Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of nanoscale order–disorder transitions on the magnetic properties of Heusler compounds for spintronics†
J.
Karel‡
 *a,
J. E.
Fischer
*a,
J. E.
Fischer
 a,
S.
Fabbrici
b,
E.
Pippel
c,
P.
Werner
c,
M.
Vinicius Castergnaro
d,
P.
Adler
a,
S.
Ouardi
a,
B.
Balke
e,
G. H.
Fecher
a,
S.
Fabbrici
b,
E.
Pippel
c,
P.
Werner
c,
M.
Vinicius Castergnaro
d,
P.
Adler
a,
S.
Ouardi
a,
B.
Balke
e,
G. H.
Fecher
 a,
J.
Morais
a,
J.
Morais
 d,
F.
Albertini
b,
S. S. P.
Parkin
c and
C.
Felser
a
d,
F.
Albertini
b,
S. S. P.
Parkin
c and
C.
Felser
a
aMax Planck Institute for Chemical Physics of Solids, Dresden, Germany
bInstitute of Materials for Electronics and Magnetism, Parma, Italy
cMax Planck Institute of Microstructure Physics, Halle, Germany
dDepartamento de Fisica, Instituto de Fisica, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil
eInstitut für Anorganische und Analytische Chemie, Johannes Gutenberg-Universität, Mainz, Germany
First published on 2nd May 2017
Abstract
Modifications in nanoscale chemical order are used to tune the magnetic properties, namely TC, of Co2FeSixAl1−x (0 < x < 1). High-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) with Z-contrast reveals nanoscale regions of L21 order within a B2 matrix in the off-stoichiometry samples. Perhaps surprisingly, the latter, more chemically disordered structure, exhibits a higher TC. Upon annealing, the off-stoichiometry samples become more homogeneous with the fraction of L21 order decreasing. The short-range order was also investigated using X-ray absorption fine structure (XAFS) measurements at the Co and Fe K edges. Since the local atomic environments of Co atoms in the L21 and B2 structures are identical, the features presented in the Co K edge XAFS data are the same in both cases. By contrast, the L21 and B2 structures exhibit different signatures at the Fe K edge owing to the different chemical environments. Fitting of these spectra confirms the nanoscale chemical disorder observed by HAADF-STEM and the expected role this disorder plays on TC. Our results point to a methodology that might be extended to modify the magnetic and electronic properties of any Heusler compound; chemical disorder can be an engineering tool to realize highly tailored properties.
Introduction
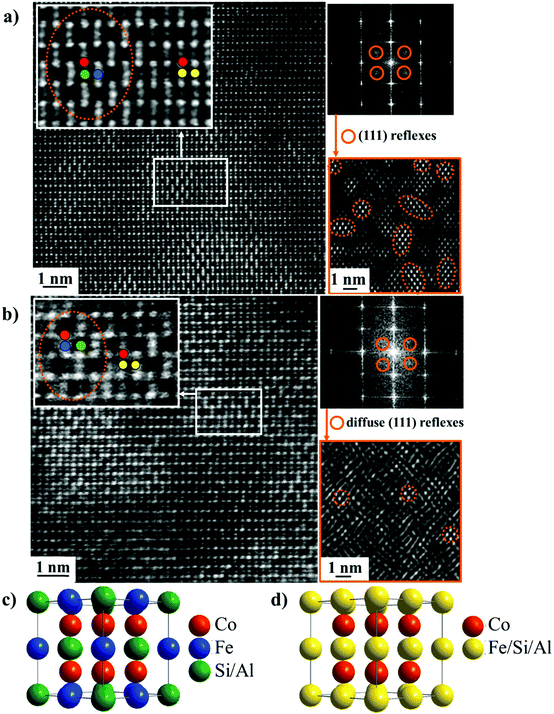
Increasingly challenging materials requirements for spintronics applications have initiated research efforts towards designing new materials with carefully tailored properties.1,2 The requirements for spin transfer torque (STT) applications are a perfect example, where an ideal candidate material possesses high spin polarization, low saturation magnetization, a high magnetic Curie temperature (TC), uniaxial magnetic anisotropy and low Gilbert damping.1 One commonly used design concept is valence electron counting (via chemical composition). Heusler compounds are a particularly attractive class of materials from this perspective.2 In the full Heusler X2YZ compounds, the X, Y and Z chemical species can be easily substituted to control the magnetic and electronic properties over a very large parameter space. Additionally, chemical disorder, despite it typically being viewed as detrimental, has also been proposed as an engineering tool for spintronic materials. For instance, Chadov et al. showed theoretically that chemical disorder in Mn3Ga-based Heusler compounds can lead to spin-selective electron localization, which produces enhanced spin polarization.3In the work presented here, we use changes in nanoscale chemical order to modify the magnetic properties of Co2FeSixAl1−x (0 < x < 1). This composition series was chosen for study since both end members, Co2FeSi and Co2FeAl, display technologically useful properties, such as high TC, low damping and half metallicity.4–9 Co2FeSi crystallizes in the L21 structure shown in Fig. 2c while Co2FeAl typically presents partial chemical order (B2 order) shown in Fig. 2d. The