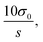Paper spray mass spectrometry for high-throughput quantification of nicotine and cotinine†
James E.
Keating
a,
John T.
Minges
b,
Scott H.
Randell
b and
Gary L.
Glish
 *a
*a
aDepartment of Chemistry, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA. E-mail: glish@unc.edu
bMarsico Lung Inst. Cyst. Fibrosis Res. Ctr., The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
First published on 28th November 2017
Abstract
The rapid release of new tobacco products requires high-throughput quantitative methods to support tobacco research. Sample preparation for LC-MS and GC-MS is time consuming and limits throughput. Paper spray tandem mass spectrometry (PS-MS/MS) is proposed and validated as a simple and rapid method for quantification of nicotine and cotinine in complex matrices to support tobacco-related research. Air liquid interface (ALI) human tracheobronchial epithelial cell (HTBEC) cultures were exposed to tobacco smoke using a Vitrocell VC-10 smoking machine. Apical culture washes (phosphate buffered saline, PBS) and basolateral media were analyzed with the PS-MS/MS method. GC-MS/MS was used as a comparative quantitative technique. The PS-MS/MS approach allowed for direct spotting of samples on the paper substrate, whereas the GC-MS/MS method required additional sample preparation in the form of solvent–solvent extraction. Limits of quantitation (LOQs) were higher with the PS-MS/MS approach than GC-MS/MS, but still below the relevant concentrations found in HTBEC smoke exposure experiments as well as most clinical applications. PS-MS/MS is readily achieved on mass spectrometers that include atmospheric pressure inlets, and allows for convenient quantification from complex matrices that would otherwise require additional sample preparation and chromatographic separation.
Introduction
Mass spectrometry provides a highly sensitive and selective method of quantification for a wide variety of analyte molecules. In tobacco-related research, quantification of small molecules, especially those related to nicotine through structure or metabolism, is of particular interest, and has been well-established.1–3 Traditionally, the complexity of sample matrices relevant to tobacco research has required pre-treatment and/or chromatographic separation prior to mass spectrometric analysis.4,5 Gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) are powerful techniques for separating analytes and interfering compounds, but are incompatible with high salt concentrations and matrix interferents inherent to the analysis of biological fluids. The use of chromatography also increases analysis time and adds complexity to the analytical system, requiring additional reagents and maintenance beyond what is required for mass spectrometry alone.For rapid and convenient quantification it is preferable to bypass sample cleanup and chromatographic separations and introduce samples directly to the mass spectrometer. The recent development of ambient ionization techniques has made this possible, and consequently these techniques have garnered a great deal of attention.6,7 Many of these techniques, including direct analysis in real time (DART) and desorption electrospray ionization (DESI), make it possible to directly analyze solids and surfaces.8,9 However, the nature of solid samples makes these ionization methods more suitable for qualitative or semi-quantitative measurements than absolute quantification due to difficulty in preparing calibration standards. Paper spray ionization has been recognized as a powerful ambient ionization technique for direct analysis of solutions of complex matrices.10,11,17
The mechanism of paper spray is analogous to the widely-used electrospray ionization, with formation of a Taylor cone following application of solvent and high voltage to a sharp-tipped piece of paper.12 Droplet size and internal energy distributions of ions resulting from paper spray have been shown to be most similar to those generated by nano-electrospray ionization.13 Sample (1–50 μL) is spotted directly on a rigid piece of paper, allowed to dry, and analyzed following application of spray solvent (10–100 μL) and high voltage (3.5–7 kV). Analyte is wicked towards the paper tip through multiple porous pathways via capillary action based on extraction compatibility with the spray solvent. Analyte wicking provides significant tolerance for interfering compounds and salts in the matrix and adds an extra degree of chemical specificity to the method. Single-use paper substrates mitigate carry-over often encountered in GC-MS and LC-MS approaches. This can improve limits of quantitation when working with analytes prone to carry-over on chromatographic systems.14,15
Paper spray mass spectrometry (PS-MS) has previously been applied to the analysis of nicotine and related alkaloids in biofluids including blood, urine, and saliva.16In vitro cytotoxicity assays are an important tool in tobacco research, enabling the testing of a large number of tobacco products over a range of experimental conditions that wouldn't be practical in vivo.17–19 Herein, quantification of nicotine and cotinine in matrices relevant to in vitro human tracheobronchial epithelial cell (HTBEC) tobacco exposure experiments was performed to further demonstrate the applicability of paper spray to tobacco research. These matrices, including the media for air-liquid interface (ALI) cultures (termed “ALI media”) and phosphate-buffered saline (PBS), contain high salt concentrations (mM range) and the ALI media contains many cell growth supplements and additives, making them unsuitable for LC and GC, as well as direct analysis by most MS techniques. The compatibility of PS and complex matrices enabled the development of quantification methods using paper spray ionization and tandem mass spectrometry (MS/MS) for rapid and selective analysis of nicotine and cotinine. The developed PS-MS/MS methods were applied to samples from HTBEC cultures exposed to whole gas phase tobacco smoke in a high-throughput Vitrocell VC-10 smoking machine and compared to an extraction-based GC-MS/MS method.
