Preparation and characterisation of an innovative injectable calcium sulphate based bone cement for vertebroplasty application†
Mehran
Dadkhah
 a,
Lucia
Pontiroli
ab,
Sonia
Fiorilli
a,
Antonio
Manca
c,
Francesca
Tallia
ad,
Ion
Tcacencu
e and
Chiara
Vitale-Brovarone
*a
a,
Lucia
Pontiroli
ab,
Sonia
Fiorilli
a,
Antonio
Manca
c,
Francesca
Tallia
ad,
Ion
Tcacencu
e and
Chiara
Vitale-Brovarone
*a
aDepartment of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Torino, Italy. E-mail: chiara.vitale@polito.it; Tel: +39 011 0904716
bOral Biology, School of Dentistry, University of Leeds, LS9 7TF Leeds, UK
cRadiology Unit, Istituto di Candiolo – Fondazione del Piemonte per l'Oncologia (FPO), IRCCS, Strada Provinciale 142 km 3.95, 10060, Candiolo (Torino), Italy
dDepartment of Materials, Imperial College London, Prince Consort Road, SW7 2BP, London, UK
eDepartment of Dental Medicine, Karolinska Institutet, Huddinge, Sweden
First published on 16th November 2016
Abstract
In this study, an innovative injectable and bioresorbable composite cement (Spine-Ghost) has been developed by combining a radiopaque glass-ceramic powder (SCNZgc) and spray-dried mesoporous bioactive particles (W-SC) into type III alpha calcium sulphate hemihydrate (α-CSH) (composition α-CSH/SCNZgc/W-SC, 70/20/10 wt%). The Spine-Ghost cement and pure α-CSH (as a reference) were characterised in terms of physical and mechanical properties and compared to a commercial reference (Cerament® – Bonesupport AB, Sweden). The Spine-Ghost cement had a setting time comparable with Cerament® showing a good injectability in the range of 8–20 minutes after the end of mixing. In addition, the Spine-Ghost cement showed a good radiopacity when compared with standard PMMA (BonOs Inject, aap Biomaterials GmbH Germany) and higher compressive strength when compared to healthy cancellous bone. The bioactivity of both Spine-Ghost and Cerament® was evaluated through in vitro soaking in simulated body fluid (SBF). Spine-Ghost samples were highly bioactive, inducing the precipitation of hydroxyapatite crystals in the first week of soaking in vitro. It was also found that the degradation kinetics of the Spine-Ghost cement were faster than those of pure α-CSH and comparable to those of Cerament® after approximately 1 month of soaking in SBF. Moreover, the Spine-Ghost cement was cytocompatible in indirect-contact culture in vitro. Overall results indicate that the Spine-Ghost cement might be a very good candidate for vertebroplasty application and could enhance new bone formation in vivo.
1. Introduction
Vertebral compression fractures (VCFs) are deformities of the vertebral body that usually do not require an open surgical approach and are often due to osteoporosis or low energy trauma. They may heal by themselves with a conservative management: brace support and bed rest, combined with the administration of analgesics and bisphosphonates. When the non-surgical approach is not effective, VCFs usually lead to untreatable pain, spine deformity and disability with a proven increase of mortality, especially in patients with poor conditions.1 An alternative minimal invasive approach to VCFs is represented by vertebroplasty (VP) and kyphoplasty (KP) that are two percutaneous spine interventions performed with the goal of relieving back pain and, when possible, restoring the vertebral height.2 Vertebroplasty, developed in the mid 1980s by Deramond and Galibert3 for a vertebral hemangioma and applied afterwards for osteoporotic fractures,4 involves the injection of a bone cement through the trabeculae of the fractured vertebral body. Cement injection is performed under continuous radiological control through a needle inserted in the vertebra by a transpedicular percutaneous approach. In kyphoplasty, developed in 2001, a balloon tamp is inserted through a vertebroplasty needle in the fractured vertebral body and then is inflated/deflated and the created cavity is filled with the bone cement5,6 injected through a needle. In randomised trials it was found that VP and KP are equally safe and significantly superior to conservative treatment.7–9Injectable bone cements (IBCs) play a critical role in determining the effectiveness of VP and KP.
Table 1 reports the list of the most relevant properties for an IBC.10,11
| • No toxicity |
| • Easy injectability and handling |
| • Sufficient radiopacity in order to be able to follow the cement flow under fluoroscopy |
| • Working time in the range of 6–10 min |
| •Appropriate setting time of about 15 min |
| • Appropriate resorption rate (neither too high nor too low) |
| • Appropriate mechanical properties for immediate reinforcement of the vertebral body, the value should be comparable to those of the healthy vertebral body (the compressive strength of cancellous bone is about 2–12 MPa) |
| • Reasonable cost |
Over the twentieth century, calcium sulphate has been considered as a bone substitute in non-load-bearing bone injuries, due to its superior characteristics such as biocompatibility, biodegradability, osteoconductivity, non-toxicity and mechanical strength12–14 comparable to cancellous bone.15 However, its clinical applications have been limited due to its poor bioactivity,16 lack of osteoinductivity17 and too fast resorption rate in vivo18,19 in comparison with bone regeneration.
Currently, the number of commercial calcium sulphate-based cements is limited: A-GRIX® (AG Digital Technology Corp), Cerament® (BoneSupport AB (Sweden)), OsteoCure® (Futura Biomedical (US)), DentoGen® (Orthogen Corporation), MIIG® X3 and Pro-Dense® (Wright Medical (US)). These cements are composed of one or more solid phases and a liquid phase, which are mixed before operating.20 Among these commercial calcium sulphate-based bone cements, the use of MIIG® in 28 patients was described by Chen and co-workers21 for the open surgical treatment of thoracolumbar burst fractures with posterior instrumentation/decompression combined with vertebroplasty. The safety and efficacy of Cerament® (Bonesupport AB, Sweden) were first described in a prospective non-randomised multicentre study on 15 patients,22 followed by a prospective study on 20 patients and a histological study on a rabbit bone defect documenting bone ingrowth.23
In recent years, several approaches have been proposed for the development of synthetic calcium-based bone cements. For example, Yang and co-workers24 combined calcium sulphate with β-tricalcium phosphate to improve the osteoregenerative capacity. In 2013, Chen and co-workers25 improved the rapid resorption of calcium sulphate by adding dicalcium silicate. In 2015, Tan and co-workers26 reported a composition of calcium sulphate cement/silica-based mesoporous materials (SBA-15) for the controlled release of BMP-2.
Bioactive glasses are a group of surface reactive biomaterials that in certain ranges of compositions are characterised by an excellent osteoconductivity and ability to stimulate bone formation. Mesoporous bioactive glasses (MBGs) are a more recent subcategory of bioactive glasses that have received considerable research interest in the last 5 years.27 This increasing interest is due to their excellent structural features such as a well-ordered mesoporous channel structure, a large specific surface area, a tunable volume and pore size (5 to 20 nm) and a narrow pore diameter distribution.28,29
Calcium phosphate and calcium sulphate cements seem to be applicable as biomaterials for vertebral stabilisation and augmentation.30,31 However these cements have some shortcomings such as poor injectability and handling, low mechanical strength, low viscosity and too fast absorption.30–32 To bypass these limitations, the purpose of our study was to develop an injectable composite cement based on type III alpha calcium sulphate hemihydrate (α-CSH) as a resorbable matrix, enriched with mesoporous glass particles and a glass-ceramic radiopaque phase. Setting time, injectability, compressive strength and in vitro bioactivity and degradability in simulated body fluid (SBF) of the developed injectable cement were assessed. Biological tests using rat bone marrow stromal cells were also carried out in vitro.