Fermi energy (EF) is near the top (bottom) of the minority states gap in Co2FeAl (Co2FeSi), and, consequently, disorder can eliminate the half-metallic behavior.4 Thus, our study is focused on the off-stoichiometry compositions in this series where EF can be shifted to the middle of the minority states gap, thus making the spin-polarization more robust to disorder.10 In this composition range, chemical disorder can then be used to tune the properties, namely TC which is crucially important in maintaining stability in spintronic devices. We will show that B2 structured Co2FeAl exhibits a TC of 1218 K, which is higher than previously reported.11 In the off-stoichiometry samples, high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) with Z-contrast reveals nanoscale puddles of L21 order within a B2 matrix. Perhaps surprisingly, the latter, more chemically disordered structure, exhibits a higher TC. Upon annealing, the off-stoichiometry samples become more homogeneous with the fraction of L21 order decreasing. Hence a simple annealing procedure to induce chemical disorder on the nanoscale can be used to enhance TC in a regime where the spin-polarization should be robust to disorder. Our results suggest a similar methodology to modify the magnetic properties can be employed in thin films, where disorder can be introduced during growth.
Experimental procedure
The compounds Co2FeSixAl1−x with x = 0, 0.2, 0.4, 0.6, 0.8 and 1.0 were synthesized by arc-melting stoichiometric quantities of the pure elements (purity at least 99.999+%) under an argon atmosphere. The ingots were remelted three times to improve the homogeneity. For annealing, the ingots were sealed in a tantalum tube, which was encapsulated in a quartz ampoule. Co2FeSi was annealed at 1000 °C for 21 days, Co2FeAl was annealed at 900 °C for 10 days and the off-stoichiometric compositions were annealed at 730 °C for 7 days; all samples were subsequently quenched in a mixture of water and ice. Samples were characterized using X-ray diffraction, scanning electron microscopy/wavelength dispersive X-ray spectroscopy (SEM/WDX), 57Fe Mössbauer spectrometry, SQUID magnetometry and AC magnetic susceptibility using thermomagnetic analysis (TMA). HAAD-STEM with Z-contrast and X-ray absorption fine structure (XAFS) were measured on samples with composition Co2FeSi0.2Al0.8 annealed at 730 °C for 7 days. Further details of the sample preparation and characterization can be found in the ESI.†Results and discussion
X-ray diffraction patterns do not indicate any impurity phases and show the compounds become more B2-like with increasing Al content, as revealed by a gradual reduction in the intensity of the (111) peak. 57Fe Mössbauer spectrometry shows only one Fe sextet for each sample, indicating a lack of Co–Fe disorder. SEM/WDS analysis reveals average compositions that agree well with the target compositions and no indication of impurity phases. Further details of the sample characterization are given in the ESI.†The total magnetic moment per formula unit m at 2 K as a function of the number of valence electrons (NVE) is shown in Fig. 1a. All the samples (except Co2FeAl) closely follow the Slater–Pauling rule represented by the solid black line, indicating half-metallic ferromagnetism.12 Co2FeAl has the B2 structure, and its deviation from Slater–Pauling behavior has been previously reported and ascribed to this partial chemical disorder.4 The fact that m varies only by changing the non-magnetic Z element is well understood by the Slater–Pauling rule, which fixes the Fermi energy in the minimum (band gap) of the minority electron density of states. Thus, the number of occupied minority electrons is also fixed, and a change in the number of valence electrons can only result in a change in the number of majority electrons, independent of what atom they reside on. Finally, the magnetic moment in the primitive cell is just given by the difference in the number of majority and minority electrons.
