Simple aliphatic oximes as nonconventional luminogens with aggregation-induced emission characteristics†
Abstract
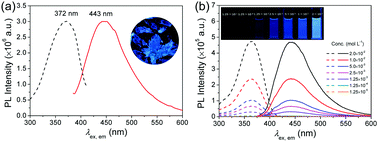
Amine-containing polymers and small molecules with only nonconventional chromophores are found to emit strong luminescence under suitable conditions, and their oxidation products are believed to be the chromophores. However, the oxidized species or groups have never been thoroughly separated and well characterized and hence the main chromophore(s) responsible for the fluorescence is not clear. In this work, three isomeric small molecular aliphatic amines are oxidized with H2O2 and then their oxidized products are separated and characterized. Both primary and secondary amines can be oxidized with H2O2, while tertiary amines cannot. Oximes (R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH) rather than hydroxylamines are proved to be the chromophores for the first time. Hexanal oxime emits very weak fluorescence in dilute solutions, but becomes highly emissive in thick solutions or in the solid state, exhibiting distinct aggregation-induced emission (AIE) characteristics. The fluorescence of hexanal oxime is also sensitive to temperature and metal ions. Crystallographic data from the single crystal structure of hexanal oxime and theoretical calculations prove that the aggregation of oxime groups (C
N–OH) rather than hydroxylamines are proved to be the chromophores for the first time. Hexanal oxime emits very weak fluorescence in dilute solutions, but becomes highly emissive in thick solutions or in the solid state, exhibiting distinct aggregation-induced emission (AIE) characteristics. The fluorescence of hexanal oxime is also sensitive to temperature and metal ions. Crystallographic data from the single crystal structure of hexanal oxime and theoretical calculations prove that the aggregation of oxime groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH) through intermolecular hydrogen bonding results in extended conjugation and a rigidified conformation, which lead to strong fluorescence in the aggregated state.
N–OH) through intermolecular hydrogen bonding results in extended conjugation and a rigidified conformation, which lead to strong fluorescence in the aggregated state.


 Please wait while we load your content...
Please wait while we load your content...