2. Materials and methods
2.1. Synthesis and characterisation of mesoporous powders and glass-ceramic particles
Mesoporous bioactive glass particles, with the theoretical composition of SiO2/CaO: 80/20 mol%, were synthesised by an aerosol-assisted spray-drying method as described elsewhere.33 All the precursors were purchased from Sigma-Aldrich and used as received.In brief, 2.03 g of P123 (EO20PO70EO20, where “EO” is poly(ethylene glycol) and “PO” is poly(propylene glycol)) was dissolved in distilled water (85 mL) until a transparent solution was obtained. In the meantime, 10.73 g of tetraethyl orthosilicate (TEOS) were pre-hydrolysed in an aqueous hydrochloric acid under vigorous stirring to obtain a transparent solution. Subsequently, pre-hydrolysed TEOS was added dropwise to the polymeric solution and stirred for one hour to obtain a transparent solution. Afterwards, 3 g of calcium nitrate tetrahydrate (CaNT) were added to the mixed aqueous solution and stirred for 20 minutes. Spray-drying of the final synthesis solution was carried out using a Büchi Mini Spray-Dryer B-290. The collected sprayed powders were then calcined at 700 °C for 5 hours. The obtained mesoporous powders will be referred to as W-SC hereafter.
The N2 adsorption–desorption isotherm of the W-SC powders was evaluated using a Quantachrome Autosorb-1 instrument. The specific surface area was calculated through the BET (Brunauer–Emmett–Teller) model in the relative pressure range of 0.04–0.1 and pore size distribution was obtained through the DFT (Density Functional Theory) method, using the NLDFT (Nonlocal Density Functional Theory) equilibrium model.
Powders were characterised by X-ray diffraction (XRD, PhilipsX'Pert diffractometer, 2θ within 10–70°) and XRD patterns were analysed using X-Pert high score software. In addition, powders were characterised using a field emission scanning electron microscope (FESEM, Merlin-Zeiss) and an energy dispersive X-ray spectrophotometer (EDS). To prepare the samples for FESEM observations, the mesoporous powders were dispersed in ultrapure isopropanol and sonicated, and then a drop of this suspension was dispensed on a copper grid with holey carbon (200 mesh).
Glass-ceramic particles (SCNZgc) with composition SiO2/CaO/Na2O/ZrO2, 57/30/6/7 mol% were prepared by melting and casting processes. Briefly, silica (SiO2), calcium carbonate (CaCO3), sodium carbonate (Na2CO3) and zirconia (ZrO2) were melted in a platinum crucible at 1600 °C, followed by rapid quenching in water. Thereafter, the glass frit was ceramised at 1150 °C for 3 hours. This process was followed by grinding and sieving to yield powders below 20 μm.34
2.2. Preparation and characterisation of the bioceramic composite cement
The composite cement was developed based on Vitale-Brovarone's patent (EP2569025). It was prepared by mixing SCNZgc particles and spray-dried mesoporous W-SC particles with α-calcium sulphate hemihydrate (α-CSH) powder. The different components were mixed using the following proportion: α-CSH/SCNZgc/W-SC: 70/20/10 wt%. The obtained powder was then mixed with distilled water using a liquid to powder ratio (L/P) of 0.4 mL g−1 to obtain an injectable suspension. Upon contact with water, the α-calcium sulphate hemihydrate becomes calcium sulphate dihydrate (CSD), which has a lower solubility and thus precipitates in a continuous process that leads to the powder setting. The obtained cement will be referred to as Spine-Ghost hereafter.The phase composition, the microstructural morphology as well as the elemental analysis of Spine-Ghost were characterised by XRD analysis and FESEM coupled with EDS, respectively. Since Spine-Ghost samples were not conductive, they were coated with a metal layer (Cr) to allow for FESEM observation.
2.3. Setting time, injectability and compressive strength
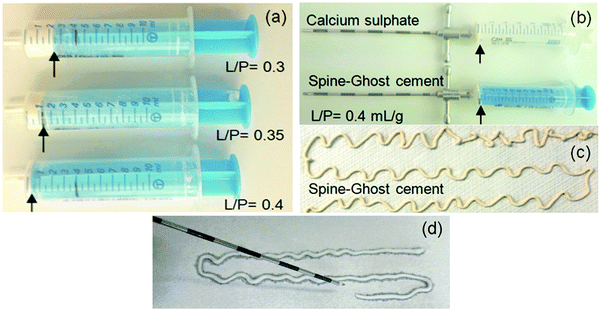
The setting times of the calcium sulphate cement (CSC as a reference), the Spine-Ghost cement and Cerament® (commercial reference) were evaluated using the Gillmore apparatus according to the ASTM C 266-08e1. The test consists of two parts: in the first one, a light rod (96.3 g) is coupled with a thick needle (2 mm), while in the second one a heavier rod (400 g) is combined with a thinner needle (1 mm). The cement sample was inserted into a mould as soon as the powder and the liquid phase were sufficiently mixed and a fluid paste was obtained. During the test, the rod and its needle were periodically allowed to fall and the needle imprint was evaluated. Specifically, the initial setting time is evaluated as the time lapse between mixing the powder and liquid phases until the lighter thick needle put in contact with the surface of the paste does not leave a visible trace any longer. The final setting time is defined as the time when the heavier thin needle can be plunged on the surface of the specimen without producing any visible impression. For each group of samples, three specimens were tested and the results were reported as mean value ± standard deviation (M ± SD).The injectability of the Spine-Ghost cement and the CSC was evaluated qualitatively by extruding a certain amount of paste through a 13 gauge vertebroplasty needle (internal diameter 1.803 mm). To optimise the L/P of the Spine-Ghost cement, different L/P ratios were evaluated. For this reason, 5 g of, respectively, Spine-Ghost and calcium sulphate hemihydrate (CSH) powders were mixed with distilled water in a syringe coupled with a vertebroplasty needle (13 gauge), L/P = 0.3, 0.35 and 0.4 mL g−1. The syringe was pressed manually under constant pressure and speed, until the paste could be extruded. The quantity of the cement remaining in the syringe was then visually compared for the different samples.
In order to evaluate the compressive strength of Spine-Ghost, CSC and Cerament®, cylindrical specimens (Φ 13.5 mm, h 10.0 mm) were prepared by using custom made polymeric moulds. The compressive test of the samples was performed under both wet and dry conditions, respectively, after one and seven days from the sample preparation, based on ASTM F2224-09. The test was carried out with a 5 kN load cell, using a crosshead speed of 1 mm min−1 by means of a Zwick/Z100 machine from Zwick/Roell. Three samples per type of cement were tested, the compressive strength was calculated for each group and the results were expressed as M ± SD. Compressive strength (σc) was defined as the maximum pressure load sustained by the sample (Fmax) divided by the cross area of the sample (A), as reported in eqn (1):
| σc = Fmax/A | (1) |
2.4. In vitro bioactivity test in simulated body fluid (SBF)
SBF solution was prepared according to the method described by Kokubo35 and has an ion concentration similar to that of human blood plasma. Cylindrical samples (Φ 13.5 mm, h 5 mm) of the CSC and the Spine-Ghost cement were prepared through the injection of the paste in the polymeric mould. After setting for 24 hours, specimens were soaked in SBF using a surface area to volume ratio of 0.048 cm−1. Samples were stored in an orbital shaker (IKA® KS 4000 i controls) for seven days at 37 °C and 120 rpm, without refreshing the solution. At specified times, specimens were rinsed with distilled water and dried at 60 °C for 5 hours. The deposition of hydroxyapatite (HA) crystals on the surface of specimens was then assessed by FESEM/EDS analysis after metallisation with Cr and wide angle X-ray diffraction (WAXRD) analysis. In addition, the bioactivity of SCNZgc particles was assessed by soaking 100 mg of SCNZgc particles in 100 mL of SBF. Samples were kept in an orbital shaker at 37 °C and 120 rpm for two weeks without refreshing the solution.36 At the end of the soaking time, the collected powders were gently rinsed with distilled water and dried at 60 °C. The morphology and composition of the glass-ceramic particles were evaluated through FESEM coupled with EDS analysis.2.5. In vitro degradation of the cements
The in vitro degradation of the Spine-Ghost, Cerament® and CSC was investigated by immersing cylindrical samples (Φ 13.5 mm, h 5.0 mm) in SBF solution with a surface area to volume ratio of 0.196 cm−1. The SBF solution was changed every 48 hours. Specimens were removed from the SBF after incubation at specific time points (1, 3, 7, 14 and 28 days). The extracted specimens were rinsed with distilled water and then dried at 60 °C for 5 hours to attain a constant weight. In order to calculate the degradation ratio, the initial (W0) and the final weights (Wt) of the specimens were recorded before and after soaking the sample in SBF (after complete drying). The pH value of the SBF was measured before the sample removal to verify that values remained within the physiological range that allows cells to survive. The degradation rate of the samples was investigated by calculating their weight loss percentage according to the following equation:| Degradation ratio = [(W0 − Wt)/W0] × 100 | (2) |
The morphology and phase composition of the soaked samples were characterised using FESEM/EDS and WAXRD, respectively.
2.6. Working and hardening time evaluation of the Spine-Ghost cement
In clinical applications, it is very important to know for how long the operator will be able to inject the bone cement and how fast the cement will harden at human body temperature. The working time should be long enough to let the operator fill the vertebral body with a slow injection to prevent leakages. The hardening time should be delayed enough to allow the needle repositioning, if required, after a first injection. On the other hand, the patient should pass from the prone to the supine position as soon as possible after the end of the procedure without waiting too long for cement hardening. With the aim of simulating the real clinical conditions, these tests were carried out in the interventional radiology operating room of Istituto di Candiolo – FPO, IRCCS (Turin, Italy). In this cancer institute, vertebroplasty is routinely performed by interventional radiologists since 2002 mainly for osteoporosis (>80% of cases) and a mean of 250 patients are treated each year for benign VCFs. Simulations were performed using a special syringe with a screw injecting system (Duro-Ject®, Cook Medical – Bloomington, IN – USA), coupled with a 13 gauge × 15 cm vertebroplasty needle (Optimed Medizinische Instrumente GmbH, Ettlingen-Germany). To this aim, distilled water and Spine-Ghost powders were mixed in a 20 mL syringe with L/P = 0.4 mL g−1 and transferred into the 10 mL syringe of the Duro-Ject® system.2.7. Radiopacity assessment of the Spine-Ghost cement
As previously mentioned (Table 1), radiopacity is one of the features that a bone cement must have to be considered suitable for vertebroplasty and kyphoplasty. Considering that the most frequent complication of VP and KP is cement leakage, the visibility of the bone cement under X-rays is crucial to stop the injection in time when an initial extravasation is detected. Small pulmonary emboli are relatively frequent37 and asymptomatic, but an important embolisation can be fatal38 as well as an endocanalar significant leakage can lead to neurological complications.39 For these reasons, radiopacity of the Spine-Ghost cement was evaluated at IRCCS and compared with the commercial control Cerament® (Bonesupport AB, Sweden), which is opacified with iohexol. Moreover, further assessment was carried out in comparison with a PMMA-based commercial bone cement with a standard radio-opacity called BonOs Inject (aap Biomaterials GmbH, Germany), opacified with 10.8 g of zirconium dioxide (out of 24 g of powder).2.8. In vitro biological assessment of the Spine-Ghost cement
For the in vitro assays, discs (5 mm diameter) made of the Spine-Ghost cement were used. Bone marrow-derived stromal cells (BMSCs) were isolated from adult male Sprague-Dawley rats as described previously40 and used at passages 3 to 5. The experiments were approved by the Stockholm South Ethical Committee in accordance with the policy on human care and use of laboratory animals. For all the in vitro assays, indirect-contact culture was used where BMSCs were seeded in triplicate on the base of 12-well plates (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2) and the biocement discs were placed into the inserts inside the wells (separated from the cells by a porous membrane with 1 μm pore size; all cell culture plastics from Corning) in a humidified atmosphere of 5% CO2 at 37 °C. The culture plastic was used as a control. The experiments were repeated twice.
000 cells per cm2) and the biocement discs were placed into the inserts inside the wells (separated from the cells by a porous membrane with 1 μm pore size; all cell culture plastics from Corning) in a humidified atmosphere of 5% CO2 at 37 °C. The culture plastic was used as a control. The experiments were repeated twice.
Cytocompatibility was assessed by tetrazolium salt colorimetric assay according to the manufacturer's instructions (Cell Proliferation Kit I (MTT), Roche Diagnostics) after 24 hours of culturing in alpha-minimum essential medium supplemented with 10% fetal bovine serum and antibiotics/antimycotics (all from Life Technologies, Inc.).
Alkaline phosphatase (ALP) activity was used as a marker for the osteogenic differentiation of BMSCs in the presence of the Spine-Ghost discs. For the ALP assay, the normal medium was replaced with commercially available osteogenic medium (Osteogenic Differentiation BulletKit™, Lonza) containing ascorbic acid, beta-glycerophosphate and dexamethasone. After 7 and 14 days of indirect-contact osteogenic culturing, the BMSCs were washed with phosphate buffer, then lysed with 0.2% Triton X-100 (Sigma-Aldrich), and sonicated. ALP activity was quantified by measuring the rate of formation of p-nitrophenol (pNP) produced by hydrolysis of p-nitrophenylphosphate (Sigma-Aldrich) in 1 M diethanolamine solution, buffered to pH 9.8 at 37 °C for 30 min using a spectrophotometer (Labsystems Multiskan MS), measured at 405 nm. The final ALP data were expressed as fold change vs. cells cultured alone.
3. Results
3.1. Material characterisation
As previously reported by the authors in ref. 33, W-SC showed a type IV nitrogen adsorption–desorption isotherm, typical of mesoporous materials, with a sharp step at approximately 0.7p/p0. The BET specific surface area is around 190 m2 g−1 and the pore volume 0.25 cm3 g−1. The average pore size, as calculated by the DFT method, was centred at 5 nm.
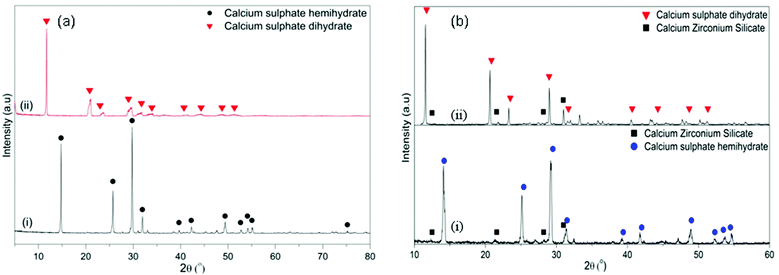
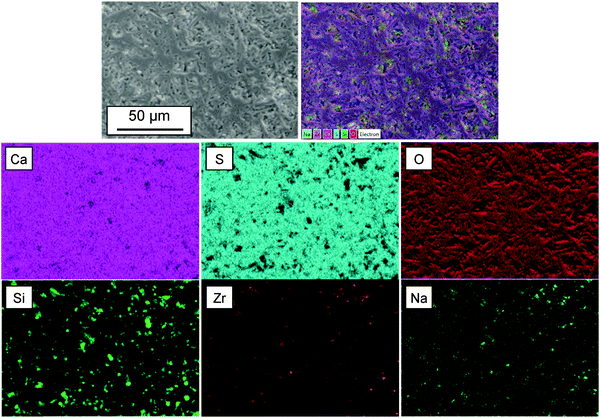
Fig. 3 shows the FESEM images of the α-CSH powder before and after hydration (CSC) and the Spine-Ghost cement after setting for 24 hours. As it can be observed in Fig. 3(a and b), the α-CSH powders had prismatic crystals with an average length and width of approximately 9 and 3 μm, respectively. The FESEM micrographs of the CSC showed the needle-like crystals of CSD (Fig. 3(c and d)). In addition, the initial composition of the CSC was confirmed by the EDS analysis (Fig. 3e), which detected Ca, O and S. FESEM micrographs of the Spine-Ghost cement are shown in Fig. 3(f and g) at different magnifications. As it can be seen, the Spine-Ghost cement was composed of micron-sized plates, spherical particles (W-SC) and some irregular particles that could be attributed to the three components of the cement calcium sulphate hemihydrate, mesoporous particles and glass-ceramic particles, respectively. The EDS qualitative analysis of the Spine-Ghost sample (Fig. 3h) showed the presence of S, O, Si, Ca, and Zr because of the different cement phases. Fig. 4 shows the EDS mapping analysis of S, O, Si, Ca, Zr and Na for the Spine-Ghost cement, which revealed a homogeneous distribution of the different phases throughout the cement, without segregation.