Experimental
Calibrators and samples
Nicotine was purchased from MilliporeSigma (St. Louis, MO), and nicotine-d4, cotinine, and cotinine-d3 were purchased from Cerilliant (Round Rock, TX). Water (Optima grade), acetonitrile (Optima grade), and acetic acid (Optima grade) were purchased from Fisher Scientific (Hampton, NH). PBS and ALI media were prepared (ALI media protocol is given in ESI†) and used in pre-exposure HTBEC smoke exposure experiments as an apical lavage and basolateral cell media, respectively, to provide true matrix blanks for method development. Calibrators for nicotine, cotinine, and isotopically labeled standards were prepared in acetonitrile. To 60 μL of pre-exposure PBS or ALI sample, 10 μL of each standard was added and vortexed (30 s) for final nicotine and cotinine concentrations ranging from 10 to 1500 ng mL−1 and isotopically labeled standard concentrations of 1000 ng mL−1. Nicotine and cotinine in pre-exposure PBS and ALI were used directly in PS-MS/MS. Nicotine calibrators for GC-MS/MS were prepared by adjusting the pH to 13 by addition of 2.5 M NaOH followed by liquid–liquid extraction of PBS and ALI samples with 1 mL of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 methyl tert-butyl ether
40 methyl tert-butyl ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane
dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate, sample drying, and reconstitution in 80 μL of acetonitrile.20
ethyl acetate, sample drying, and reconstitution in 80 μL of acetonitrile.20
Smoke exposures
Briefly, ALI HTBEC 6.5 mm Transwell (Costar, 3470) cell cultures were created as previously described.21 Exposure to 3R4F reference cigarette smoke was performed in a Vitrocell VC10 exposure system under ISO 3402 standard conditions (https://www.iso.org/standard/28324.html). Tobacco smoke was diluted with humidified air to final smoke percentages of 58.3%, 35.9%, 21.9%, 15.7%, 12.3%, and 0% (air only). Cells from four non-smoking lung donors, procured under UNC Biomedical IRB-approved protocol # 12-2293, were exposed to each smoke dilution in quadruplicate. Apical culture washings with PBS and conditioned ALI basolateral media were collected following tobacco smoke exposure. Samples were prepared for analysis by adding nicotine-d4 and cotinine-d3 to final concentrations of 1000 ng mL−1. The solvent–solvent extraction method described above was used to further prepare exposure samples for GC-MS/MS analysis.Instrumentation
PS-MS/MS experiments were performed with a Prosolia Velox 360 coupled to a Thermo Scientific LTQ-FT mass spectrometer. The mass spectrometer was set to recognize the Velox 360 as a nano-electrospray ionization source. GC-MS/MS experiments were performed on a Bruker EVOQ gas chromatograph-triple quadrupole mass spectrometer. A Vitrocell VC-10 smoking machine was used for in vitro HTBEC smoke exposure experiments.Instrument parameters
Paper spray was performed by manually pipetting 10 μL of sample onto the Velox sample cartridge. A solvent pump on the Velox 360 dispenses 100 μL of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1% acetonitrile
0.1% acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid spray solution and spray voltage (7.5 kV for PBS, 5.5 kV for ALI) is applied. MS/MS analysis was performed for 2 minutes per cartridge by monitoring the following transitions: m/z 163.2 to m/z 132.2 for nicotine, m/z 167.2 to m/z 136.2 for nicotine-d4, m/z 177.2 to m/z 98.2 for cotinine, and m/z 180.2 to m/z 101.2 for cotinine-d3. The inlet capillary of the Thermo LTQ-FT was held at 200 °C, and the sheath, auxiliary, and sweep gases were turned off. Mass spectrometer settings were chosen based on standard nESI source parameters.
acetic acid spray solution and spray voltage (7.5 kV for PBS, 5.5 kV for ALI) is applied. MS/MS analysis was performed for 2 minutes per cartridge by monitoring the following transitions: m/z 163.2 to m/z 132.2 for nicotine, m/z 167.2 to m/z 136.2 for nicotine-d4, m/z 177.2 to m/z 98.2 for cotinine, and m/z 180.2 to m/z 101.2 for cotinine-d3. The inlet capillary of the Thermo LTQ-FT was held at 200 °C, and the sheath, auxiliary, and sweep gases were turned off. Mass spectrometer settings were chosen based on standard nESI source parameters.
GC-MS/MS was performed with 1 μL splitless injections with an injector temperature of 280 °C, transfer line temperature of 250 °C, and electron ionization source temperature of 200 °C. Helium (Airgas, 99.999% purity) was used as a carrier gas with a linear velocity of 30 cm s−1. The oven temperature was ramped from 70 to 260 °C for a total run-time of 14 minutes. MS/MS analysis was performed by monitoring the following transitions: m/z 162.2 to m/z 84.1 for nicotine and m/z 166.2 to m/z 84.1 for nicotine-d4.
Results and discussion
PS-MS/MS method development
The duration of a PS-MS/MS analysis is defined by application of high voltage to the Velox sample cartridge for the formation of electrospray. At the end of an experiment the voltage is turned off and the cartridge is discarded. Signal stability of the nicotine-d4 product ion (m/z 136.2) during PS-MS/MS analysis is shown in Fig. 1. Signal intensity reaches a maximum rapidly upon application of high voltage (HV) and decreases during analysis, eventually reaching zero intensity if HV remains on.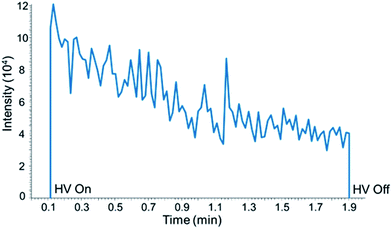 | ||
| Fig. 1 Extracted ion chronogram of nicotine-d4 product ion (m/z 136.2) showing signal intensity over 2 minute PS-MS/MS analysis. | ||
Application of 100 μL of spray solvent generates signal for approximately 5 minutes before reaching zero intensity. This is attributed to loss and evaporation of the 90/10/0.1% acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid spray solvent from the cartridge. Replenishing the spray solvent restores the signal intensity, and some custom-built paper spray sources include the ability to continuously add spray solvent for longer run times.22 The variation in signal intensity over time is corrected by using the ratio of analyte to isotopically labeled standard product ions for quantification, and for single-use cartridges a short run time is sufficient. Data analysis is performed by integrating the signal intensity over the 2 minute analysis for each analyte and isotopically labeled standard.
acetic acid spray solvent from the cartridge. Replenishing the spray solvent restores the signal intensity, and some custom-built paper spray sources include the ability to continuously add spray solvent for longer run times.22 The variation in signal intensity over time is corrected by using the ratio of analyte to isotopically labeled standard product ions for quantification, and for single-use cartridges a short run time is sufficient. Data analysis is performed by integrating the signal intensity over the 2 minute analysis for each analyte and isotopically labeled standard.
PS-MS/MS and GC-MS/MS calibration
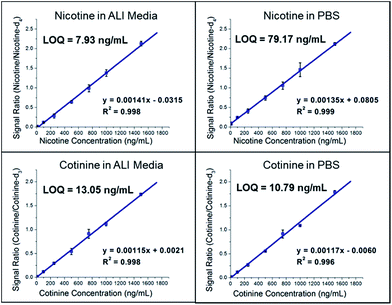
Quantification of nicotine and cotinine in pre-exposure PBS and ALI was completed in triplicate using the PS-MS/MS methods developed and outlined above. Calibration (pre-exposure) matrices were designed to match the exposure sample matrices by collecting apical washes (PBS) and basal media (ALI) from HTBEC cells that had not been exposed to tobacco smoke. Matrix matching ensures consistency between calibrators and exposure samples, as materials from the cells are likely to be present after contact between the cells and solution. Calibrators for each analyte/matrix combination exhibited linearity within the entire calibration region found to be relevant to the HTBEC smoke exposure experiments. Plots for each are shown in Fig. 2. Limits of quantitation (LOQs) are calculated as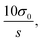 where σ0 is the standard deviation of the blank (internal standard only) measurements and s is the sensitivity of the method. Quantification of nicotine using the GC-MS/MS method was completed to provide results from a standard method to compare PS-MS/MS performance. LOQ's from the two methods are as follows: 79.17 ng mL−1 (PS-MS/MS) vs. 24.35 ng mL−1 (GC-MS/MS) for nicotine in PBS and 7.39 ng mL−1 (PS-MS/MS) vs. 5.42 ng mL−1 (GC-MS/MS) for nicotine in ALI.
where σ0 is the standard deviation of the blank (internal standard only) measurements and s is the sensitivity of the method. Quantification of nicotine using the GC-MS/MS method was completed to provide results from a standard method to compare PS-MS/MS performance. LOQ's from the two methods are as follows: 79.17 ng mL−1 (PS-MS/MS) vs. 24.35 ng mL−1 (GC-MS/MS) for nicotine in PBS and 7.39 ng mL−1 (PS-MS/MS) vs. 5.42 ng mL−1 (GC-MS/MS) for nicotine in ALI.
An indirect comparison of the PS-MS/MS method to a clinically applicable test to distinguish smokers from non-smokers using cotinine concentrations in urine reports a cutoff of 49.7 ng mL−1, which is well above LOQ's for cotinine in PBS (10.79 ng mL−1) and ALI (13.05 ng mL−1).23 Urine was selected for comparison with ALI and PBS due to its high salt concentration, which is believed to be the main contributor to higher LOQs for the PS-MS/MS method over the GC-MS/MS method. As the relevant nicotine concentrations for the tobacco smoke exposure experiments are well above the LOQ's of both methods we conclude that PS-MS/MS is a suitable quantitative technique for these analyses. Unless an application requires the lower LOQ provided by the GC-MS/MS calibration experiment then the dramatically reduced sample preparation and analysis time of the PS-MS/MS method is preferable.
PS-MS/MS and GC-MS/MS HTBEC smoke exposure results
Nicotine in PBS apical lavages from the HTBEC smoke exposure experiments were quantified using the PS-MS/MS and GC-MS/MS methods. Average concentrations (n = 4) for each dilution of tobacco smoke for each method along with associated uncertainty values (RSDs) appear in Table 1.High RSD values (>20%) are related to inconsistency of smoke exposure, rather than the quantitative methods. This is supported by the low RSD's (2–13%) observed during calibration experiments using process matrix blanks of PBS.
To verify that the PS-MS/MS and GC-MS/MS methods are equivalent, a Passing–Bablok regression was used.24 Passing–Bablok tests for statistically significant differences in the slope and intercept from 1 and 0, respectively, of a linear regression between results from two analytical methods. The linear relationship between the calibration results from each apical lavage sample (n = 24) from GC-MS/MS and PS-MS/MS (x and y variables, respectively) was confirmed using a cumulative sum test (p = 0.249).
Results from the Passing–Bablok model calculations are as follows: slope = 1.125 (0.998 to 1.292, 95% CI) and intercept = 8.4 (−22.1 to 33.5, 95% CI). The bounds of the slope and intercept at the 95% confidence include the ideal values of 1 and 0, suggesting that the results from the PS-MS/MS and GC-MS/MS nicotine quantification from PBS apical lavages are statistically equivalent in the concentration range relevant to HTBEC smoke exposure.
Conclusions
Coupling high-throughput quantification with PS-MS/MS to high-throughput tobacco smoke exposure experiments with the Vitrocell VC-10 greatly increases the number of experiments that can be completed in a set period of time. Reducing the time required for sample preparation as well as analysis time of the method itself can permit screening of additional parameters for in vitro as well as in vivo studies (i.e. greater diversity of tobacco-product brands, concentration of tobacco smoke, etc.) to allow tobacco research to keep pace with the growing number of products. New and emerging tobacco products, especially electronic cigarettes (e-cigarettes) are of particular interest, in part because of the rapid release of new products as well as the relative lack of toxicity data.25–28The validation of PS-MS/MS as a quantitative technique for analysis of nicotine and cotinine in complex matrices is a step towards a comprehensive toxicity screening platform with greater sampling capacity than what is available with current chromatography-based mass spectrometry methods. The simplicity of coupling PS to mass spectrometers with atmospheric pressure interfaces, which includes the vast majority of LC-MS instruments, makes it an attractive option for increasing sample throughput in tobacco-related research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Heart, Lung, and Blood Institute at the National Institute of Health (Grant #5-P50-HL-120100-01). We thank Prosolia Inc. for providing the Velox 360 PaperSpray source, Bruker Daltonics for providing the EVOQ 456 GC-MS platform, and Dr Russ Grant for helpful discussions.Notes and references
- B. H. Jung, B. C. Chung, S. J. Chung, M.-H. Lee and C. K. Shim, J. Pharm. Biomed. Anal., 1999, 20, 195–202 CrossRef CAS PubMed.
- J. T. Bernert, W. E. Turner and J. L. Pirkle, et al. , Clin. Chem., 1997, 43, 2281–2291 CAS.
- T. P. Moyer, J. R. Charlson, R. J. Enger, L. C. Dale, J. O. Ebbert, D. R. Schroeder and R. D. Hurt, Clin. Chem., 2002, 48, 1460–1471 CAS.
- B. A. Tomkins, G. J. Van Berkel, R. A. Jenkins and R. W. Counts, J. Anal. Toxicol., 2006, 30, 178–186 CrossRef CAS PubMed.
- R. Dams, M. A. Huestis, W. E. Lambert and C. M. Murphy, J. Am. Soc. Mass Spectrom., 2003, 14, 1290–1294 CrossRef CAS PubMed.
- R. G. Cooks, Z. Ouyang, Z. Takats and J. M. Wiseman, Science, 2006, 311, 1566–1570 CrossRef CAS PubMed.
- M. Z. Huang, C. H. Yuan, S.-C. Cheng, Y.-T. Cho and J. Shiea, Annu. Rev. Anal. Chem., 2010, 3, 43–65 CrossRef CAS PubMed.
- R. B. Cody, J. A. Laramée and H. D. Durst, Anal. Chem., 2005, 77, 2297–2302 CrossRef CAS PubMed.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Science, 2004, 306, 471–473 CrossRef CAS PubMed.
- J. Liu, H. Wang, N. E. Manicke, J. M. Lin, R. G. Cooks and Z. Ouyang, Anal. Chem., 2010, 82, 2463–2471 CrossRef CAS PubMed.
- H. Wang, N. E. Manicke, Q. Yang, L. Zheng, R. Shi, R. G. Cooks and Z. Ouyang, Anal. Chem., 2011, 83, 1197–1201 CrossRef CAS PubMed.
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Science, 1989, 246, 64–71 CAS.
- H. Wang, J. Liu, R. G. Cooks and Z. Ouyang, Angew. Chem., Int. Ed., 2010, 122, 889–892 CrossRef.
- P. T. Vallano, S. B. Shugarts, E. J. Woolf and B. K. Matuszewski, J. Pharm. Biomed. Anal., 2005, 36, 1073–1078 CrossRef CAS PubMed.
- R. Bakhtiar and T. K. Majumdar, J. Pharmacol. Toxicol. Methods, 2007, 55, 262–278 CrossRef CAS PubMed.
- H. Wang, Y. Ren, M. N. McLuckey, N. E. Manicke, J. Park, L. Zheng, R. Shi, R. G. Cooks and Z. Ouyang, Anal. Chem., 2013, 85, 11540–11544 CrossRef CAS PubMed.
- W. Sun, R. Wu and J. A. Last, Toxicology, 1995, 100, 163–174 CrossRef CAS PubMed.
- E. Elmore, T. T. Luc, V. E. Steele, G. J. Kelloff and J. L. Redpath, Methods Cell Sci., 2000, 22, 17–24 CrossRef CAS PubMed.
- F. Cervellati, X. M. Muresan, C. Sticozzi, R. Gambari, G. Montagner, H. J. Forman, C. Torricelli, E. Maioli and G. Valacchi, Toxicol. In Vitro, 2014, 28, 999–1005 CrossRef CAS PubMed.
- L. Carlos, F. Gomes, F. Oliveira and L. L. Pucci, Rev. Cienc. Farm. Basica Apl., 2013, 34, 177–182 Search PubMed.
- M. Leslie Fulcher, S. Gabriel, K. A. Burns, J. R. Yankaskas and S. H. Randell, in Human Cell Culture Protocols, Humana Press, New Jersey, 2005, pp. 183–206 Search PubMed.
- S. L. Reeber, S. Gadi, S.-B. Huang and G. L. Glish, Anal. Methods, 2015, 7, 9808–9816 RSC.
- M. J. Jarvis, H. Tunstall-Pedoe, C. Feyerabend, C. Vesey and Y. Saloojee, Am. J. Public Health, 1987, 77, 1435–1438 CrossRef CAS PubMed.
- H. Passing and W. Bablok, Clin. Chem. Lab. Med., 1983, 21, 709 CAS.
- B. M. Kuehn, J. Am. Med. Assoc., 2009, 302, 937 CrossRef CAS PubMed.
- D. L. Palazzolo, Front. Public Health, 2013, 1, 56 Search PubMed.
- M. J. Schroeder and A. C. Hoffman, Tob. Control, 2014, 23(2), 30–35 CrossRef PubMed.
- R. Z. Behar, et al. , Nicotine Tob. Res., 2014, 17, 1–8 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ay02204b |
| This journal is © The Royal Society of Chemistry 2018 |