Enhancing the electrocatalytic activity of CoO for the oxidation of 5-hydroxymethylfurfural by introducing oxygen vacancies†
Abstract
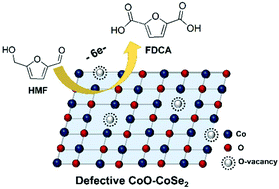
The electrochemical oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) is a highly attractive strategy to generate valuable biomass-based oxygenated chemicals. Robust, stable and inexpensive electrocatalysts are crucial for this interesting reaction. In this work, we found that the electrocatalytic performance of cobalt oxide (CoO) could be significantly improved by introducing oxygen vacancies via Se doping. The resulting CoO-CoSe2 with a CoO/CoSe2 molar ratio of 23 : 1 showed excellent performance and stability for the electro-oxidation of HMF to FDCA, and a FDCA yield of 99% could be achieved with a faradaic efficiency of 97.9% at a potential of 1.43 V vs. RHE. A systematic study indicates that the introduction of rich oxygen vacancies could enhance the catalytic activity and the selectivity to FDCA by increasing the electrochemical surface area and reducing charge transfer resistance. As far as we know, this is the first work to develop a highly stable metal oxide as the active component for this reaction.


 Please wait while we load your content...
Please wait while we load your content...