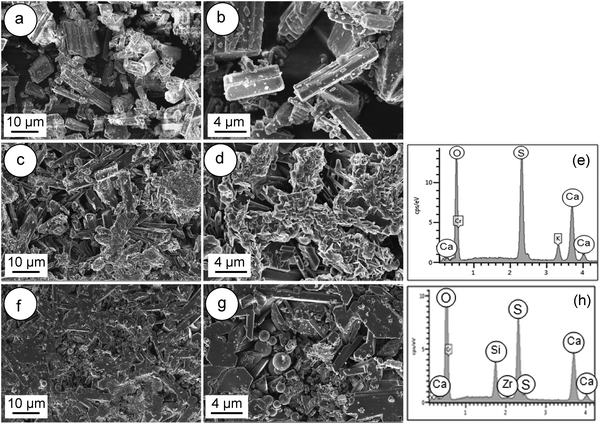 | ||
| Fig. 3 FESEM images of (a and b) the α-calcium sulphate hemihydrate powder (α-CSH); (c–e – EDS) the calcium sulphate cement (CSC); (f–h – EDS) the Spine-Ghost cement. | ||
3.2. Injectability, setting time and compressive strength of cements
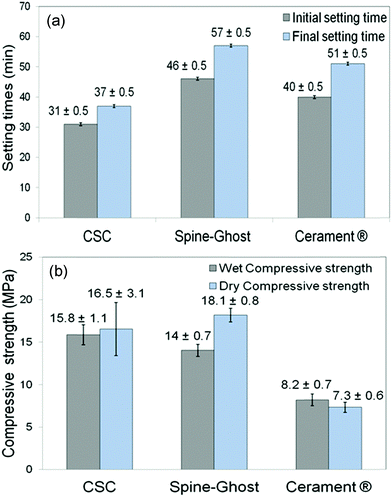
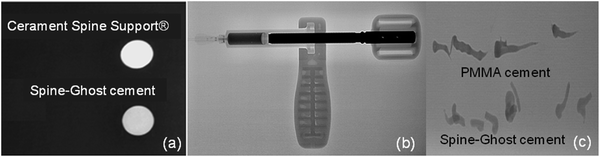
Fig. 5a displays the results of a qualitative injectability test of the Spine-Ghost cement through a 13 gauge vertebroplasty needle with various L/P ratios (0.3, 0.35 and 0.4 mL g−1). It was observed that the quantity of the residual paste inside the syringe was more than 2 mL for a L/P ratio of 0.3 due to the very high viscosity of the cement that hinders its complete injection. Instead, the amount of residual paste after injection was less than 1 mL for a L/P ratio of 0.4 (Fig. 5b). Therefore, the selected L/P ratio was set as 0.4 mL g−1 for the preparation of the optimised Spine-Ghost samples (Fig. 5b). Fig. 5c confirms that with the optimised liquid to powder ratio, Spine-Ghost showed a suitable injectability, comparable to the injectability of Cerament® as shown in Fig. 5d.The setting times of the CSC, Spine-Ghost and Cerament® are reported in Fig. 6a. According to the obtained results, the longest initial and final setting times were observed for the Spine-Ghost cement with (46 ± 0.5) and (57 ± 0.5) minutes, respectively. However, the composite cement hardened within 1 hour, which is a time comparable to Cerament®, taken as a commercial reference. Instead, the CSC exhibited the lowest initial (31 ± 0.5 min) and final (37 ± 0.5 min) setting times.
Wet and dry compressive strengths of the CSC, Spine-Ghost and Cerament® cements were measured after setting of the samples for one and seven days, respectively (Fig. 6b). These results suggested that the CSC had a relatively higher wet compressive strength (approximately 15.8 ± 1.1 MPa) compared to the Spine-Ghost cement (14 ± 0.7 MPa). Conversely, Spine-Ghost exhibited the highest compressive strength after setting for seven days (18.1 ± 0.8 MPa). These results clearly demonstrate that the Spine-Ghost cement has a higher compressive strength under both wet and dry conditions compared to Cerament®.
3.3. Osteointegration test: in vitro bioactivity
The in vitro bioactivity of a material for bone regeneration is assessed through the observation of the precipitation of a crystalline hydroxyapatite (HA) layer, resulting from dissolution, diffusion, ion exchange and precipitation steps. Firstly, an ion exchange between Ca2+ and H+ or H3O+ from solution occurs, which causes the formation of surface silanol groups (Si–OH). Subsequently, Ca2+ and PO43− groups adsorb to the surface to nucleate an amorphous calcium phosphate-rich layer. Finally, the incorporation of OH− and CO32− anions from the solution leads to the growth and crystallisation of the amorphous calcium phosphate layer and to the formation of a HA layer on the surface of the sample.29,41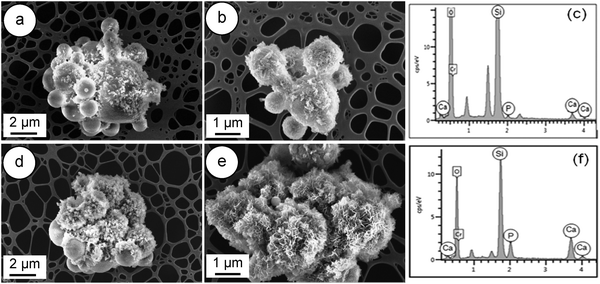 | ||
| Fig. 7 FESEM images of W-SC: (a and b) after immersion for 8 hours in SBF; (d and e) after 24 hours of soaking in SBF and related EDS spectra (c and f). | ||
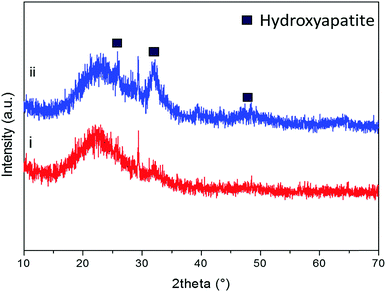
As previously reported by the authors,33 after soaking in SBF, the diffraction peaks of hydroxyapatite can be identified at approximately 2θ/26°, 29° and 32° after soaking the powders in SBF for 8 hours (Fig. 8(i)). As it can be observed in Fig. 8(ii), by increasing the soaking time up to 24 hours, the intensity of the hydroxyapatite peaks at 26°and 32°/2θ increased and new peaks at 2θ/40°, 47° and 49° appeared.
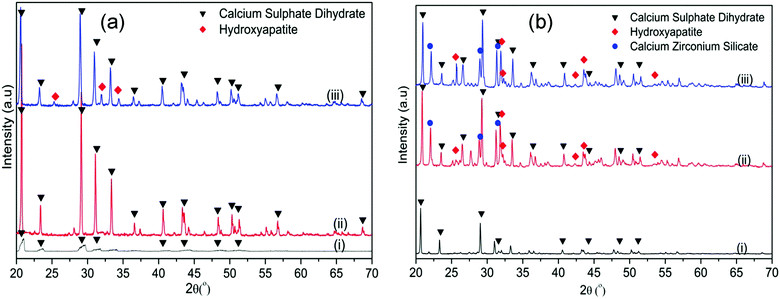
These results were further supported by the WAXRD analysis of the samples before and after soaking in SBF (Fig. 10). In the CSC, the only diffraction peaks that could be detected after 1 day of soaking were attributed to CSD (Fig. 10a(ii)). After 1 week of soaking (Fig. 10a(iii)), besides the CSD, peaks of HA could be identified at 2θ/25.88°, 31.79° and 34.07°. Conversely, in the WAXRD pattern of the Spine-Ghost sample after 1 day of immersion (Fig. 10b(ii)), the peaks of HA were immediately detected. The diffraction peaks of HA increased in intensity by increasing the soaking time up to 1 week (Fig. 10b(iii)).
3.4. Resorption kinetics of cements and pH value changes in SBF
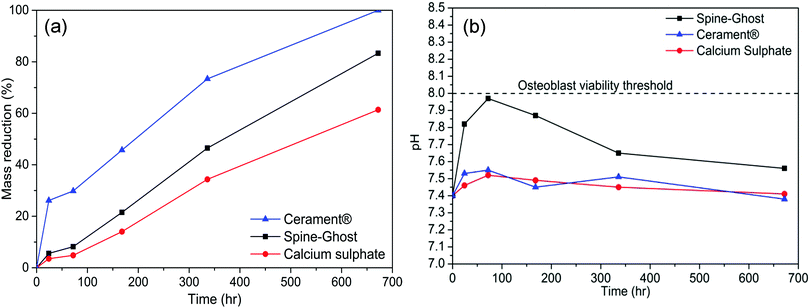
In order to assess the resorption kinetics, CSC, Spine-Ghost and Cerament® specimens were soaked in SBF for various time periods (1, 3, 7, 14, and 28 days). The resorption kinetics of cements is shown in Fig. 11: their dimensions were reduced gradually by a surface erosion process. The degradation ratio of the samples was also evaluated by calculating the percentage of mass reduction of the cement specimens (Fig. 12(a)). It was found that about 83.3% of the Spine-Ghost mass was dissolved in the SBF after 28 days of soaking. On the other hand, the degradation kinetics of the CSC were significantly slower for the 28 days of soaking with a mass loss of 61.5%, while the mass reduction of Cerament® was 100% after soaking for 28 days.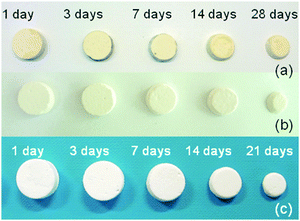 | ||
| Fig. 11 Photographs of (a) calcium sulphate cement (CSC); (b) Spine-Ghost; (c) Cerament® after soaking in SBF for 1, 3, 7, 14, 21 and 28 days. | ||
The pH values of the SBF solution collected throughout the 28 days of soaking are reported in Fig. 12(b): a sharp increase in the pH value from 7.4 to 7.97 was observed within 72 hours, followed by a gradual decrease to 7.56 after 28 days of soaking. At variance, the immersion of the CSC in SBF led to a slow increase of the pH values from 7.4 to 7.52 within 3 days, followed by a decrease from 7.52 to 7.41 between 3 and 28 days of soaking. For Cerament®, the pH value increased up to 7.55 in the first 3 days and decreased gradually to 7.38 after 28 days of soaking.
Fig. 13 shows FESEM images of the surface of Spine-Ghost and Cerament® cements after 28 days of soaking in SBF. A noticeable layer of bone-like apatite was observed on the surface of the Spine-Ghost cement (Fig. 13(a and b)), as well as on Cerament® (Fig. 13(d and e)).
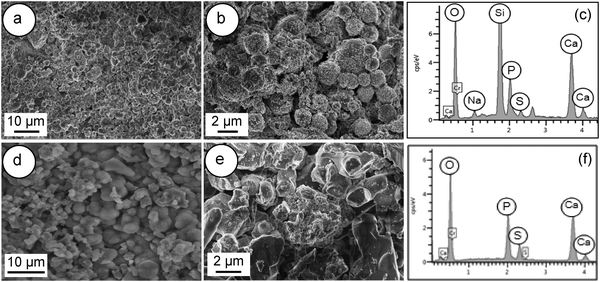 | ||
| Fig. 13 FESEM images and EDS of: (a–c) Spine-Ghost cement; (d–f) Cerament®; after soaking in SBF for 28 days. | ||
3.5. Simulating the clinical conditions
For clinical applications it is crucial to evaluate the hardening time at both room temperature (from 20 to 22 °C) and at body temperature. To assess the hardening time of Spine-Ghost, the paste was kept in an oven at 37 °C. At this temperature, the complete hardening of the Spine-Ghost cement was observed after about 40 minutes from the beginning of mixing.
3.6. In vitro biological assessment of the Spine-Ghost cement
The Spine-Ghost cement was well tolerated by the BMSCs after 24 h of culturing (89.3 ± 7.8% viability vs. cells cultured alone).However, the Spine-Ghost cement did not enhance the ALP activity of the BMSCs (Fig. 15). The Spine-Ghost might not be able to support the osteogenic differentiation of the BMSCs in vitro in the present set-up.
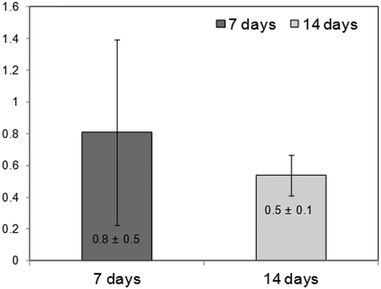 | ||
| Fig. 15 Fold change ALP activity on Spine-Ghost vs. cells cultured alone (=1), indirect-contact culture, 1 μm pore-size inserts, and 12 well-plate. | ||
The high dissolubility and inorganic ion accumulation of the Spine-Ghost cement in aqueous medium are likely to be the main factor to influence the cell behaviour in vitro.
4. Discussion
Calcium sulphate is desirable as an ideal regenerative material as:• It resorbs completely in a relatively short period of time.
• It provides a resorbable scaffold for bone growth.
• It may induce osteoblastic activity by the release of sufficient calcium ions.
• The dissolution rate of calcium sulphate makes it suitable as a delivery vehicle (growth factors, drugs, and antibiotics).42
One of the most popular commercial bone cements is Cerament® (Bonesupport AB, Sweden), which is composed of a mixture of synthetic calcium sulphate hemihydrate, hydroxyapatite and a non-ionic iodinated radiological contrast agent (iohexol).22 After setting, this cement has a compressive strength similar to that of healthy cancellous bone43 and a final setting time in the range of one hour.22 These results show that this injectable material is used as an alternative to PMMA for vertebroplasty.
The self-setting properties of bone cements such as injectability and setting time have crucial effects on their applicability in clinics.44 Injectability is an important property for a bioactive cement, because it grants an easy delivery of the paste through a needle or cannula.45 Injectable bone cements provide the possibility of injecting the paste into skeletal sites of limited accessibility, filling complicated cavities46 and containing defects such as periodontal bony defects, cysts, tumor removal sites and graft substitution gaps.45
Nevertheless, it is not possible to achieve a suitable injectability using a pure calcium sulphate cement due to the lack of inherent viscosity:44 during the injection from the delivery system, the cement paste suffers ‘filter pressing’, so that a separation of the powder and the liquid phase occurs.47 Furthermore, a pure calcium sulphate cement will have a too short final setting time (37 ± 0.5 min), which makes it unsuitable for clinical applications. For these reasons, pure injectable calcium sulphate cements have never been reported, whereas, if other phases are added, it is possible to develop a suitable cement such as Cerament®. In the present study, a novel injectable calcium sulphate-based cement (Spine-Ghost) was developed by incorporating mesoporous powders (W-SC) and glass-ceramic particles (SCNZ) into a calcium sulphate matrix. According to the obtained results, the quantity of the residual Spine-Ghost cement in the syringe, using the optimised L/P ratio (0.4 mL g−1), was less than 1 mL after injection through a 13 gauge vertebroplasty needle. It should be considered that, due to the shear thickening of the paste close to the neck of the syringe, a small amount of cement would always remain in this region even for the cement with full injectability.45 As it can be seen in Fig. 5(c and d) the injectability of the Spine-Ghost cement with the optimum liquid to powder ratio (0.4 mL g−1) is comparable to Cerament®. Moreover it should be mentioned that, during the injection of the Spine-Ghost cement filter pressing or phase separation was not observed (Fig. 5(c)). The absence of any phase separation effect was also confirmed by the EDS mapping analysis in which, in every section of the cement, a homogenous distribution of the different phases was observed.
The setting time of an injectable bone cement is a significant factor for its clinical applications.48 A bone cement with too short or too long setting times is not suitable to be utilised in clinical surgery.49 In fact, in clinical applications, the material needs to be injected before it begins to set and the cement has to reach its final setting time before the treated vertebra is loaded. The setting reaction of the calcium sulphate cement (CSC) can be divided in the following steps: (a) mixing of CSH with a solution leads to the formation of a suspension, (b) saturation of the solution by the dissolution of CSH, (c) precipitation of CSD after the super-saturation of the solution, (d) growth of CSD nuclei to larger crystals and (e) hardening of the cement due to the interlocking of the CSD crystals. Steps (c) and (e) can be identified as the initial and final setting times, respectively.50 Our data showed that the longest initial and final setting times were observed for the Spine-Ghost cement (46 ± 0.5 and 57 ± 0.5 min, respectively) compared to both the CSC (31 ± 0.5 and 37 ± 0.5 min) and Cerament® (40 ± 0.5 and 51 ± 0.5, min). However, the differences were quiet limited and the Spine-Ghost cement could be set within 1 hour, which was comparable with Cerament® from a clinical point of view. In fact, a bone cement with a slightly longer setting time improves the injectability and handling of the cement during the operation before the paste starts to set. Furthermore, the hardening time of the Spine-Ghost cement was evaluated by incubating the cement at a temperature of approximately 37 °C. The obtained results showed that the full hardening time of the Spine-Ghost cement was about 40 min from the beginning of the mixing which was comparable with Cerament® (full hardening within 45 min) supporting the suitability for clinical use.23
Since bone has to withstand high stresses and a challenging body environment, the mechanical properties of a hardened bone cement have been one of the main crucial factors for its clinical use.47
In clinical applications, the compressive strength of a cement material should be analogous or comparable with that of cancellous bone, which is in the range of 2–12 MPa.51,52 It is evident from the results that the hardening process improved the mechanical strength of the cements. Moreover, it is clear that the compressive strength of all the specimens was enhanced by increasing the testing time up to seven days. This increased strength can be explained by the growth of calcium sulphate dihydrate into larger crystals and by the interlocking of these crystals upon hardening of the cement. In this work, the compressive strength of Spine-Ghost under both wet and dry conditions (14 ± 0.7 and 18.1 ± 0.8 MPa, respectively) was higher than the one shown by Cerament® (8.2 ± 0.7 and 7.3 ± 0.6 MPa, respectively) ensuring an enhanced mechanical support compared to the commercial reference. Siemund and co-workers53 described their initial experience with Cerament® in seven patients that underwent vertebroplasty for osteoporotic VCFs, reporting a mean vertebral height loss after treatment of 3.6 mm (range 1.5–5.5) or 21% (range 8–32). These data can be due to incomplete vertebral filling (average injected volume: 1.9 mL, range 0.2–4.0) as a consequence of needle plugging or to a preliminary formulation of Cerament®, but the doubt of cement failure cannot be ruled out. The increase in the mechanical strength of Spine-Ghost can be attributed to the incorporation of the glass-ceramic particles and to the size and flowability of the W-SC particles. The small and spherical W-SC particles can fill the gaps between the hydrating calcium sulphate particles increasing the overall cement density and play a role in the reduction of the crack propagation in the composite. These results are comparable with in-use bone cements which have a compressive strength of around 11 MPa54 and are situated slightly below the upper limit of the compressive strength of cancellous bone (2–12 MPa).51,52 It should also be underlined that Spine-Ghost shows a satisfactory radiopacity (Fig. 14) in comparison with both a standard PMMA similarly opacified with zirconium dioxide (BonOs Inject) and with the calcium sulphate cement Cerament®, chosen as a commercial reference.
The radiopacity of Cerament®, which will fade away over time in the early follow-up, is obtained with an iodinated contrast medium, whereas Spine-Ghost owes its radiopacity to the glass-ceramic phase containing ZrO2 that will keep the bone cement visible until its complete resorption. The long-lasting radiopacity of Spine-Ghost allows for the checking of the integrity of the bone cement during follow-up in the case of pain relapse.
If we compare the safety profile of Cerament® and Spine-Ghost, we should underline that the first contains a liquid iodinated contrast medium (iohexol) that is commonly used intravenously for Computed Tomography. The prevalence of “allergic” reactions to nonionic iodinated contrast medium is generally quite low (reported from 0.3% to 3%)55 but in the cases56 of 2% the reaction can be severe and more difficult to manage during an intervention performed in the prone position. Most of the anaphylactoid reactions are also delayed and can mix up with other common post-procedural symptoms like nausea and pain, thus complicating the patient's management during recovery. In a study on 1000 patients57 who underwent Computed Tomography with intravenous contrast medium, the total adverse reactions that occurred with iohexol were 2.5% that was significantly higher than those that occurred with a different iodinated contrast medium (iodixanol, less than 1%, p < 0.05).
Another important characteristic of biomaterials is their bioactivity, which is defined as the ability of these materials to chemically bond with living bone tissue.58 Hydroxyapatite (HA) is a calcium phosphate based material with a chemical composition and a crystal structure similar to the mineral component of human bone and teeth.59 It plays a significant role in establishing a strong chemical bond with bone tissue. Generally, the ability of a biomaterial to induce hydroxyapatite formation in SBF solution is an accepted way to assess the bioactivity of that material in vitro, and can be used as an index of predictability for its in vivo behaviour. In our study, with the aim of imparting bioactive properties to the bone cement, a spray-dried mesoporous bioactive glass with SiO2/CaO binary composition (W-SC),33 characterised by a spherical morphology, a large exposed surface area and a pore volume, was properly incorporated inside the cement matrix.
The high surface area of W-SC led to an extremely high bioactivity assessed by means of an in vitro bioactivity test showing the formation of a hydroxyapatite layer on the surface of the mesoporous powders after only 8 hours of soaking in SBF. This result is very encouraging and could indicate a potential simulation of bone regeneration in vivo based on the pivotal role of W-SC in the nucleation of HA crystals. In the present study, the very low bioactivity of the calcium sulphate cement was noticeably improved by incorporating the W-SC powders into the CSC. In fact, the in vitro results showed that Spine-Ghost induced the precipitation of a considerable amount of apatite only after 24 hours of immersion, with much faster kinetics if compared to calcium sulphate (Fig. 9). The excellent bioactivity of Spine-Ghost is due to the fast and remarkable release of osteoinductive ions such as Ca and Si from the mesoporous spray-dried powders (W-SC), which play an active biological role in influencing the rate of bone regeneration.60 The release of Si ions in SBF led to a high concentration of surface silanol groups that resulted in a faster nucleation and mineralisation of apatite crystals.29 Besides, as an added value, W-SC powders, because of their well-ordered accessible pores, could potentially provide the ability of loading and releasing drugs and biomolecules in a controlled fashion directly at the intervention site.
Another important feature of a bone cement is its resorbability, which allows new bone tissue to grow into the defect and to replace the cement in a few months.19,25 Over the last few decades, highly resorbable bone substitutes have received considerable interest in bone surgery as they can allow for the reduction of inflammation, stiffness and pain.19,20 In fact, resorption kinetics of degradable materials should match the new bone formation so that these materials are gradually substituted by the new bone tissue, in order to guarantee a continuous support to the vertebra.26 The degradation rate of calcium sulphate-based cements is more rapid than the rate of new bone formation, which often leads to its replacement with fibrous tissue. Therefore, designing calcium sulphate-based bone cements with suitable degradability is a major target. In our study, the degradation rates of calcium sulphate, Spine-Ghost and Cerament® cements were evaluated by their weight loss after soaking in SBF for various time periods. The Spine-Ghost cement showed a weight loss ratio of 83.3% after 28 days of soaking (Fig. 12(a)) and thus faster degradability compared to calcium sulphate (61.5%). The results demonstrated that the Spine-Ghost cement was fully degraded after 32 days of immersion in SBF, hence after a slightly longer time than Cerament®, which showed 100% resorption after 28 days of soaking. It should be underlined that these mass losses were evaluated according to the ISO standards by completely soaking the sample in SBF whereas, once injected, the cement will not be completely surrounded by the physiological fluids and thus the clinical practice generally shows slower resorption rates. Furthermore, the results showed that the pH of the SBF solution increased within 3 days of soaking due to Ca ion release and the formation of phosphate, calcite or apatite.29 It is noteworthy that the immersion of the Spine-Ghost samples in SBF solution led to a slightly alkaline environment, which is an advantage for the nucleation rate of hydroxyapatite crystals.61
These very encouraging results about the mechanical properties, setting times, bioactivity, degradability and biocompatibility of the Spine-Ghost cement, which proved to be equal or superior to the commercial reference, are the groundwork on which more extensive biological tests in vitro and in vivo (in sheep) are ongoing.
5. Conclusions
In the present study, a novel calcium sulphate hemihydrate-based composite cement (Spine-Ghost) was described. The Spine-Ghost cement was prepared by mixing glass-ceramic particles (to impart radiopacity) and mesoporous particles (to impart bioactivity) with alpha calcium sulphate (as a resorbable matrix), in order to improve the mechanical properties, setting times and bioactivity of the composite cement. The obtained results revealed that the setting time of the Spine-Ghost cement was comparable to that of Cerament® and Spine-Ghost showed improved injectability and handling. By simulating the clinical conditions, it was found that the Spine-Ghost cement was easily injectable in the range of 8–20 minutes after mixing. Furthermore, the comparison of Spine-Ghost with other commercial products based on PMMA and on calcium sulphate (BonOs Inject® and Cerament®, respectively) showed that this novel composite cement had a very good radiopacity, allowing us to easily follow the cement flow during the clinical procedure. Our findings showed that Spine-Ghost had the highest compressive strength compared to both the reference (pure calcium sulphate) and Cerament®. Moreover, the Spine-Ghost cement showed superior bioactivity by inducing the bone-like apatite formation on the surface of the cement only after one day of soaking in SBF solution. It was also found that the Spine-Ghost cement had a higher degradability in comparison with pure calcium sulphate after soaking for 28 days, which was comparable to Cerament®. Moreover, the Spine-Ghost cement was cytocompatible in indirect-contact culture in vitro. Altogether, the results indicate that the Spine-Ghost cement might be a very good candidate for vertebroplasty applications and could enhance new bone formation in vivo.Acknowledgements
The research leading to these results has received funding from the European Union Seventh Framework Program (FP7/2007-2013) under grant agreement no. [280575]-Restoration.References
- D. Bliuc, N. D. Nguyen, V. E. Milch, T. V. Nguyen, A. Eisman and J. R. Center, JAMA, J. Am. Med. Assoc., 2009, 30, 513–521 CrossRef PubMed.
- A. B. Dublin, J. Hartman, R. E. Latchaw, J. K. Hald and M. H. Reid, Am. J. Neuroradiol., 2005, 26, 489–492 Search PubMed.
- P. Galibert, H. Deramond, P. Rosat and D. Le Gars, Neuro-Chirurgie, 1987, 33, 166–168 CAS.
- C. Debussche-Depriester, H. Deramond, P. Fardellone, A. Heleg, L. Sebert, L. Cartz and P. Galibert, Neuroradiology, 1991, 33, 149–152 CrossRef.
- S. M. Belkoff, J. M. Mathis, D. C. Fenton, R. M. Scribner, M. E. Reiley and K. Talmadge, Spine, 2001, 26, 151–156 CrossRef CAS PubMed.
- S. R. Garfin, H. A. Yuan and M. A. Reiley, Spine, 2001, 26, 1511–1515 CrossRef CAS PubMed.
- M. Dohm, C. M. Black, A. Dacre, J. B. Tillman and G. Fueredi, Neuroradiology, 2014, 35, 2227–2236 CrossRef CAS PubMed.
- C. A. Klazen, P. N. Lohle, J. de Vries, F. H. Jansen, A. V. Tielbeek, M. C. Blonk, A. Venmans, W. J. Van Rooij, M. C. Schoemaker, J. R. Juttmann, T. H. Lo, H. J. Verhaar, Y. van der Graaf, K. J. van Everdingen, A. F. Muller, O. E. Elgersma, D. R. Halkema, H. Fransen, X. Janssens and E. Buskens, Lancet, 2010, 376, 1085–1092 CrossRef.
- D. Wardlaw, S. R. Cummings, J. V. Meirhaeghe, L. Bastian, J. B. Tillman, J. Ranstam, R. Eastell, P. Shabe, K. Talmadge and S. Boonen, Lancet, 2009, 373, 1016–1024 CrossRef.
- G. Lewis, J. Biomed. Mater. Res., 2006, 76, 456–468 CrossRef PubMed.
- P. F. Heini and U. Berlemann, Eur. Spine, 2001, 10, 205–213 CrossRef PubMed.
- A. Perry, A. Mahar, J. Massie, N. Arrieta, S. Garfin and C. Kim, Spine, 2005, 5, 489–493 CrossRef PubMed.
- C. Yashavantha Kumar, K. B. Nalini, J. Menon, D. K. Patro and B. H. Banerji, J. Clin. Diagn. Res., 2013, 7, 2926–2928 Search PubMed.
- Y. F. Tsai, C. C. Wu, F. Y. Fan, H. C. Cheng, Y. C. Liaw, Y. K. Huang, L. H. Hsu and K. C. Yand, Process Biochem., 2014, 49, 2285–2291 CrossRef CAS.
- D. J. Hak, J. Am. Acad. Orthop. Surgeons, 2007, 15, 525–536 CrossRef.
- G. Yang, J. Liu, F. Li, Z. Pan, X. Ni, Y. Shen, H. Xu and Q. Huang, Mater. Sci. Eng., 2014, 35, 70–76 CrossRef CAS PubMed.
- R. Guarnieri, G. Pecora, M. Fini, N. N. Aldini, R. Giardino, G. Orsini and A. Piattelli, J. Periodontol., 2004, 75, 902–908 CrossRef PubMed.
- Z. He, Q. Zhai, M. Hu, C. Cao, J. Wang, H. Yang and B. Li, J. Orthopaed. Transl., 2015, 3, 1–11 Search PubMed.
- G. Hu, L. Xiao, H. Fu, D. Bi, H. Ma and P. Tong, J. Mater. Sci.: Mater. Med., 2010, 21, 627–634 CrossRef CAS PubMed.
- M. Bohner, Eur. Cells Mater., 2010, 20, 1–12 CAS.
- C. Chen, G. LV, B. Xu, X. Zhang and X. Ma, Eur. Spine, 2014, 23, 1548–1557 CrossRef PubMed.
- M. Rauschmann, T. Vogl, A. Verheyden, R. Pflugmacher, T. Werba, S. Schmidt and J. Hierholzer, Eur. Spine, 2010, 19, 887–892 CrossRef PubMed.
- H. P. Hatten JR and M. J. Voor, Interv. Neuroradiol., 2012, 18, 105–113 CAS.
- H. L. Yang, X. S. Zhu, L. Chen, C. M. Chen, D. C. Mangham, L. A. Coulton and S. S. Aiken, J. Biomed. Mater. Res., 2012, 100, 1911–1921 CrossRef CAS PubMed.
- C. C. Chen, C. W. Wang, N. S. Hsueh and S. J. Ding, J. Alloys Compd., 2014, 585, 25–31 CrossRef CAS.
- H. Tan, S. Yang, P. Dai, W. Li and B. Yue, Nanomedicine, 2015, 10, 4341–4350 CAS.
- C. Wu and J. Chang, Interface Focus, 2012, 2, 292–306 CrossRef PubMed.
- X. Yan, C. Yu, X. Zhou, J. Tang and D. Zhao, Angew. Chem., Int. Ed., 2004, 43, 5980–5984 CrossRef CAS PubMed.
- M. Mozafari, F. Moztarzadeh and M. R. Tahriri, J. Non-Cryst. Solids, 2010, 356, 1470–1478 CrossRef CAS.
- T. H. Lim, G. T. Brebach, S. M. Renner, W. J. Kim, J. G. Kim, R. E. Lee and G. B. Andersson, Spine, 2002, 27, 1297–1302 CrossRef PubMed.
- I. A. Grafe, M. Baier, G. Noldge, C. Weiss, K. Da Fonseca, J. Hillmeier, M. Libicher, G. Rudofsky, C. Metzner, P. Nawroth, P. J. Meeder and C. Kasperk, Spine, 2008, 33, 1284–1290 CrossRef PubMed.
- E. F. Burguera, H. H. K. Xu and L. Sun, J. Biomed. Mater. Res., Part B, 2007, 84, 493–502 Search PubMed.
- L. Pontiroli, M. Dadkhah, G. Novajra, I. Tcacencu, S. Fiorilli and C. Vitale-Brovarone, Mater. Lett. Search PubMed , under review.
- C. Vitale-Brovarone, L. Pontiroli, G. Novajra, I. Tcacencu, J. Reis and A. Manca, Key Eng. Mater., 2015, 631, 43–47 CrossRef.
- T. Kokubo and H. Takadama, Biomaterials, 2006, 27, 2907–2915 CrossRef CAS PubMed.
- A. L. B. Maçon, T. B. Kim, E. M. Valliant, K. Goetschius, R. K. Brow, D. E. Day, A. Hoppe, A. R. Boccaccini, I. Y. Kim, C. Ohtsuki, T. Kokubo, A. Osaka, M. Vallet-Regí, D. Arcos, L. Fraile, A. J. Salinas, A. V. Teixeira, Y. Vueva, R. M. Almeida, M. Miola, C. Vitale-Brovarone, E. Verné, W. Höland and J. R. Jones, J. Mater. Sci.: Mater. Med., 2015, 26, 1–10 CrossRef PubMed.
- R. Schmidt, B. Cakir and T. Mattes, Eur. Spine, 2005, 14, 466–473 CrossRef CAS PubMed.
- F. Monticelli, H. J. Meyer and E. Tutsch-Bauer, Forensic Sci. Int., 2005, 149, 35–38 CrossRef CAS PubMed.
- Y. J. Chen, T. S. Tan and W. H. Chen, Spine, 2006, 31, 379–382 CrossRef PubMed.
- G. Tour, M. Wendel and I. Tcacencu, J. Tissue Eng. Regener. Med., 2014, 8, 841–849 CrossRef CAS PubMed.
- N. Letaïef, A. Lucas-Girot, H. Oudadesse, R. Dorbez-Sridi and P. Boullay, Microporous Mesoporous Mater., 2014, 195, 102–111 CrossRef.
- M. V. Thomas and D. A. Puleo, J. Biomed. Mater. Res., Part B, 2009, 88, 597–610 CrossRef PubMed.
- M. Nilsson, J. S. Wang, L. Wielanek, K. E. Tanner and L. Lidgren, J. Bone Jt. Surg., 2004, 86, 120–125 CAS.
- Z. Huan and J. Chang, Acta Biomater., 2007, 3, 952–960 CrossRef CAS PubMed.
- S. Sandhya, S. S. Babu, K. V. Nishad, H. Varma and M. Komath, J. Mater. Sci.: Mater. Med., 2015, 26–31 CAS.
- C. V. Rahman, A. Saeed, L. J. White, T. W. A. Gould, G. T. S. Kirby, M. J. Sawkins, C. Alexander, F. R. A. J. Rose and K. M. Shakesheff, Chem. Mater., 2012, 24, 781–795 CrossRef CAS.
- L. Zhao, M. D. Weir and H. H. K. Xu, Biomaterials, 2010, 31, 6502–6510 CrossRef CAS PubMed.
- Z. Chen, H. Liu, X. Liu, X. Lian, Z. Guo, H. J. Jiang and F. Z. Cui, Mater. Sci. Eng., C, 2013, 33, 1048–1053 CrossRef CAS PubMed.
- W. Liu, C. Wu, W. Liu, W. Zhai and J. Chang, J. Biomed. Mater. Res., Part B, 2012, 10, 279–286 Search PubMed.
- K. J. Anusavice, Phillips' science of dental materials, Elsevier, Saunders, 11th edn, 2008 Search PubMed.
- M. N. Rahaman, D. E. Day, B. S. Bal, Q. Fu, S. B. Jung, L. F. Bonewald and A. P. Tomsia, Acta Biomater., 2011, 7, 2355–2373 CrossRef CAS PubMed.
- J. S. Temenoff and A. G. Mikos, Biomaterials, 2000, 21, 2405–2412 CrossRef CAS PubMed.
- R. Siemund, L. T. Nilsson, M. Cronqvist and B. Stromqvist, Interv. Neuroradiol., 2009, 15, 335–340 Search PubMed.
- S. V. Dorozhkin, Int. J. Mater. Chem., 2011, 1, 1–48 CrossRef.
- A. Rosado Ingelmo, I. Doña Diaz, R. Cabañas Moreno, M. C. Moya Quesada, C. García-Avilés, I. García Nuñez, J. I. Martínez Tadeo, R. Mielgo Ballesteros, N. Ortega-Rodríguez, M. A. Padial Vilchez, L. Sánchez-Morillas, C. Vila Albelda, E. Moreno Rodilla and M. J. Torres Jaén, J. Invest. Allergol. Clin. Immunol., 2016, 26, 144–155 CrossRef CAS PubMed.
- C. L. Wang, R. H. Cohan, J. H. Ellis, E. M. Caoili, G. Wang and I. R. Francis, Am. J. Roentgenol., 2008, 191, 409–415 CrossRef PubMed.
- Y. Xiao, G. Zeng, X. Liu, C. Peng, C. Lai and P. Zhou, SpringerPlus, 2016, 5, 148–153 CrossRef PubMed.
- S. M. Kenny and M. Buggy, J. Mater. Sci.: Mater. Med., 2003, 14, 923–938 CrossRef CAS PubMed.
- A. Ruksudjarit, K. Pengpat, G. Rujijanagul and T. Tunkasiri, Curr. Appl. Phys., 2007, 8, 270–272 CrossRef.
- L. L. Hench, J. Eur. Ceram. Soc., 2009, 29, 1257–1265 CrossRef CAS.
- J. Liu, K. Mao, Z. Liu, X. Wang, F. Cui, W. Guo, K. Mao and S. Yang, PLoS One, 2013, 8, e75668 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tb02139e |
| This journal is © The Royal Society of Chemistry 2017 |