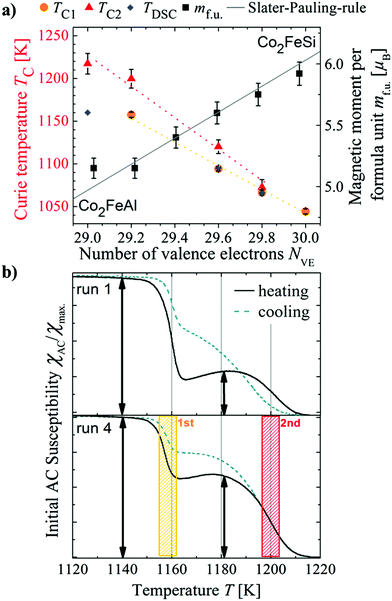 | ||
| Fig. 1 (a) Curie temperature, transition temperatures observed in the DSC measurement and magnetic moment per formula unit as a function of the number of valence electrons. (b) Examples of TMA measurements on one piece of the sample Co2FeSi0.2Al0.8. The upper picture shows the first measurement on a virgin sample, below the fourth measurement carried out on the same piece is depicted. The relative intensity of the second transition grows with increased number of heating and cooling cycles until reaching an equilibrium state in which the transition temperature and the transition's intensity remain stable. The values shown for the TC in Table S1 (ESI†) and (a) are determined from the equilibrium signal. Once this equilibrium is reached, the differences between the transition temperatures in the heating and cooling curves disappear. | ||
Fig. 1a also shows a decrease in TC of nearly 200 K with an increase of only 1 valence electron; this behavior is in contradiction to the theoretically predicted and experimentally observed trends in Co2-based Heusler materials where TC tends to increase with increasing NVE.13,14 However, these predictions and observations were shown over a much larger range of NVE and in compounds with the L21 structure, whereas here the Al containing members of the series partly or completely exhibit the B2 structure. Evidently, the degree of order in the sample is the more important factor when studying the trend in the TC over such a small range in NVE. In addition, the off-stoichiometry samples exhibit two transition temperatures (Fig. 1b), originating from two separate magnetic phases. The endpoints of the composition range, by contrast, present a single magnetic transition temperature, with Co2FeAl demonstrating the highest reported TC of 1218 K.
Fig. 1b displays susceptibility measurements after 4 annealing cycles for x = 0.2; one cycle consists of heating and cooling slowly from 300 K to 1226 K. These curves show that after annealing, the magnetic phase with the higher TC grows. The lower transition temperature corresponds to endothermic transitions below the melting point found in differential scanning calorimetry measurements and to magnetic transition temperatures found by other groups.11,15,16 The second transition has not been observed before and does not correspond to structural transition temperatures found by Umetsu et al.11
To probe the origin of these separate transitions, two samples of Co2FeSi0.2Al0.8 were studied in detail. One sample (#1) was synthesized and annealed using the procedure described above. A second sample (#2), from the same ingot as #1, was additionally heated and cooled slowly four times between room temperature and 1226 K, to simulate the conditions in the TMA apparatus. HAADF-STEM (Z-contrast) images were taken along the (110) zone axis (Fig. 2a and b). Filtering these images using the (111) reflections leads to the highlighted L21 ordered areas (orange circles in Fig. 2a and b). A sharp contrast exists between the L21 ordered areas and the B2 areas in sample #1, and we assign the transitions observed in the AC susceptibility curves to the Curie temperatures of the L21 (TC1) and B2 (TC2) phases based on these results. The first transition is sharper, and the second transition is broadened, which is consistent with TC1 corresponding to the ordered phase, while TC2 originates from the phase with more chemical disorder. Upon annealing (sample #2), the fraction of the L21 phase decreases, and the contrast between the L21 and B2 phases is not as sharp. This observation suggests a homogenization of the structure, which is more B2-like after annealing.
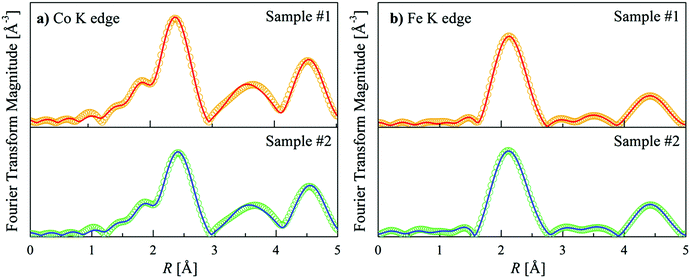
The short-range order was also investigated using XAFS measurements at the Co and Fe K edges. In this case, a new sample was prepared following the same synthesis route and annealing as was done for samples #1 and #2. The main structural parameters were obtained by fitting the Fourier transforms (FT) of the extended X-ray absorption fine structure (EXAFS) signals at both edges (see ESI†). Since the local atomic environments of Co atoms in the L21 and B2 structures are identical, the features presented in the Co K edge EXAFS data will be the same in both cases. By contrast, the L21 and B2 structures present different signatures at the Fe K edge owing to the different chemical environments, which lead to distinct photoelectron scattering processes within the sample and, thus, distinct EXAFS signals.
FEFF 9.617 was used to calculate the scattering amplitudes and phase shifts resulting from the photoelectron scattering processes in both the B2 and L21 structures for Co2FeZ. The best fits of the FTs of samples #1 and #2 at the Co and Fe edges are presented in Fig. 3a and b, respectively, and the structural parameters obtained from this analysis are given in Table S3 and S4 of the ESI.† The coordination numbers obtained for both samples are in agreement with those expected for Fe in Co2FeZ in a B2 structure, despite small variations in the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio at longer distances. For sample #1, the number of Z atoms as second nearest neighbors was 13% higher than expected for a perfect B2 structure, and the number of Fe atoms at the same distance was 16% lower. Moreover, the number of Z atoms as third nearest neighbors was 7% lower than expected for a perfect B2 structure, and the number of Fe atoms was 10% higher. These modifications in the local environment surrounding the Fe atoms indicate that some fraction of Fe is present in the L21 structure in sample #1. The changes are less evident at longer distances than at the shorter ones, indicating the presence of small islands of Co2FeZ with the L21 structure dispersed in the predominant B2 matrix. In sample #2, the EXAFS analysis pointed to a smaller contribution of L21 structure compared to sample #1. These results are consistent with the nanoscale chemical disorder observed by HAADF-STEM and the expected role this disorder plays on the modifications in TC. An increase in TC with introduced chemical disorder was recently observed in Co1+xFe2−xSi Heusler compounds; namely the largest TC was reported for the Co1.5Fe1.5Si composition.18 Analysis of the Fe local atomic environments via Mössbauer spectroscopy indicated that a change in the site occupancy and resultant modification of the exchange energies (due to variations in the magnetic moment and exchange integrals) was the likely origin of the enhanced TC. Similarly, the greater TC observed here is likely related to the modifications in the local atomic environments in the B2 structure with respect to the L21 structure.
Z ratio at longer distances. For sample #1, the number of Z atoms as second nearest neighbors was 13% higher than expected for a perfect B2 structure, and the number of Fe atoms at the same distance was 16% lower. Moreover, the number of Z atoms as third nearest neighbors was 7% lower than expected for a perfect B2 structure, and the number of Fe atoms was 10% higher. These modifications in the local environment surrounding the Fe atoms indicate that some fraction of Fe is present in the L21 structure in sample #1. The changes are less evident at longer distances than at the shorter ones, indicating the presence of small islands of Co2FeZ with the L21 structure dispersed in the predominant B2 matrix. In sample #2, the EXAFS analysis pointed to a smaller contribution of L21 structure compared to sample #1. These results are consistent with the nanoscale chemical disorder observed by HAADF-STEM and the expected role this disorder plays on the modifications in TC. An increase in TC with introduced chemical disorder was recently observed in Co1+xFe2−xSi Heusler compounds; namely the largest TC was reported for the Co1.5Fe1.5Si composition.18 Analysis of the Fe local atomic environments via Mössbauer spectroscopy indicated that a change in the site occupancy and resultant modification of the exchange energies (due to variations in the magnetic moment and exchange integrals) was the likely origin of the enhanced TC. Similarly, the greater TC observed here is likely related to the modifications in the local atomic environments in the B2 structure with respect to the L21 structure.
Nanoscale chemical disorder could present further opportunities to engineer functional materials and realize new properties. For instance, recent work has reported ultrafast demagnetization in amorphous CoFeB due to enhanced spin–lattice scattering resulting from structural disorder.19 The methodology presented in our work might therefore be extended to engineer materials with carefully tailored nanoscale chemical disorder for use in all optical magnetic switching. Moreover, control of chemical disorder can lead to the discovery of new materials properties, such as the giant intrinsic exchange bias reported in Mn–Pt–Ga as a result of anti-site disorder.20
Conclusion
In summary, modifications in TC in off-stoichiometry Co2FeSixAl1−x (0 < x < 1) compounds result from nanoscale chemical disorder. This composition range was specifically examined since the spin-polarization should be more robust to disorder. HAAD-STEM and EXAFS revealed L21 ordered nano-puddles in a B2 matrix in the as-prepared sample with x = 0.2; the fraction of B2 chemical order grew after annealing, resulting in an enhanced TC. Moreover, we present a record TC of 1218 K for B2-structured Co2FeAl. Our results point to a methodology that might be extended to modify the magnetic and electronic properties of any Heusler compound, thin film or bulk; chemical disorder can be an engineering tool to realize highly tailored properties.Acknowledgements
The authors gratefully acknowledge support of this work by the Brazilian Synchrotron Light Laboratory (proposals XPD-17015 and XAFS1-17012), CNPq and CAPES-PROBRAL.References
- J. Winterlik, S. Chadov, A. Gupta, V. Alijani, T. Gasi, K. Filsinger, B. Balke, G. H. Fecher, C. A. Jenkins, F. Casper, J. Kueler, G.-D. Liu, L. Gao, S. S. P. Parkin and C. Felser, Adv. Mater., 2012, 24, 6283 CrossRef CAS PubMed.
- T. Graf, C. Felser and S. S. P. Parkin, Prog. Solid State Chem., 2011, 39, 1 CrossRef CAS.
- S. Chadov, S. W. D’Souza, L. Wollmann, J. Kiss, G. H. Fecher and C. Felser, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 094203 CrossRef.
- T. M. Nakatani, A. Rajanikanth, Z. Gercsi, Y. K. Takahasi, K. Inomata and K. Hono, J. Appl. Phys., 2007, 102, 033916 CrossRef.
- Q. L. Ma, X. M. Zhang, T. Miyazaki and S. Mizukami, Sci. Rep., 2015, 5, 7863 CrossRef CAS PubMed.
- T. Kubota, S. Tsunegi, M. Oogane, S. Mizukami, T. Miyazaki, H. Naganuma and Y. Ando, Appl. Phys. Lett., 2009, 94, 122504 CrossRef.
- S. Mizukami, D. Watanabe, M. Oogane, Y. Ando, Y. Miura, M. Shirai and T. Miyazaki, J. Appl. Phys., 2009, 105, 07D306 CrossRef.
- M. Belmeguenai, M. S. Gabor, T. Petrisor, Jr., F. Zighem, S. M. Cherif and C. Tiusan, J. Appl. Phys., 2015, 117, 023906 CrossRef.
- H. C. Herper, B. Krumme, D. Ebke, C. Antoniak, C. Weis, A. Warland, A. Hütten, H. Wende and P. Entel, J. Appl. Phys., 2011, 109, 07E128 CrossRef.
- G. H. Fecher and C. Felser, J. Phys. D: Appl. Phys., 2007, 40, 1582 CrossRef CAS.
- R. Y. Umetsu, A. Okubo and R. Kainuma, J. Appl. Phys., 2012, 111, 073909 CrossRef.
- I. Galanakis, P. H. Dederichs and N. Papanikolaou, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 174429 CrossRef.
- J. Kübler, G. H. Fecher and C. Felser, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 024414 CrossRef.
- G. H. Fecher, H. C. Kandpal, S. Wurmehl, C. Felser and G. Schönhense, J. Appl. Phys., 2006, 99, 08J106 CrossRef.
- X. Zhu, Y. Dai and C. Luo, J. Magn. Magn. Mater., 2016, 398, 7 CrossRef CAS.
- B. Balke, G. H. Fecher and C. Felser, Appl. Phys. Lett., 2007, 90, 242503 CrossRef.
- J. J. Rehr, J. J. Kas, F. D. Vila, M. P. Prange and K. Jorissen, Phys. Chem. Chem. Phys., 2010, 12, 5503 RSC.
- J. E. Fischer, J. Karel, S. Fabbrici, P. Adler, S. Ouardi, G. H. Fecher, F. Albertini and C. Felser, Phys. Rev. B, 2016, 94, 024418 CrossRef.
- S. Bonetti, M. C. Hoffmann, M.-J. Sher, Z. Chen, S.-H. Yang, M. G. Samant, S. S. P. Parkin and H. A. Dürr, Phys. Rev. Lett., 2016, 117, 087205 CrossRef CAS PubMed.
- A. K. Nayak, M. Nicklas, S. Chadov, P. Khuntia, C. Shekhar, A. Kalache, M. Baenitz, Y. Skourski, V. K. Guduru, A. Puri, U. Zeitler, J. M. D. Coey and C. Felser, Nat. Mater., 2015, 14, 679 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7tc01241a |
| ‡ Present address: Department of Materials Science and Engineering, Monash University, Clayton, Victoria, Australia. E-mail: julie.karel@monash.edu |
| This journal is © The Royal Society of Chemistry 2017 |