Soroka and J. M. Katz, J. Clin. Microbiol., 2011, 49, 2210–2215 CrossRef PubMed.
- Y. T. Kim, J. H. Jung, Y. K. Choi and T. S. Seo, Biosens. Bioelectron., 2014, 61, 485–490 CrossRef CAS PubMed.
- J. H. Jung, S. J. Oh, Y. T. Kim, S. Y. Kim, W. J. Kim, J. Jung and T. S. Seo, Anal. Chim. Acta, 2015, 853, 541–547 CrossRef CAS PubMed.
- K. Mitamura, H. Shimizu, M. Yamazaki, M. Ichikawa, K. Nagai, J. Katada, A. Wada, C. Kawakami and N. Sugaya, J. Virol. Methods, 2013, 194, 123–128 CrossRef CAS PubMed.
- N. Wiriyachaiporn, W. Maneeprakorn, C. Apiwat and T. Dharakul, Microchim. Acta, 2015, 182, 85–93 CrossRef CAS.
- T. T. Le, P. Chang, D. J. Benton, J. W. McCauley, M. Iqbal and A. E. G. Cass, Anal. Chem., 2017, 89, 6781–6786 CrossRef CAS PubMed.
- S. Bamrungsap, C. Apiwat, W. Chantima, T. Dharakul and N. Wiriyachaiporn, Microchim. Acta, 2014, 181, 223–230 CrossRef CAS.
- D. T. Lau, H. Ma, S. M. Lemon, E. Doo, M. G. Ghany, E. Miskovsky, G. L. Woods, Y. Park and J. H. Hoofnagle, J. Viral Hepatitis, 2003, 10, 331–334 CrossRef.
- K. S. Myint, M. Guan, H. Y. Chen, Y. Lu, D. Anderson, T. Howard, H. Noedl and M. P. Mammen Jr., Am. J. Trop. Med. Hyg., 2005, 73, 942–946 CAS.
- H. Y. Chen, Y. Lu, T. Howard, D. Anderson, P. Y. Fong, W. P. Hu, C. P. Chia and M. Guan, Clin. Diagn. Lab. Immunol., 2005, 12, 593–598 CAS.
- T. Salminen, E. Juntunen, N. Khanna, K. Pettersson and S. M. Talha, J. Virol. Methods, 2016, 228, 67–73 CrossRef CAS PubMed.
- L. Li, L. Zhou, Y. Yu, Z. Zhu, C. Lin, C. Lu and R. Yang, Diagn. Microbiol. Infect. Dis., 2009, 63, 165–172 CrossRef CAS PubMed.
- S. Huaibin, Y. Hang, N. Jin Zhong, X. Shasha, Z. Changhua, M. Lan and L. Lin Song, Nanotechnology, 2011, 22, 375602 CrossRef PubMed.
- J. Shen, Y. Zhou, F. Fu, H. Xu, J. Lv, Y. Xiong and A. Wang, Talanta, 2015, 142, 145–149 CrossRef CAS PubMed.
- US Food and Drug Administration, https://www.fda.gov/downloads/biologicsbloodvaccines/bloodbloodproducts/approvedproducts/premarketapprovalspmas/ucm364698.pdf, (accessed January 2018).
- S. Workman, S. K. Wells, C. P. Pau, S. M. Owen, X. F. Dong, R. LaBorde and T. C. Granade, J. Virol. Methods, 2009, 160, 14–21 CrossRef CAS PubMed.
- H. H. Lee, M. A. Dineva, Y. L. Chua, A. V. Ritchie, I. Ushiro-Lumb and C. A. Wisniewski, J. Infect. Dis., 2010, 201(Suppl 1), S65–S72 CrossRef CAS PubMed.
- B. A. Rohrman, V. Leautaud, E. Molyneux and R. R. Richards-Kortum, PLoS One, 2012, 7, e45611 CAS.
- Z. A. Parpia, R. Elghanian, A. Nabatiyan, D. R. Hardie and D. M. Kelso, JAIDS, J. Acquired Immune Defic. Syndr., 2010, 55, 413–419 CrossRef CAS PubMed.
- C. N. Loynachan, M. R. Thomas, E. R. Gray, D. A. Richards, J. Kim, B. S. Miller, J. C. Brookes, S. Agarwal, V. Chudasama, R. A. McKendry and M. M. Stevens, ACS Nano, 2018, 12, 279–288 CrossRef CAS PubMed.
- J. Hu, L. Wang, F. Li, Y. L. Han, M. Lin, T. J. Lu and F. Xu, Lab Chip, 2013, 13, 4352–4357 RSC.
- X. Fu, Z. Cheng, J. Yu, P. Choo, L. Chen and J. Choo, Biosens. Bioelectron., 2016, 78, 530–537 CrossRef CAS PubMed.
- H. Tsutsumi, K. Ouchi, M. Ohsaki, T. Yamanaka, Y. Kuniya, Y. Takeuchi, C. Nakai, H. Meguro and S. Chiba, J. Clin. Microbiol., 1999, 37, 2007–2009 CAS.
- A. Wada, Y. Sakoda, T. Oyamada and H. Kida, J. Virol. Methods, 2011, 178, 82–86 CrossRef CAS PubMed.
- X. Li, D. Lu, Z. Sheng, K. Chen, X. Guo, M. Jin and H. Han, Talanta, 2012, 100, 1–6 CrossRef CAS PubMed.
- N. A. Shaikh, J. Ge, Y. X. Zhao, P. Walker and M. Drebot, Clin. Chem., 2007, 53, 2031–2034 CAS.
- A. R. Sambol and S. H. Hinrichs, J. Virol. Methods, 2009, 157, 223–226 CrossRef CAS PubMed.
- S. D. Blacksell, J. A. Doust, P. N. Newton, S. J. Peacock, N. P. Day and A. M. Dondorp, Trans. R. Soc. Trop. Med. Hyg., 2006, 100, 775–784 CrossRef CAS PubMed.
- S. D. Blacksell, P. N. Newton, D. Bell, J. Kelley, M. P. Mammen Jr., D. W. Vaughn, V. Wuthiekanun, A. Sungkakum, A. Nisalak and N. P. Day, Clin. Infect. Dis, 2006, 42, 1127–1134 CrossRef PubMed.
- T. T. Nga, K. T. Thai, H. L. Phuong, P. T. Giao, Q. Hung le, T. Q. Binh, V. T. Mai, N. Van Nam and P. J. de Vries, Clin. Vaccine Immunol., 2007, 14, 799–801 CrossRef CAS PubMed.
- P. Dussart, L. Petit, B. Labeau, L. Bremand, A. Leduc, D. Moua, S. Matheus and L. Baril, PLoS Neglected Trop. Dis., 2008, 2, e280 Search PubMed.
- M. Moorthy, S. Chandy, K. Selvaraj and A. M. Abraham, Indian J. Med. Microbiol., 2009, 27, 254–256 CrossRef CAS PubMed.
- R. Lima Mda, R. M. Nogueira, H. G. Schatzmayr and F. B. dos Santos, PLoS Neglected Trop. Dis., 2010, 4, e738 Search PubMed.
- L. Osorio, M. Ramirez, A. Bonelo, L. A. Villar and B. Parra, Virol. J., 2010, 7, 361 CrossRef PubMed.
- V. Tricou, H. T. Vu, N. V. Quynh, C. V. Nguyen, H. T. Tran, J. Farrar, B. Wills and C. P. Simmons, BMC Infect. Dis., 2010, 10, 142 CrossRef PubMed.
- Y. Zhang, J. Bai and J. Y. Ying, Lab Chip, 2015, 15, 1465–1471 RSC.
- MD Doctors Direct, http://www.mddoctorsdirect.com/sites/default/files/product-pdf/46FK10_Chikungunya-IgM(D)_CE(Rev.4).pdf, (accessed January 2018).
- CTKbiotech Online, http://ctkbiotech.com/ctk-product/chikungunya-igm-combo-rapid-test-ce/, (accessed January 2018).
- S. D. Blacksell, A. Tanganuchitcharnchai, R. G. Jarman, R. V. Gibbons, D. H. Paris, M. S. Bailey, N. P. Day, R. Premaratna, D. G. Lalloo and H. J. de Silva, Clin. Vaccine Immunol., 2011, 18, 1773–1775 CrossRef CAS PubMed.
- H. Kosasih, S. Widjaja, E. Surya, S. H. Hadiwijaya, D. P. Butarbutar, U. A. Jaya, Nurhayati, B. Alisjahbana and M. Williams, Southeast Asian J. Trop. Med. Public Health, 2012, 43, 55–61 CAS.
- C. M. Prat, O. Flusin, A. Panella, B. Tenebray, R. Lanciotti and I. Leparc-Goffart, Emerging Infect. Dis., 2014, 20, 2129–2132 CrossRef CAS PubMed.
- E. I. Laderman, E. Whitworth, E. Dumaual, M. Jones, A. Hudak, W. Hogrefe, J. Carney and J. Groen, Clin. Vaccine Immunol., 2008, 15, 159–163 CrossRef CAS PubMed.
- J. Li, M. Zou, Y. Chen, Q. Xue, F. Zhang, B. Li, Y. Wang, X. Qi and Y. Yang, Anal. Chim. Acta, 2013, 782, 54–58 CrossRef CAS PubMed.
- P. Brangel, A. Sobarzo, C. Parolo, B. S. Miller, P. D. Howes, S. Gelkop, J. J. Lutwama, J. M. Dye, R. A. McKendry, L. Lobel and M. M. Stevens, ACS Nano, 2018, 12, 63–73 CrossRef CAS PubMed.
- D. Duan, K. Fan, D. Zhang, S. Tan, M. Liang, Y. Liu, J. Zhang, P. Zhang, W. Liu, X. Qiu, G. P. Kobinger, G. F. Gao and X. Yan, Biosens. Bioelectron., 2015, 74, 134–141 CrossRef CAS PubMed.
- A. E. Hagstrom, G. Garvey, A. S. Paterson, S. Dhamane, M. Adhikari, M. K. Estes, U. Strych, K. Kourentzi, R. L. Atmar and R. C. Willson, PLoS One, 2015, 10, e0126571 Search PubMed.
- I. Bosch, H. de Puig, M. Hiley, M. Carre-Camps, F. Perdomo-Celis, C. F. Narvaez, D. M. Salgado, D. Senthoor, M. O'Grady, E. Phillips, A. Durbin, D. Fandos, H. Miyazaki, C. W. Yen, M. Gelvez-Ramirez, R. V. Warke, L. S. Ribeiro, M. M. Teixeira, R. P. Almeida, J. E. Munoz-Medina, J. E. Ludert, M. L. Nogueira, T. E. Colombo, A. C. B. Terzian, P. T. Bozza, A. S. Calheiros, Y. R. Vieira, G. Barbosa-Lima, A. Vizzoni, J. Cerbino-Neto, F. A. Bozza, T. M. L. Souza, M. R. O. Trugilho, A. M. B. de Filippis, P. C. de Sequeira, E. T. A. Marques, T. Magalhaes, F. J. Diaz, B. N. Restrepo, K. Marin, S. Mattar, D. Olson, E. J. Asturias, M. Lucera, M. Singla, G. R. Medigeshi, N. de Bosch, J. Tam, J. Gomez-Marquez, C. Clavet, L. Villar, K. Hamad-Schifferli and L. Gehrke, Sci. Transl. Med., 2017, 9, eaan1589 CrossRef PubMed.
- J. H. Lee, H. S. Seo, J. H. Kwon, H. T. Kim, K. C. Kwon, S. J. Sim, Y. J. Cha and J. Lee, Biosens. Bioelectron., 2015, 69, 213–225 CrossRef CAS PubMed.
- C. W. Yen, H. de Puig, J. O. Tam, J. Gomez-Marquez, I. Bosch, K. Hamad-Schifferli and L. Gehrke, Lab Chip, 2015, 15, 1638–1641 RSC.
- X. Wang, N. Choi, Z. Cheng, J. Ko, L. Chen and J. Choo, Anal. Chem., 2017, 89, 1163–1169 CrossRef CAS PubMed.
- S. Kersting, V. Rausch, F. F. Bier and M. von Nickisch-Rosenegk, Malar. J, 2014, 13, 99 CrossRef PubMed.
- M. S. Cordray and R. R. Richards-Kortum, Am. J. Trop. Med. Hyg., 2012, 87, 223–230 CrossRef CAS PubMed.
- A. H. Moody and P. L. Chiodini, Br. J. Biomed. Sci., 2002, 59, 228–231 CrossRef CAS PubMed.
- S. Knecht, D. Ricklin, A. N. Eberle and B. Ernst, J. Mol. Recognit., 2009, 22, 270–279 CrossRef CAS PubMed.
- B. Gryseels, K. Polman, J. Clerinx and L. Kestens, Lancet, 2006, 368, 1106–1118 CrossRef.
- S. Cui and G. Tong, J. Vet. Diagn. Invest., 2008, 20, 567–571 CrossRef PubMed.
- D. Peng, S. Hu, Y. Hua, Y. Xiao, Z. Li, X. Wang and D. Bi, Vet. Immunol. Immunopathol., 2007, 117, 17–25 CrossRef CAS PubMed.
- D. H. Whang and T. Um, Korean J. Lab. Med., 2005, 25, 186–191 Search PubMed.
- Y. Khudyakov and S. Kamili, Virus Res., 2011, 161, 84–92 CrossRef CAS PubMed.
- M. H. K. Ansari, M. D. Omrani and V. Movahedi, Hepatitis Mon., 2007, 7, 87–91 Search PubMed.
- P. Yager, E. Fu, T. Liang, B. Lutz and J. L. Osborn, 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences 2011, MicroTAS 2011, vol. 3, pp. 2092–2095.
- G. Rezza, L. Nicoletti, R. Angelini, R. Romi, A. C. Finarelli, M. Panning, P. Cordioli, C. Fortuna, S. Boros, F. Magurano, G. Silvi, P. Angelini, M. Dottori, M. G. Ciufolini, G. C. Majori, A. Cassone and C. s. group, Lancet, 2007, 370, 1840–1846 CrossRef CAS.
- F. Staikowsky, F. Talarmin, P. Grivard, A. Souab, I. Schuffenecker, K. Le Roux, M. Lecuit and A. Michault, PLoS One, 2009, 4, e7603 Search PubMed.
- W. Van Bortel, F. Dorleans, J. Rosine, A. Blateau, D. Rousset, S. Matheus, I. Leparc-Goffart, O. Flusin, C. Prat, R. Cesaire, F. Najioullah, V. Ardillon, E. Balleydier, L. Carvalho, A. Lemaitre, H. Noel, V. Servas, C. Six, M. Zurbaran, L. Leon, A. Guinard, J. van den Kerkhof, M. Henry, E. Fanoy, M. Braks, J. Reimerink, C. Swaan, R. Georges, L. Brooks, J. Freedman, B. Sudre and H. Zeller, Euro. Surveill., 2014, 19, 20759 CrossRef PubMed.
- E. Burdino, G. Calleri, P. Caramello and V. Ghisetti, Emerging Infect. Dis., 2016, 22, 1837–1839 CrossRef PubMed.
- Centers for Disease Control and Prevention, https://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html (accessed January 2018).
- W. H. O. Ebola Response Team, B. Aylward, P. Barboza, L. Bawo, E. Bertherat, P. Bilivogui, I. Blake, R. Brennan, S. Briand, J. M. Chakauya, K. Chitala, R. M. Conteh, A. Cori, A. Croisier, J. M. Dangou, B. Diallo, C. A. Donnelly, C. Dye, T. Eckmanns, N. M. Ferguson, P. Formenty, C. Fuhrer, K. Fukuda, T. Garske, A. Gasasira, S. Gbanyan, P. Graaff, E. Heleze, A. Jambai, T. Jombart, F. Kasolo, A. M. Kadiobo, S. Keita, D. Kertesz, M. Kone, C. Lane, J. Markoff, M. Massaquoi, H. Mills, J. M. Mulba, E. Musa, J. Myhre, A. Nasidi, E. Nilles, P. Nouvellet, D. Nshimirimana, I. Nuttall, T. Nyenswah, O. Olu, S. Pendergast, W. Perea, J. Polonsky, S. Riley, O. Ronveaux, K. Sakoba, R. Santhana Gopala Krishnan, M. Senga, F. Shuaib, M. D. Van Kerkhove, R. Vaz, N. Wijekoon Kannangarage and Z. Yoti, N. Engl. J. Med., 2014, 371, 1481–1495 CrossRef PubMed.
- H. Bourhy, P. E. Rollin, J. Vincent and P. Sureau, J. Clin. Microbiol., 1989, 27, 519–523 CAS.
- A. Nishizono, P. Khawplod, K. Ahmed, K. Goto, S. Shiota, K. Mifune, T. Yasui, K. Takayama, Y. Kobayashi, K. Mannen, V. Tepsumethanon, C. Mitmoonpitak, S. Inoue and K. Morimoto, Microbiol. Immunol., 2008, 52, 243–249 CrossRef CAS PubMed.
- B. Kang, J. Oh, C. Lee, B. K. Park, Y. Park, K. Hong, K. Lee, B. Cho and D. Song, J. Virol. Methods, 2007, 145, 30–36 CrossRef CAS PubMed.
- S. Kasempimolporn, W. Saengseesom, S. Huadsakul, S. Boonchang and V. Sitprija, J. Vet. Diagn. Invest., 2011, 23, 1197–1201 CrossRef PubMed.
- D. K. Yang, E. K. Shin, Y. I. Oh, K. W. Lee, C. S. Lee, S. Y. Kim, J. A. Lee and J. Y. Song, J. Vet. Sci., 2012, 13, 43–48 CrossRef PubMed.
- P. Sharma, C. K. Singh and D. Narang, Vet. World, 2015, 8, 135–138 CrossRef CAS PubMed.
- World Health Organization, http://www.who.int/hiv/topics/vct/about/en/ (accessed January, 2018).
- G. Wu and M. H. Zaman, Bull. W. H. O., 2012, 90, 914–920 CrossRef PubMed.
- H. Arai, B. Petchclai, K. Khupulsup, T. Kurimura and K. Takeda, J. Clin. Microbiol., 1999, 37, 367–370 CAS.
- S. Aidoo, W. K. Ampofo, J. A. Brandful, S. V. Nuvor, J. K. Ansah, N. Nii-Trebi, J. S. Barnor, F. Apeagyei, T. Sata, D. Ofori-Adjei and K. Ishikawa, J. Clin. Microbiol., 2001, 39, 2572–2575 CrossRef CAS PubMed.
- R. Ribeiro-Rodrigues, L. Ferreira da Silva Pinto Neto, C. B. Cunha, V. P. Cabral and R. Dietze, Clin. Diagn. Lab. Immunol., 2003, 10, 303–307 CAS.
- T. C. Granade, B. S. Parekh, P. M. Tih, T. Welty, E. Welty, M. Bulterys, G. Ndikintum, G. Nkuoh and S. Tancho, Clin. Diagn. Lab. Immunol., 2005, 12, 855–860 CAS.
- Centers for Disease Control and Prevention, https://hiv-tests-advantages-disadvantages.pdf (accessed January, 2018).
- S. Phillips, T. C. Granade, C. P. Pau, D. Candal, D. J. Hu and B. S. Parekh, Clin. Diagn. Lab. Immunol., 2000, 7, 698–699 CAS.
- F. Ketema, C. Zeh, D. C. Edelman, R. Saville and N. T. Constantine, J. Acquired Immune Defic. Syndr., 2001, 27, 63–70 CrossRef CAS PubMed.
- H. Syed Iqbal, P. Balakrishnan, K. G. Murugavel and S. Suniti, J. Clin. Lab. Anal., 2008, 22, 178–185 CrossRef CAS PubMed.
- Y. Wang, H. Xu, M. Wei, H. Gu, Q. Xu and W. Zhu, Mater. Sci. Eng., C, 2009, 29, 714–718 CrossRef CAS.
- T. C. Granade, S. Workman, S. K. Wells, A. N. Holder, S. M. Owen and C. P. Pau, Clin. Vaccine Immunol., 2010, 17, 1034–1039 CrossRef CAS PubMed.
- M. D. Lindsley, N. Mekha, H. C. Baggett, Y. Surinthong, R. Autthateinchai, P. Sawatwong, J. R. Harris, B. J. Park, T. Chiller, S. A. Balajee and N. Poonwan, Clin. Infect. Dis, 2011, 53, 321–325 CrossRef PubMed.
- E. Uchio, K. Aoki, W. Saitoh, N. Itoh and S. Ohno, Ophthalmology, 1997, 104, 1294–1299 CrossRef CAS PubMed.
- T. Fujimoto, T. Okafuji, T. Okafuji, M. Ito, S. Nukuzuma, M. Chikahira and O. Nishio, J. Clin. Microbiol., 2004, 42, 5489–5492 CrossRef CAS PubMed.
- G. L. Campbell, A. A. Marfin, R. S. Lanciotti and D. J. Gubler, Lancet Infect. Dis., 2002, 2, 519–529 CrossRef PubMed.
- K. A. Gyure, J. Neuropathol. Exp. Neurol., 2009, 68, 1053–1060 CrossRef PubMed.
- P. Koraka, H. Zeller, M. Niedrig, A. D. Osterhaus and J. Groen, Microbes Infect., 2002, 4, 1209–1215 CrossRef CAS PubMed.
- A. K. Malan, P. J. Stipanovich, T. B. Martins, H. R. Hill and C. M. Litwin, Am. J. Clin. Pathol., 2003, 119, 508–515 CrossRef CAS PubMed.
- A. K. Malan, T. B. Martins, H. R. Hill and C. M. Litwin, J. Clin. Microbiol., 2004, 42, 727–733 CrossRef CAS PubMed.
- G. E. Fridley, H. Le and P. Yager, Anal. Chem., 2014, 86, 6447–6453 CrossRef CAS PubMed.
- J. Hu, S. Wang, L. Wang, F. Li, B. Pingguan-Murphy, T. J. Lu and F. Xu, Biosens. Bioelectron., 2014, 54, 585–597 CrossRef CAS PubMed.
- V. Venkatraman and A. J. Steckl, Biosens. Bioelectron., 2015, 74, 150–155 CrossRef CAS PubMed.
- J. Li and J. Macdonald, Biosens. Bioelectron., 2016, 85, 998–999 CrossRef CAS PubMed.
- X. Cao, Y. Ye and S. Liu, Anal. Biochem., 2011, 417, 1–16 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |