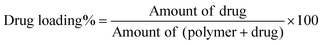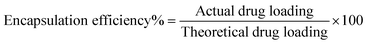Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biodegradable nitric oxide precursor-loaded micro- and nanoparticles for the treatment of Staphylococcus aureus biofilms
Sayeed
Hasan
a,
Nicky
Thomas
 a,
Benjamin
Thierry
b and
Clive A.
Prestidge
*a
a,
Benjamin
Thierry
b and
Clive A.
Prestidge
*a
aSchool of Pharmacy and Medical Sciences, Sansom Institute for Health Research, University of South Australia, Adelaide, South Australia, Australia. E-mail: clive.prestidge@unisa.edu.au
bFuture Industries Institute, University of South Australia, Mawson Lakes, South Australia, Australia
First published on 9th January 2017
Abstract
Bacteria in biofilms are more difficult to eradicate than planktonic bacteria and result in treatment challenges for many chronic infectious diseases. Nitric oxide (NO) is an endogenous molecule that offers potential as an alternative to conventional antibiotics; however its sustained topical delivery to biofilms is not readily achieved. With this in mind, we report the development of biodegradable poly(lactide-co-glycolide) (PLGA) based microparticles (MP) and nanoparticles (NP) for encapsulation of the NO precursor isosorbide mononitrate (ISMN) and the controlled delivery to Staphylococcus aureus (S. aureus) biofilms. Firstly, water-in-oil-in-water (w/o/w) emulsification/solvent evaporation methods for PLGA NP and MP syntheses were experimentally optimised with respect to particle size and ISMN loading/encapsulation efficiency. The influence of various experiment parameters, such as the volume of inner aqueous phase, concentration of surfactants, mixing time on the particle size, drug loading and encapsulation efficiency were investigated systematically. Both PLGA MP and NP formulations enabled sustained ISMN release in physiological media over 3 to 5 days. PLGA MP with diameters of ∼3 μm and ISMN loading of 2.2% (w/w) were identified as the optimum delivery system and demonstrated significant antibacterial activity in S. aureus biofilms. This behaviour is considered to be due to targeted biofilm delivery through a combination of effective penetration and sustained release of ISMN.
Introduction
The increasing prevalence of Staphylococcus aureus (S. aureus) biofilm-associated infections such as skin and bone infections,1,2 endocarditis,3 sinus4 and device-related infections (catheters, implantable prosthetics)5,6 are placing significant burden on healthcare systems and society.7 Biofilms are complex, functional communities of one or more species of microorganisms that are encased in extracellular polymeric substance (EPS) and are attached to both a solid surface and to each other.8 The slow growth rates,9 low antibiotic penetration,10 high cell density,11 excessive extracellular matrices,12 pH alterations,13 mutations14 and altered nutrient requirements15 are well-known properties that give rise to greater antimicrobial resistance in microbial biofilms.11,16 The emergence of bacterial resistance and the innate tolerance of microbial biofilms to antibiotic therapy have intensified the problems in their eradication. Therefore, recent research has focused on the development of alternative antimicrobial therapies to treat bacterial biofilms.Nitric oxide (NO) is a potential alternative for antimicrobial therapy to combat both free-floating (planktonic) bacteria and biofilm-associated bacteria as a result of its intrinsic antimicrobial properties.17 NO has been investigated for the treatment of chronic infectious diseases that are frequently associated with bacterial biofilms.15 NO is an endogenously produced gas and has been considered as a promising therapeutic agent in free radical biology investigations due to its remarkable vasorelaxation, antimicrobial and anti-tumour activity.18 The clinical application of NO is, however limited due to its short half-life, instability during storage, potential toxicity, lack of efficient localized and systemic delivery and poor dose control.19 To overcome these limitations, various NO donors with a range of stabilities have developed to deliver therapeutic levels of NO in biological systems. These NO donors are typically low molecular weight compounds including nitrates, nitrites, N-nitroso, C-nitroso, metal NO complexes and diazeniumdiolates.20 The most widely used NO donors are organic nitrates such as glycerol trinitrate (GTN) and isosorbide mononitrate (ISMN),21 which have been widely used in the treatment of cardiac diseases. However, the therapeutic potential of NO against biofilms is concentration dependent. Anti-biofilm effects of NO are achieved only at higher concentrations, whereas at suboptimal NO concentrations, S. aureus biofilm growth is enhanced.22 The dualistic properties of NO must therefore be taken into consideration when designing a topical delivery agent.23 To this end, obtaining therapeutic NO levels at an appropriate exposure time is the key challenge towards achieving potent anti-biofilm activity.
To date, there are few reports on the development of particle-based NO delivery systems in which NO donors either encapsulated into or covalently attached to macro- or micromolecular structures and their antibacterial activity in bacterial biofilms. Hetrick et al. synthesized two NONOate derived silica nanoparticle systems, N-methylaminopropyltrimethoxysilane/tetraethyl orthosilicate (MAP3/TEOS) and N-(6-aminohexyl) aminopropyltrimethoxysilane (AHAP3/TEOS) and reported increased MAP3/TEOS loading and faster NO release compared to AHAP3/TEOS particles.24 The MAP3/TEOS particles exhibited a 1000-fold increase in bactericidal efficacy against P. aeruginosa biofilms. Additionally, a significant reduction of planktonic bacteria was reported by using macromolecule NONOate modified silica nanoparticles compared to micromolecule N-diazeniumdiolate-modified proline (PROLI/NO).25 Friedman et al. introduced a new silane-based hydrogel nanoparticle platform. The core of this nanoparticle contained nitrite encapsulated within a composite matrix composed of tetramethyl orthosilicate, polyethylene glycol (PEG), chitosan and a glass forming disaccharide. During synthesis, nitrite is thermally reduced to NO.26 In a subsequent study, Martinez et al. evaluated the antimicrobial potential of silane based hydrogel nanoparticles.27 This study demonstrated that the NO releasing nanoparticles reduced 99.9% of bacterial growth for cutaneous methicillin-resistant Staphylococcus aureus (MRSA) in a murine infection model. However, the toxicity of silica may limit their potential as a future therapeutic agent.
There is increasing interest in the development of ISMN-based formulations for anti-biofilm applications, owing to its well- established efficacy and safety profile. Recently, our group reported on a novel ISMN liposomal formulation for the treatment of S. aureus biofilm-associated chronic rhinosinusitis.23 Following the exposure of S. aureus biofilm to various liposomal ISMN formulations, multilamellar vesicles (MLV) were found to have greater anti-biofilm effects compared to non-liposomal ISMN and unilamellar vesicle (ULV) liposomes. An increase in the lipid content of liposomal ISMN also improved the anti-biofilm efficacy of both MLV and ULV liposomes. A potential drawback of these systems is the presence of both free ISMN and encapsulated ISMN in the formulation that may cause uncontrolled NO donor delivery to biofilms and potential NO side effects such as hypotension.22 Furthermore, liposomal formulations suffer the drawback of poor stability, uncontrolled drug release and low encapsulation efficiency of water-soluble drugs due to rapid drug leakage. The potential use of a successful therapeutic depends on the bioavailability of the therapeutic agents at the site of action. Poly(lactide-co-glycolide) or PLGA micro/nanoparticles have been widely used for encapsulating therapeutic drugs in controlled release applications. Given that PLGA presents inherent advantages over other systems including controlled release rates, biocompatibility/biodegradability, low cytotoxicity and favourable degradation characteristics.28 Most importantly, PLGA are a family of FDA-approved biodegradable polymers that have been extensively studied as delivery vehicles for drugs, proteins and peptides.29,30 Therefore, PLGA-based formulations are a feasible approach for overcoming the challenges of NO delivery to bacterial biofilms.
Here, we explore the potential of PLGA particles to encapsulate and release ISMN in a controlled manner to kill microbial cells within in vitro S. aureus biofilms. We demonstrate that the rate of ISMN release can be tuned by the PLGA particle size and ISMN loading, and quantify anti-biofilm activities of the optimised ISMN-PLGA particulate formulations.
Materials and methods
Materials
The biodegradable polymer PLGA (LA/GA ratio 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, MW 30
50, MW 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–60
000–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), polyvinyl alcohol (PVA; 80% hydrolysed, MW 9000–10
000 Da), polyvinyl alcohol (PVA; 80% hydrolysed, MW 9000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and Tween 80 were purchased from Sigma Aldrich, Australia. Isosorbide mononitrate (ISMN) (purity >95%, MW 191.14) was procured from Bosche Scientific, USA. All other chemical reagents used including acetone, methanol, dichloromethane (Merck, Germany) were of analytical grade. High purity water was obtained from a Milli-Q Direct water purification system (Millipore, USA) and was used throughout the study.
000 Da) and Tween 80 were purchased from Sigma Aldrich, Australia. Isosorbide mononitrate (ISMN) (purity >95%, MW 191.14) was procured from Bosche Scientific, USA. All other chemical reagents used including acetone, methanol, dichloromethane (Merck, Germany) were of analytical grade. High purity water was obtained from a Milli-Q Direct water purification system (Millipore, USA) and was used throughout the study.
ISMN-loaded PLGA particle preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g (Haake Z36HK, Wehingen, Germany) for 20 min, washed with Milli-Q water three times, and freeze-dried overnight and stored at 4 °C until further use.
000 × g (Haake Z36HK, Wehingen, Germany) for 20 min, washed with Milli-Q water three times, and freeze-dried overnight and stored at 4 °C until further use.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 20 min and washed three times with Milli-Q water. Finally, the products were freeze-dried and stored at 4 °C.
000 × g for 20 min and washed three times with Milli-Q water. Finally, the products were freeze-dried and stored at 4 °C.
Characterisation of PLGA particles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant was directly injected into a high performance liquid chromatography (HPLC) system (UFLC XR, Shimadzu, Japan). Chromatographic separation was performed on a LiChrospher RP C18 column (5 μm, 4.6 mm ID × 150 mm, Grace Davidson Discovery Science, Rowville, VIC) at a detection wavelength of 215 nm. The concentration of ISMN was calculated from a standard curve, prepared by measuring the peak areas of known concentrations of ISMN in methanol/water mixtures. Drug loading and encapsulation efficiency were calculated as follows:
000 rpm for 10 min. The supernatant was directly injected into a high performance liquid chromatography (HPLC) system (UFLC XR, Shimadzu, Japan). Chromatographic separation was performed on a LiChrospher RP C18 column (5 μm, 4.6 mm ID × 150 mm, Grace Davidson Discovery Science, Rowville, VIC) at a detection wavelength of 215 nm. The concentration of ISMN was calculated from a standard curve, prepared by measuring the peak areas of known concentrations of ISMN in methanol/water mixtures. Drug loading and encapsulation efficiency were calculated as follows:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g followed by dilution of the supernatants with mobile phase and ISMN quantification by HPLC as described above. Fresh PBS (1 mL) was added to the particle suspension after each sampling time point and agitation was continued. All release experiments were performed in triplicate.
000 × g followed by dilution of the supernatants with mobile phase and ISMN quantification by HPLC as described above. Fresh PBS (1 mL) was added to the particle suspension after each sampling time point and agitation was continued. All release experiments were performed in triplicate.
In vitro bacteria studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 dilution). This suspension served as the inoculum for the formation of biofilms.
15 dilution). This suspension served as the inoculum for the formation of biofilms.
Results and discussion
Synthesis and optimization of ISMN loaded PLGA particles
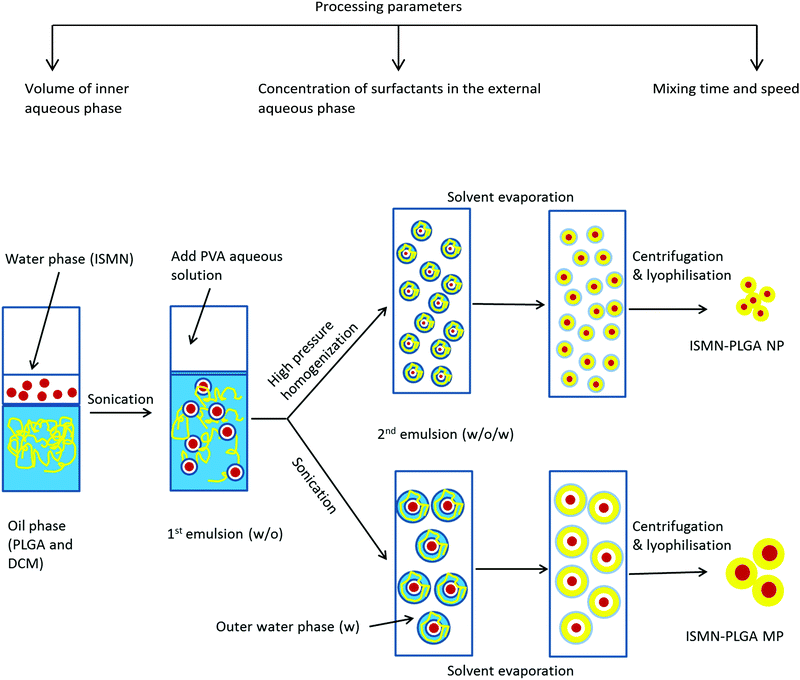
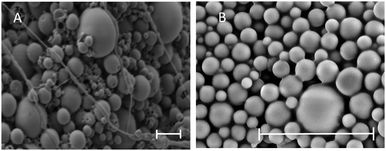
When considering PLGA particle preparation, the selection of a suitable encapsulation method is largely dependent on the physiochemical properties of the drug. For hydrophilic drug encapsulation, the double emulsion solvent evaporation technique is the most commonly utilized. However, the efficient encapsulation of a low molecular weight and water-soluble molecules such as ISMN into PLGA is a significant challenge. The common double emulsion technique39 suffers from poor encapsulation efficiency for water soluble drugs owing to their rapid migration and therefore loss into the aqueous phase. To improve the encapsulation of ISMN into PLGA particles we adjusted specific processing parameters including the volume of the internal aqueous phase, concentration of surfactants and mixing time and evaluated their effect on the particle size, ISMN loading and encapsulation efficiency.40,41| Microparticles | Nanoparticles | |||||
|---|---|---|---|---|---|---|
| Volume of internal phase (mL) | Average sizeb (μm) | DL (%) | EE (%) | Average size (nm) | DL (%) | EE (%) |
| a All batches were emulsified using an ultrasonicator (30 W) for 60 s, 10 mL PLGA (1%) dichloromethane solution was used as organic phase and 1% PVA (w/v) used as outer aqueous phase. b SEM, At least six representative PLGA MPs were used for the calculation, Data are given as mean ± SD (n = 3). | ||||||
| 0.1 | 9.8 ± 1.8 | 0.8 ± 0.28 | 8.1 ± 5.0 | 172 ± 11 | 0.14 ± 0.03 | 1.6 ± 0.6 |
| 0.3 | 5.6 ± 2.2 | 1.9 ± 0.25 | 21.2 ± 1.5 | 255 ± 35 | 0.23 ± 0.03 | 2.7 ± 0.2 |
| 0.5 | 2.9 ± 1.4 | 2.2 ± 0.33 | 23.6 ± 1.4 | 264 ± 21 | 0.41 ± 0.02 | 5.8 ± 0.1 |
| 1 | 2.8 ± 1.7 | 1.3 ± 0.20 | 15.4 ± 0.7 | 556 ± 32 | 0.49 ± 0.07 | 6.3 ± 0.2 |
| Microparticle | Nanoparticle | |||||
|---|---|---|---|---|---|---|
| PVA concentration (w/v) | Average size (μm) | DL (%) | EE (%) | Size (nm) | DL (%) | EE (%) |
| a All batches were emulsified using 0.5 mL ISMN aqueous solution (ISMN; 60 mg mL−1) was used as internal aqueous phase and 10 mL PLGA (1%) dichloromethane solution was used as organic phase. | ||||||
| 0.1% | 3.8 ± 2.6 | 1.7 ± 0.51 | 18.1 ± 2.6 | 271 ± 17 | 0.33 ± 0.62 | 4.7 ± 0.3 |
| 0.5% | 3.4 ± 1.3 | 2.0 ± 0.63 | 21.1 ± 1.9 | 265 ± 9 | 0.36 ± 0.02 | 5.4 ± 0.1 |
| 1% | 2.9 ± 1.4 | 2.2 ± 0.33 | 23.6 ± 1.4 | 264 ± 21 | 0.41 ± 0.02 | 5.8 ± 0.1 |
| Ultra-sonication time (s) | Average size (μm) | DL (%) | EE (%) |
|---|---|---|---|
| a All batches were emulsified using an ultrasonicator (30 W) for 60 s. | |||
| 10 | 5.6 ± 1.1 | 0.8 ± 0.34 | 8.9 ± 6.1 |
| 30 | 4.7 ± 2.5 | 1.3 ± 0.29 | 14.1 ± 3.6 |
| 60 | 2.9 ± 1.4 | 2.2 ± 0.33 | 23.6 ± 1.4 |
| 90 | 3.1 ± 1.5 | 2.2 ± 0.17 | 24.8 ± 6 |
| 120 | 3.6 ± 2.6 | 2.3 ± 0.32 | 25.6 ± 6.6 |
| Homogenization pressure (bar) | Number of cycles | Size (nm) | DL (%) | EE (%) |
|---|---|---|---|---|
| 500 | 1 | 271 ± 16 | 0.40 ± 0.29 | 5.6 ± 0.6 |
| 500 | 3 | 264 ± 21 | 0.41 ± 0.02 | 5.8 ± 0.3 |
| 500 | 5 | 243 ± 43 | 0.37 ± 0.21 | 4.8 ± 0.8 |
| 1000 | 1 | 139 ± 62 | 0.21 ± 0.02 | 2.7 ± 0.2 |
| 1000 | 3 | 121 ± 31 | 0.16 ± 0.04 | 2.2 ± 0.1 |
| 1000 | 5 | 101 ± 34 | 0.16 ± 0.08 | 2.2 ± 0.4 |
Based on the above data, the optimal processing parameters were defined as: 0.5 mL for the inner aqueous phase (ISMN, 60 mg mL−1), 1% PVA concentration (w/v), 1% PLGA in dichloromethane and either 60 seconds ultra-sonication for MP or 3 cycles of homogenization at 500 bars for NP.
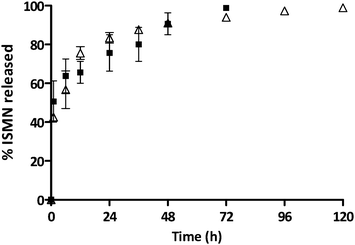 | ||
| Fig. 5 The percentage of ISMN release from PLGA-MPs (Δ) and PLGA-NPs (■) at 37 °C in PBS (pH 7.4), data represent mean ± SD, n = 3. | ||
In contrast, the sustained release phase results from several factors such as an increase in the diffusion pathways with time due to polymer swelling, degradation and erosion.47
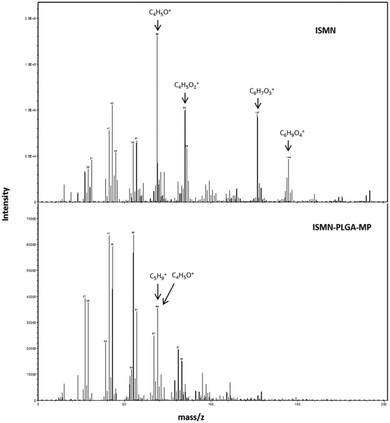
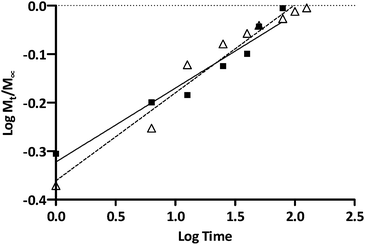
To further elucidate ISMN release mechanisms from these PLGA particles, ISMN release data were fitted to the Korsmeyer–Peppas equation as shown in Fig. 6,48i.e. Mt/M∞ = ktn, where Mt/M∞ is the fraction of ISMN released at time t, k is the rate constant and n is the drug release exponent. In this context, n values between 0.45 and 0.89 are indicative of non-Fickian release which refers to a combination of both diffusion and erosion drug release mechanisms.49 The observed n values for the release of ISMN from PLGA-MPs and PLGA-NPs of 0.74 and 0.66 respectively (R2 > 0.95) is confirmation of non-Fickian behaviour. Such release characteristics could be attributed to the increase in strong entanglement bonds between the polymer matrixes which resisted the erosion by the dissolution medium in the initial hours of ISMN release.47 The drug release from PLGA micro/nanoparticles is a complex process attributed to diffusion followed by degradation and is controlled by the molar ratio of the lactic and glycolic acid in the polymer chain, molecular weight of the polymer, the degree crystallinity and physico-chemical properties of the drug.30 Initial release of ISMN from both PLGA micro- and nanoparticles is considered a burst release of deposited or weakly bound ISMN molecules on the sub-surface of micro- and nanoparticles. Jameela et al.50 explained that when particles are prepared by the double emulsion method, most hydrophilic drugs exhibit higher tendency to migrate to the aqueous medium, thereby concentrating at the surface or sub-surface of the particles and rapidly released. A potential limiting factor in the preparation of ISMN-containing PLGA micro/nanoparticle is the hydrophobic nature of the PLGA polymer in contrast to the hydrophilic characteristics of ISMN. Generally, encapsulation of hydrophobic drug molecules in PLGA preparations is preferred due to strong hydrophobic interactions between the polymer and drug during formulation. Given the high aqueous solubility of ISMN (60 mg mL−1), it is not surprising that previous reports suggesting its presence on the outer surface or subsurface of particles, forming a molecular layer and thereby susceptible to be easily and rapidly released.51 Besides the polymer type, the drug loading also influenced the drug release rate. PLGA micro- and nanoparticle had different ISMN loadings (2.2% and 0.4% respectively) and these two ISMN release profiles have similar shape, but the PLGA-MPs with a higher ISMN loading leads to an increased initial rapid release.52 After the initial ISMN release from PLGA-MPs, there was a sustained ISMN release, indicating the constant degradation of the PLGA and the diffusion of encapsulated ISMN from the inner core of the PLGA-MPs.53 In contrast, faster release of ISMN from the PLGA-NPs could result from larger surface area and the shorter diffusion pathways compared to microparticles.
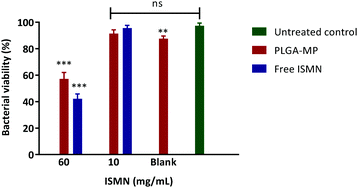
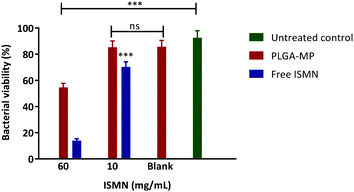
| MIC (mg mL−1) | MBEC (mg mL−1) | ||
|---|---|---|---|
| Free ISMN | 3.75 | 15 | |
| MP-encapsulated ISMN | 7.5 | 30 | |
Conclusion
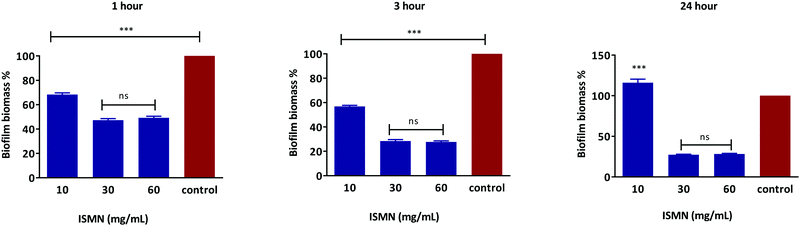
A double emulsion solvent evaporation method has been successfully optimised to encapsulate the NO donor ISMN into PLGA nanoparticles and microparticles. Considerable sustained release of ISMN has been demonstrated. PLGA microparticles but not nanoparticles were able to deliver sufficient levels of ISMN to planktonic and biofilms of S. aureus to cause significant anti-bacterial effects. ISMN-PLGA-MP formulations have potential for the treatment of a number of biofilm-based infections.Acknowledgements
This project has been supported by The National Health and Medical Research Council (NHMRC project grant GNT1047576 and GNT1090898). The University of South Australia and School of Pharmacy and Medical Sciences are acknowledged for providing a scholarship to SH. This work was performed in part at the South Australian node of the Australian National Fabrication Facility (ANFF) under the National Collaborative Research Infrastructure Strategy to provide nano- and microfabrication facilities for Australia's researchers.References
- G. A. James, E. Swogger, R. Wolcott, E. D. Pulcini, P. Secor, J. Sestrich, J. W. Costerton and P. S. Stewart, Wound Repair Regen., 2008, 16, 37–44 CrossRef PubMed.
- R. A. Brady, J. G. Leid, J. H. Calhoun, J. W. Costerton and M. E. Shirtliff, FEMS Immunol. Med. Microbiol., 2008, 52, 13–22 CrossRef CAS PubMed.
- B. A. Cunha, M. V. Gill and J. M. Lazar, Infect. Dis. Clin. North Am., 1996, 10, 811–834 CrossRef CAS PubMed.
- A. Foreman and P.-J. Wormald, Laryngoscope, 2010, 120, 1701–1706 CrossRef PubMed.
- R. O. Darouiche, N. Engl. J. Med., 2004, 350, 1422–1429 CrossRef CAS PubMed.
- G. E. Thwaites, J. D. Edgeworth, E. Gkrania-Klotsas, A. Kirby, R. Tilley, M. E. Török, S. Walker, H. F. L. Wertheim, P. Wilson and M. J. Llewelyn, Lancet Infect. Dis., 2011, 11, 208–222 CrossRef PubMed.
- P. M. Hawkey, J. Antimicrob. Chemother., 2008, 62, i1–i9 CrossRef CAS PubMed.
- B. Vu, M. Chen, R. Crawford and E. Ivanova, Molecules, 2009, 14, 2535 CrossRef CAS PubMed.
- N. Høiby, T. Bjarnsholt, M. Givskov, S. Molin and O. Ciofu, Int. J. Antimicrob. Agents, 2010, 35, 322–332 CrossRef PubMed.
- I. d'Angelo, C. Conte, M. I. La Rotonda, A. Miro, F. Quaglia and F. Ungaro, Adv. Drug Delivery Rev., 2014, 75, 92–111 CrossRef PubMed.
- T.-F. C. Mah and G. A. O'Toole, Trends Microbiol., 2001, 9, 34–39 CrossRef CAS PubMed.
- C. A. Fux, J. W. Costerton, P. S. Stewart and P. Stoodley, Trends Microbiol., 2005, 13, 34–40 CrossRef CAS PubMed.
- P. S. Stewart, J. Bacteriol., 2003, 185, 1485–1491 CrossRef CAS PubMed.
- N. Høiby, O. Ciofu and T. Bjarnsholt, Future Microbiol., 2010, 5, 1663–1674 CrossRef PubMed.
- J. W. Costerton, P. S. Stewart and E. P. Greenberg, Science, 1999, 284, 1318–1322 CrossRef CAS PubMed.
- C. A. Gordon, N. A. Hodges and C. Marriott, J. Antimicrob. Chemother., 1988, 22, 667–674 CrossRef CAS PubMed.
- G. N. Marcells, P. S. Phillips, N. A. Cohen, R. J. Harvey and R. Sacks, Otolaryngol.–Head Neck Surg., 2011, 144, 159–169 CrossRef.
- D. A. Sanchez, J. Nosanchuk and A. Friedman, Nanomedicine, 2012, 7, 933–936 CrossRef CAS PubMed.
- J. Saraiva, S. S. Marotta-Oliveira, S. A. Cicillini, J. D. O. Eloy and J. M. Marchetti, J. Drug Delivery, 2011, 936438 Search PubMed.
- P. G. Wang, M. Xian, X. Tang, X. Wu, Z. Wen, T. Cai and A. J. Janczuk, Chem. Rev., 2002, 102, 1091–1134 CrossRef CAS PubMed.
- A. Friedman and J. Friedman, Expert Opin. Drug Delivery, 2009, 6, 1113–1122 CrossRef CAS PubMed.
- C. Jardeleza, A. Foreman, L. Baker, S. Paramasivan, J. Field, L. W. Tan and P. J. Wormald, International Forum of Allergy and Rhinology, 2011, 1, 438–444 CrossRef PubMed.
- C. Jardeleza, S. Rao, B. Thierry, P. Gajjar, S. Vreugde, C. A. Prestidge and P. J. Wormald, PLoS One, 2014, 9, e92117 Search PubMed.
- E. M. Hetrick, J. H. Shin, H. S. Paul and M. H. Schoenfisch, Biomaterials, 2009, 30, 2782–2789 CrossRef CAS PubMed.
- E. M. Hetrick, J. H. Shin and M. H. Schoenfisch, 8th World Biomaterials Congress 2008, 2008, 4, 1845 Search PubMed.
- A. J. Friedman, G. Han, M. S. Navati, M. Chacko, L. Gunther, A. Alfieri and J. M. Friedman, Nitric Oxide, 2008, 19, 12–20 CrossRef CAS PubMed.
- L. R. Martinez, G. Han, M. Chacko, M. R. Mihu, M. Jacobson, P. Gialanella, A. J. Friedman, J. D. Nosanchuk and J. M. Friedman, J. Invest. Dermatol., 2009, 129, 2463–2469 CrossRef CAS PubMed.
- J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329–347 CrossRef CAS PubMed.
- R. Jain, N. H. Shah, A. W. Malick and C. T. Rhodes, Drug Dev. Ind. Pharm., 1998, 24, 703–727 CrossRef CAS PubMed.
- H. K. Makadia and S. J. Siegel, Polymers, 2011, 3, 1377–1397 CrossRef CAS PubMed.
- K. Dillen, J. Vandervoort, G. Van den Mooter, L. Verheyden and A. Ludwig, Int. J. Pharm., 2004, 275, 171–187 CrossRef CAS PubMed.
- J. Liu, Z. Qiu, S. Wang, L. Zhou and S. Zhang, Biomed. Mater., 2010, 5, 065002 CrossRef PubMed.
- J. O'Sullivan, M. Park and J. Beevers, J. Cereal Sci., 2016, 69, 77–84 CrossRef.
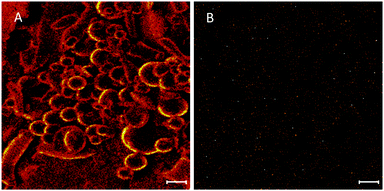
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- I. Wiegand, K. Hilpert and R. E. W. Hancock, Nat. Protoc., 2008, 3, 163–175 CrossRef CAS PubMed.
- J. H. Merritt, D. E. Kadouri and G. A. O'Toole, Current Protocols in Microbiology, 2011 Search PubMed.
- E. Peeters, H. J. Nelis and T. Coenye, J. Microbiol. Methods, 2008, 72, 157–165 CrossRef CAS PubMed.
- I. Vandecandelaere, H. Van Acker and T. Coenye, Methods Mol. Biol., 2016, 1333, 53–66 Search PubMed.
- N. Thomas, C. Thorn, K. Richter, B. Thierry and C. Prestidge, J. Pharm. Sci., 2016, 105, 3115–3122 CrossRef CAS PubMed.
- U. Bilati, E. Allémann and E. Doelker, Pharm. Dev. Technol., 2003, 8, 1 CrossRef CAS PubMed.
- U. Bilati, E. Allémann and E. Doelker, J. Microencapsulation, 2005, 22, 205–214 CrossRef CAS PubMed.
- T. Verrecchia, G. Spenlehauer, D. V. Bazile, A. Murry-Brelier, Y. Archimbaud and M. Veillard, J. Controlled Release, 1995, 36, 49–61 CrossRef CAS.
- A. Rafati, A. Boussahel, K. M. Shakesheff, A. G. Shard, C. J. Roberts, X. Chen, D. J. Scurr, S. Rigby-Singleton, P. Whiteside, M. R. Alexander and M. C. Davies, J. Controlled Release, 2012, 162, 321–329 CrossRef CAS PubMed.
- J. G. Y. Chan, H. K. Chan, C. A. Prestidge, J. A. Denman, P. M. Young and D. Traini, Eur. J. Pharm. Biopharm., 2013, 83, 285–292 CrossRef CAS PubMed.
- A. M. Belu, M. C. Davies, J. M. Newton and N. Patel, Anal. Chem., 2000, 72, 5625–5638 CrossRef CAS PubMed.
- N. Faisant, J. Siepmann, J. Richard and J. P. Benoit, Eur. J. Pharm. Biopharm., 2003, 56, 271–279 CrossRef CAS PubMed.
- A. N. Ford Versypt, D. W. Pack and R. D. Braatz, J. Controlled Release, 2013, 165, 29–37 CrossRef CAS PubMed.
- R. W. Korsmeyer, R. Gurny, E. Doelker, P. Buri and N. A. Peppas, Int. J. Pharm., 1983, 15, 25–35 CrossRef CAS.
- J. Vysloužil, P. Doležel, M. Kejdušová, V. Košál, L. Beneš and K. Dvoráčková, Pharm. Dev. Technol., 2016, 21, 214–221 CrossRef PubMed.
- S. R. Jameela, N. Suma and A. Jayakrishnan, J. Biomater. Sci., Polym. Ed., 1997, 8, 457–466 CrossRef CAS PubMed.
- C. Ahlneck and G. Zografi, Int. J. Pharm., 1990, 62, 87–95 CrossRef CAS.
- F. Cui, D. Cun, A. Tao, M. Yang, K. Shi, M. Zhao and Y. Guan, J. Controlled Release, 2005, 107, 310–319 CrossRef CAS PubMed.
- H. K. Makadia and S. J. Siegel, Polymers, 2011, 3, 1377–1397 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |